Voltage-Dependent Calcium Channels, Calcium Binding Proteins, and Their Interaction in the Pathological Process of Epilepsy
Abstract
:1. Introduction
2. The Function and Distribution of VDCCs in the Central Nervous System
3. VDCCs in the Pathological Process of Epilepsy
4. The Function and Distribution of CBPs in the Central Nervous System
5. CBPs in the Pathological Process of Epilepsy
5.1. Background
5.2. CaM in the Pathological Process of Epilepsy
5.3. CB in the Pathological Process of Epilepsy
5.4. CR in the Pathological Process of Epilepsy
5.5. PV in the Pathological Process of Epilepsy
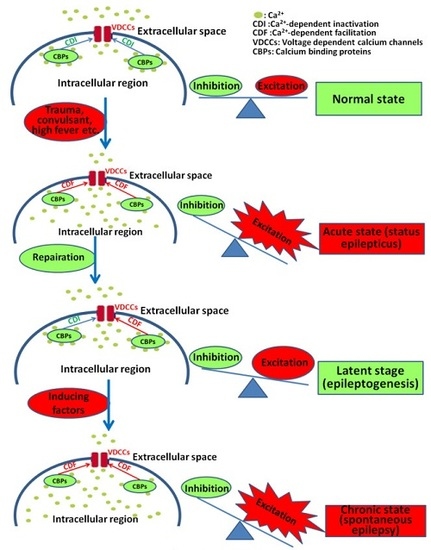
6. Regulation of CBPs on VDCCs and the Implication of the Interaction between CBPs and VDCCs in the Pathological Process of Epilepsy
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Larkin, J.G.; Besag, F.M.; Cox, A.; Williams, J.; Brodie, M.J. Nifedipine for epilepsy? A double-blind, placebo-controlled trial. Epilepsia 1992, 33, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Steinlein, O.K. Calcium signaling and epilepsy. Cell Tissue Res. 2014, 357, 385–393. [Google Scholar] [CrossRef] [PubMed]
- William, A.; CatterallCatterall, W.A. Voltage-gated calcium channels. Cold Spring Harbor Perspect. Biol. 2011, 3, a003947. [Google Scholar]
- Spitzer, N.C. Calcium: First messenger. Nat. Neurosci. 2008, 11, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, C.P.; Bading, H. Nuclear calcium signaling. Adv. Exp. Med. Biol. 2012, 970, 377–405. [Google Scholar] [PubMed]
- Bading, H.; Ginty, D.D.; Greenberg, M.E. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 1993, 260, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Chawla, S.; Johnson, C.M.; Bading, H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature 1997, 385, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Bading, H. CREB/CBP and SRE-interacting transcriptional regulators are fast on-off switches: Duration of calcium transients specifies the magnitude of transcriptional responses. J. Neurochem. 2001, 79, 849–858. [Google Scholar] [CrossRef] [PubMed]
- West, A.E.; Chen, W.G.; Dalva, M.B.; Dolmetsch, R.E.; Kornhauser, J.M.; Shaywitz, A.J.; Takasu, M.A.; Tao, X.; Greenberg, M.E. Calcium regulation of neuronal gene expression. Proc. Natl. Acad. Sci. USA 2001, 98, 11024–11031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellstrom, B.; Savignac, M.; Gomez-Villafuertes, R.; Naranjo, J.R. Ca2+-operated transcriptional networks: Molecular mechanisms and in vivo models. Physiol. Rev. 2008, 88, 421–449. [Google Scholar] [CrossRef] [PubMed]
- Redmond, L. Translating neuronal activity into dendrite elaboration: Signaling to the nucleus. Neuro-Signals 2008, 16, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Fearnley, C.; Smyrnias, I.; MacDonald, F.; Roderick, H.L. An update on nuclear calcium signalling. J. Cell Sci. 2009, 122, 2337–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.J.; Zou, M.; Lu, L.; Lau, D.; Ditzel, D.A.; Delucinge-Vivier, C.; Aso, Y.; Descombes, P.; Bading, H. Nuclear calcium signaling controls expression of a large gene pool: Identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet. 2009, 5, e1000604. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.W.; Lipscombe, D.; Madison, D.V.; Bley, K.R.; Fox, A.P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988, 11, 431–438. [Google Scholar] [CrossRef]
- Catterall, W.A.; Few, A.P. Calcium channel regulation and presynaptic plasticity. Neuron 2008, 59, 882–901. [Google Scholar] [CrossRef] [PubMed]
- Djamshidian, A.; Grassl, R.; Seltenhammer, M.; Czech, T.; Baumgartner, C.; Schmidbauer, M.; Ulrich, W.; Zimprich, F. Altered expression of voltage-dependent calcium channel α1 subunits in temporal lobe epilepsy with Ammon’s horn sclerosis. Neuroscience 2002, 111, 57–69. [Google Scholar] [CrossRef]
- Faas, G.C.; Vreugdenhil, M.; Wadman, W.J. Calcium currents in pyramidal CA1 neurons in vitro after kindling epileptogenesis in the hippocampus of the rat. Neuroscience 1996, 75, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Jeub, M.; Lie, A.; Blumcke, I.; Elger, C.E.; Beck, H. Loss of dynorphin-mediated inhibition of voltage-dependent Ca2+ currents in hippocampal granule cells isolated from epilepsy patients is associated with mossy fiber sprouting. Neuroscience 1999, 94, 465–471. [Google Scholar] [CrossRef]
- Hendriksen, H.; Kamphuis, W.; Lopes da Silva, F.H. Changes in voltage-dependent calcium channel alpha1-subunit mRNA levels in the kindling model of epileptogenesis. Brain Res. Mol. Brain Res. 1997, 50, 257–266. [Google Scholar] [CrossRef]
- Lie, A.A.; Blumcke, I.; Volsen, S.G.; Wiestler, O.D.; Elger, C.E.; Beck, H. Distribution of voltage-dependent calcium channel beta subunits in the hippocampus of patients with temporal lobe epilepsy. Neuroscience 1999, 93, 449–456. [Google Scholar] [CrossRef]
- Sander, J.W.; Trevisol-Bittencourt, P.C. Nifedipine as an add-on drug in the management of refractory epilepsy. Epilepsy Res. 1990, 6, 82–84. [Google Scholar] [CrossRef]
- Narayanan, J.; Frech, R.; Walters, S.; Patel, V.; Frigerio, R.; Maraganore, D.M. Low dose verapamil as an adjunct therapy for medically refractory epilepsy—An open label pilot study. Epilepsy Res. 2016, 126, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Nicita, F.; Spalice, A.; Papetti, L.; Nikanorova, M.; Iannetti, P.; Parisi, P. Efficacy of verapamil as an adjunctive treatment in children with drug-resistant epilepsy: A pilot study. Seizure 2014, 23, 36–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otoom, S.; Hasan, Z. Nifedipine inhibits picrotoxin-induced seizure activity: Further evidence on the involvement of L-type calcium channel blockers in epilepsy. Fundam. Clin. Pharmacol. 2006, 20, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Schaub, M.C.; Heizmann, C.W. Calcium, troponin, calmodulin, S100 proteins: From myocardial basics to new therapeutic strategies. Biochem. Biophys. Res. Commun. 2008, 369, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.L.; Ames, J.B.; Swindells, M.B.; Ikura, M. Diversity of conformational states and changes within the EF-hand protein superfamily. Proteins 1999, 37, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Burgoyne, R.D.; O’Callaghan, D.W.; Hasdemir, B.; Haynes, L.P.; Tepikin, A.V. Neuronal Ca2+-sensor proteins: Multitalented regulators of neuronal function. Trends Neurosci. 2004, 27, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, R.D. Neuronal calcium sensor proteins: Generating diversity in neuronal Ca2+ signalling. Nat. Rev. Neurosc. 2007, 8, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Nejatbakhsh, N.; Feng, Z.P. Calcium binding protein-mediated regulation of voltage-gated calcium channels linked to human diseases. Acta Pharmacol. Sin. 2011, 32, 741–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuhlke, R.D.; Pitt, G.S.; Tsien, R.W.; Reuter, H. Ca2+-sensitive inactivation and facilitation of L-type Ca2+ channels both depend on specific amino acid residues in a consensus calmodulin-binding motif in the α1C subunit. J. Biol. Chem. 2000, 275, 21121–21129. [Google Scholar] [CrossRef] [PubMed]
- Pitt, G.S.; Zuhlke, R.D.; Hudmon, A.; Schulman, H.; Reuter, H.; Tsien, R.W. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J. Biol. Chem. 2001, 276, 30794–30802. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; DeMaria, C.D.; Erickson, M.G.; Mori, M.X.; Alseikhan, B.A.; Yue, D.T. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron 2003, 39, 951–960. [Google Scholar] [CrossRef]
- Christel, C.; Lee, A. Ca2+-dependent modulation of voltage-gated Ca2+ channels. Biochim. Biophys. Acta 2012, 1820, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Dick, I.E.; Tadross, M.R.; Liang, H.; Tay, L.H.; Yang, W.; Yue, D.T. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature 2008, 451, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Long, L.; Tang, Y.C.; Hu, H.T.; Tang, F.R. Ca(v)1.2, Ca(v)1.3, and Ca(v)2.1 in the mouse hippocampus during and after pilocarpine-induced status epilepticus. Hippocampus 2007, 17, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Yang, Z.B.; Wang, H.; Tang, F.R. Co-localization of L-type voltage dependent calcium channel α1D subunit (Ca(v)1.3) and calbindin (CB) in the mouse central nervous system. Neurosci. Lett. 2014, 561, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kuchukhidze, G.; Wieselthaler-Holzl, A.; Drexel, M.; Unterberger, I.; Luef, G.; Ortler, M.; Becker, A.J.; Trinka, E.; Sperk, G. Calcium-binding proteins in focal cortical dysplasia. Epilepsia 2015, 56, 1207–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staley, K.; Hellier, J.L.; Dudek, F.E. Do interictal spikes drive epileptogenesis? Neuroscientist 2005, 11, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Staley, K.J.; White, A.; Dudek, F.E. Interictal spikes: Harbingers or causes of epilepsy? Neurosci. Lett. 2011, 497, 247–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudek, F.E.; Rogawski, M.A. Calcium currents burst back: A possible role for dendrites in epileptogenesis. Epilepsy Curr. 2007, 7, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, G.; D’Antuono, M.; de Guzman, P.; De Lannoy, L.; Biagini, G.; Avoli, M. In vitro ictogenesis and parahippocampal networks in a rodent model of temporal lobe epilepsy. Neurobiol. Dis. 2010, 39, 372–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staley, K.J.; Dudek, F.E. Interictal spikes and epileptogenesis. Epilepsy Curr. 2006, 6, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Spacey, S.D.; Materek, L.A.; Szczygielski, B.I.; Bird, T.D. Two novel CACNA1A gene mutations associated with episodic ataxia type 2 and interictal dystonia. Arch. Neurol. 2005, 62, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.P.; Carman, J.S.; Hoffer, B.J.; Wyatt, R.J. Effect of altered calcium ion concentration on interictal spike generation in the hippocampal slice. Exp. Neurol. 1980, 68, 489–499. [Google Scholar] [CrossRef]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Ann. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Nowycky, M.C.; Fox, A.P.; Tsien, R.W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature 1985, 316, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Cohen, S.; Li, B.; Tsien, R.W. Exploring the dominant role of Cav1 channels in signalling to the nucleus. Biosci. Rep. 2012, 33, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Sallan, M.C.; Visa, A.; Shaikh, S.; Nager, M.; Herreros, J.; Canti, C. T-type Ca2+ Channels: T for Targetable. Cancer Res. 2018, 78, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Leal, K.; Nanou, E. Calcium channels and short-term synaptic plasticity. J. Biol. Chem. 2013, 288, 10742–10749. [Google Scholar] [CrossRef] [PubMed]
- Neher, E.; Sakaba, T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 2008, 59, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Luebke, J.I.; Dunlap, K.; Turner, T.J. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron 1993, 11, 895–902. [Google Scholar] [CrossRef]
- Turner, T.J.; Adams, M.E.; Dunlap, K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc. Natl. Acad. Sci. USA 1993, 90, 9518–9522. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.; Kirschstein, T.; Kukley, M.; Pereverzev, A.; von der Brelie, C.; Schneider, T.; Beck, H. Functional specialization of presynaptic Cav2.3 Ca2+ channels. Neuron 2003, 39, 483–496. [Google Scholar] [CrossRef]
- Kamp, M.A.; Krieger, A.; Henry, M.; Hescheler, J.; Weiergraber, M.; Schneider, T. Presynaptic ’Ca2.3-containing’ E-type Ca channels share dual roles during neurotransmitter release. Eur. J. Neurosci. 2005, 21, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Sinnegger-Brauns, M.J.; Huber, I.G.; Koschak, A.; Wild, C.; Obermair, G.J.; Einzinger, U.; Hoda, J.C.; Sartori, S.B.; Striessnig, J. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol. Pharmacol. 2009, 75, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Hell, J.W.; Westenbroek, R.E.; Warner, C.; Ahlijanian, M.K.; Prystay, W.; Gilbert, M.M.; Snutch, T.P.; Catterall, W.A. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J. Cell Biol. 1993, 123, 949–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Biase, V.; Obermair, G.J.; Szabo, Z.; Altier, C.; Sanguesa, J.; Bourinet, E.; Flucher, B.E. Stable membrane expression of postsynaptic CaV1.2 calcium channel clusters is independent of interactions with AKAP79/150 and PDZ proteins. J. Neurosci. 2008, 28, 13845–13855. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.A.; Christel, C.J.; Jiao, Y.; Abiria, S.; Kim, K.Y.; Usachev, Y.M.; Obermair, G.J.; Colbran, R.J.; Lee, A. Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J. Neurosci. 2010, 30, 5125–5135. [Google Scholar] [CrossRef] [PubMed]
- Westenbroek, R.E.; Ahlijanian, M.K.; Catterall, W.A. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature 1990, 347, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, M.; Wadman, W.J. Enhancement of calcium currents in rat hippocampal CA1 neurons induced by kindling epileptogenesis. Neuroscience 1992, 49, 373–381. [Google Scholar] [CrossRef]
- Vigues, S.; Gastaldi, M.; Chabret, C.; Massacrier, A.; Cau, P.; Valmier, J. Regulation of calcium channel α1A subunit splice variant mRNAs in kainate-induced temporal lobe epilepsy. Neurobiol. Dis. 1999, 6, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Ikonomovic, M.D.; Abrahamson, E.E.; Kharlamov, E.A.; Hentosz, T.M.; Armstrong, D.M. Alterations in hippocampal voltage-gated calcium channel alpha 1 subunit expression patterns after kainate-induced status epilepticus in aging rats. Epilepsy Res. 2003, 57, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Westenbroek, R.E.; Sakurai, T.; Elliott, E.M.; Hell, J.W.; Starr, T.V.; Snutch, T.P.; Catterall, W.A. Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J. Neurosci. 1995, 15, 6403–6418. [Google Scholar] [CrossRef] [PubMed]
- Westenbroek, R.E.; Hell, J.W.; Warner, C.; Dubel, S.J.; Snutch, T.P.; Catterall, W.A. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron 1992, 9, 1099–1115. [Google Scholar] [CrossRef]
- Chung, Y.H.; Shin, C.; Park, K.H.; Cha, C.I. Immunohistochemical study on the distribution of the voltage-gated calcium channel alpha(1B) subunit in the mature rat brain. Brain Res. 2000, 866, 274–280. [Google Scholar] [CrossRef]
- Xu, J.H.; Long, L.; Wang, J.; Tang, Y.C.; Hu, H.T.; Soong, T.W.; Tang, F.R. Nuclear localization of Ca(v)2.2 and its distribution in the mouse central nervous system, and changes in the hippocampus during and after pilocarpine-induced status epilepticus. Neuropathol. Appl. Neurobiol. 2010, 36, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Soong, T.W.; Stea, A.; Hodson, C.D.; Dubel, S.J.; Vincent, S.R.; Snutch, T.P. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science 1993, 260, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.; Marubio, L.M.; Deal, C.R.; Hans, M.; Brust, P.F.; Philipson, L.H.; Miller, R.J.; Johnson, E.C.; Harpold, M.M.; Ellis, S.B. Structure and functional characterization of neuronal alpha 1E calcium channel subtypes. J. Biol. Chem. 1994, 269, 22347–22357. [Google Scholar] [PubMed]
- Yokoyama, C.T.; Westenbroek, R.E.; Hell, J.W.; Soong, T.W.; Snutch, T.P.; Catterall, W.A. Biochemical properties and subcellular distribution of the neuronal class E calcium channel alpha 1 subunit. J. Neurosci. 1995, 15, 6419–6432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siwek, M.; Henseler, C.; Broich, K.; Papazoglou, A.; Weiergraber, M. Voltage-gated Ca(2+) channel mediated Ca(2+) influx in epileptogenesis. Adv. Exp. Med. Biol. 2012, 740, 1219–1247. [Google Scholar] [PubMed]
- Craig, P.J.; Beattie, R.E.; Folly, E.A.; Banerjee, M.D.; Reeves, M.B.; Priestley, J.V.; Carney, S.L.; Sher, E.; Perez-Reyes, E.; Volsen, S.G. Distribution of the voltage-dependent calcium channel alpha1G subunit mRNA and protein throughout the mature rat brain. Eur. J. Neurosci. 1999, 11, 2949–2964. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.D.; Llinas, R. Hippocampal pyramidal cells: Significance of dendritic ionic conductances for neuronal function and epileptogenesis. J. Neurophysiol. 1979, 42, 476–496. [Google Scholar] [CrossRef] [PubMed]
- Yaari, Y.; Konnerth, A.; Heinemann, U. Spontaneous epileptiform activity of CA1 hippocampal neurons in low extracellular calcium solutions. Exp. Brain Res. 1983, 51, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.T. Ca2+ channels and epilepsy. Eur. J. Pharmacol. 2002, 447, 211–225. [Google Scholar] [CrossRef]
- Heinemann, U.; Hamon, B. Calcium and epileptogenesis. Exp. Brain Res. 1986, 65, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Blalock, E.M.; Chen, K.C.; Vanaman, T.C.; Landfield, P.W.; Slevin, J.T. Epilepsy-induced decrease of L-type Ca2+ channel activity and coordinate regulation of subunit mRNA in single neurons of rat hippocampal ‘zipper’ slices. Epilepsy Res. 2001, 43, 211–226. [Google Scholar] [CrossRef]
- Bernstein, G.M.; Mendonca, A.; Wadia, J.; Burnham, W.M.; Jones, O.T. Kindling induces an asymmetric enhancement of N-type Ca2+ channel density in the dendritic fields of the rat hippocampus. Neurosci. Lett. 1999, 268, 155–158. [Google Scholar] [CrossRef]
- Bernstein, G.M.; Mendonca, A.; Wadia, J.; Burnham, W.M.; Jones, O.T. Kindling induces a long-term enhancement in the density of N-type calcium channels in the rat hippocampus. Neuroscience 1999, 94, 1083–1095. [Google Scholar] [CrossRef]
- Beck, H.; Steffens, R.; Elger, C.E.; Heinemann, U. Voltage-dependent Ca2+ currents in epilepsy. Epilepsy Res. 1998, 32, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Sochivko, D.; Becker, A.; Chen, J.; Jiang, Y.; Yaari, Y.; Beck, H. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J. Neurosci. 2002, 22, 3645–3655. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.; Steffens, R.; Heinemann, U.; Elger, C.E. Properties of voltage-activated Ca2+ currents in acutely isolated human hippocampal granule cells. J. Neurophysiol. 1997, 77, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Beuckmann, C.T.; Sinton, C.M.; Miyamoto, N.; Ino, M.; Yanagisawa, M. N-type calcium channel alpha1B subunit (Cav2.2) knock-out mice display hyperactivity and vigilance state differences. J. Neurosci. 2003, 23, 6793–6797. [Google Scholar] [CrossRef] [PubMed]
- N’Gouemo, P.; Yasuda, R.; Faingold, C.L. Seizure susceptibility is associated with altered protein expression of voltage-gated calcium channel subunits in inferior colliculus neurons of the genetically epilepsy-prone rat. Brain Res. 2010, 1308, 153–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Borman, B.; Lakaye, B.; Minet, A.; Zorzi, W.; Vergnes, M.; Marescaux, C.; Grisar, T. Expression of mRNA encoding alpha1E and alpha1G subunit in the brain of a rat model of absence epilepsy. Neuroreport 1999, 10, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Lakaye, B.; Thomas, E.; Minet, A.; Grisar, T. The genetic absence epilepsy rat from Strasbourg (GAERS), a rat model of absence epilepsy: Computer modeling and differential gene expression. Epilepsia 2002, 43 (Suppl. 5), 123–129. [Google Scholar] [CrossRef] [PubMed]
- Weiergraber, M.; Kamp, M.A.; Radhakrishnan, K.; Hescheler, J.; Schneider, T. The Ca(v)2.3 voltage-gated calcium channel in epileptogenesis--shedding new light on an enigmatic channel. Neurosci. Biobehav. Rev. 2006, 30, 1122–1144. [Google Scholar] [CrossRef] [PubMed]
- Weiergraber, M.; Henry, M.; Radhakrishnan, K.; Hescheler, J.; Schneider, T. Hippocampal seizure resistance and reduced neuronal excitotoxicity in mice lacking the Cav2.3 E/R-type voltage-gated calcium channel. J. Neurophysiol. 2007, 97, 3660–3669. [Google Scholar] [CrossRef] [PubMed]
- Weiergraber, M.; Henry, M.; Krieger, A.; Kamp, M.; Radhakrishnan, K.; Hescheler, J.; Schneider, T. Altered seizure susceptibility in mice lacking the Ca(v)2.3 E-type Ca2+ channel. Epilepsia 2006, 47, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Jun, K.; Piedras-Renteria, E.S.; Smith, S.M.; Wheeler, D.B.; Lee, S.B.; Lee, T.G.; Chin, H.; Adams, M.E.; Scheller, R.H.; Tsien, R.W.; et al. Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc. Natl. Acad. Sci. USA 1999, 96, 15245–15250. [Google Scholar] [CrossRef] [PubMed]
- Noebels, J.L.; Sidman, R.L. Inherited epilepsy: Spike-wave and focal motor seizures in the mutant mouse tottering. Science 1979, 204, 1334–1336. [Google Scholar] [CrossRef] [PubMed]
- Bomben, V.C.; Aiba, I.; Qian, J.; Mark, M.D. Isolated P/Q Calcium Channel Deletion in Layer VI Corticothalamic Neurons Generates Absence Epilepsy. J. Neurosci. 2016, 36, 405–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouvenceau, A.; Eunson, L.H.; Spauschus, A.; Ramesh, V.; Zuberi, S.M.; Kullmann, D.M.; Hanna, M.G. Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. Lancet 2001, 358, 801–807. [Google Scholar] [CrossRef]
- Tsakiridou, E.; Bertollini, L.; de Curtis, M.; Avanzini, G.; Pape, H.C. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J. Neurosci. 1995, 15, 3110–3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talley, E.M.; Solorzano, G.; Depaulis, A.; Perez-Reyes, E.; Bayliss, D.A. Low-voltage-activated calcium channel subunit expression in a genetic model of absence epilepsy in the rat. Brain Res. Mol. Brain Res. 2000, 75, 159–165. [Google Scholar] [CrossRef]
- Kim, D.; Song, I.; Keum, S.; Lee, T.; Jeong, M.J.; Kim, S.S.; McEnery, M.W.; Shin, H.S. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron 2001, 31, 35–45. [Google Scholar] [CrossRef]
- Ernst, W.L.; Zhang, Y.; Yoo, J.W.; Ernst, S.J.; Noebels, J.L. Genetic enhancement of thalamocortical network activity by elevating alpha 1g-mediated low-voltage-activated calcium current induces pure absence epilepsy. J. Neurosci. 2009, 29, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.J.; Pitsch, J.; Sochivko, D.; Opitz, T.; Staniek, M.; Chen, C.C.; Campbell, K.P.; Schoch, S.; Yaari, Y.; Beck, H. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J. Neurosci. 2008, 28, 13341–13353. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravani, H.; Bladen, C.; Parker, D.B.; Snutch, T.P.; McRory, J.E.; Zamponi, G.W. Effects of Cav3.2 channel mutations linked to idiopathic generalized epilepsy. Ann. Neurol. 2005, 57, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Heron, S.E.; Khosravani, H.; Varela, D.; Bladen, C.; Williams, T.C.; Newman, M.R.; Scheffer, I.E.; Berkovic, S.F.; Mulley, J.C.; Zamponi, G.W. Extended spectrum of idiopathic generalized epilepsies associated with CACNA1H functional variants. Ann. Neurol. 2007, 62, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, J.; Pan, H.; Zhang, Y.; Wu, H.; Xu, K.; Liu, X.; Jiang, Y.; Bao, X.; Yao, Z.; et al. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann. Neurol. 2003, 54, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Eckle, V.S.; Shcheglovitov, A.; Vitko, I.; Dey, D.; Yap, C.C.; Winckler, B.; Perez-Reyes, E. Mechanisms by which a CACNA1H mutation in epilepsy patients increases seizure susceptibility. J. Physiol. 2014, 592, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, O. Genes and molecular mechanisms involved in the epileptogenesis of idiopathic absence epilepsies. Seizure 2012, 21, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, Y.; Wang, J.; Pan, H.; Wu, H.; Xu, K.; Liu, X.; Jiang, Y.; Shen, Y.; Wu, X. New variants in the CACNA1H gene identified in childhood absence epilepsy. Neurosci. Lett. 2006, 406, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, R.D.; Weiss, J.L. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem. J. 2001, 353, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baimbridge, K.G.; Celio, M.R.; Rogers, J.H. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992, 15, 303–308. [Google Scholar] [CrossRef]
- Cheung, W.Y. Cyclic 3′,5′-nucleotide phosphodiesterase. Demonstration of an activator. Biochem. Biophys. Res. Commun. 1970, 38, 533–538. [Google Scholar] [CrossRef]
- Kakiuchi, S.; Yamazaki, R. Calcium dependent phosphodiesterase activity and its activating factor (PAF) from brain studies on cyclic 3′,5′-nucleotide phosphodiesterase (3). Biochem. Biophys. Res. Commun. 1970, 41, 1104–1110. [Google Scholar] [CrossRef]
- Kawasaki, H.; Kretsinger, R.H. Calcium-binding proteins. 1: EF-hands. Protein Profile 1994, 1, 343–517. [Google Scholar] [PubMed]
- Yan, H.; Wang, C.; Marx, S.O.; Pitt, G.S. Calmodulin limits pathogenic Na+ channel persistent current. J. Gen. Physiol. 2017, 149, 277–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Zhuang, F.; Li, H.; Zheng, K.; Hong, Z.; Feng, W.; Zhou, W.; Chen, J. Calmodulin regulates KCNQ2 function in epilepsy. Am. J. Transl. Res. 2016, 8, 5610–5618. [Google Scholar] [PubMed]
- Arif, S.H. A Ca(2+)-binding protein with numerous roles and uses: Parvalbumin in molecular biology and physiology. BioEssays 2009, 31, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Baude, A.; Bleasdale, C.; Dalezios, Y.; Somogyi, P.; Klausberger, T. Immunoreactivity for the GABAA receptor alpha1 subunit, somatostatin and Connexin36 distinguishes axoaxonic, basket, and bistratified interneurons of the rat hippocampus. Cereb. Cortex 2007, 17, 2094–2107. [Google Scholar] [CrossRef] [PubMed]
- Camp, A.J.; Wijesinghe, R. Calretinin: Modulator of neuronal excitability. Int. J. Biochem. Cell Biol. 2009, 41, 2118–2121. [Google Scholar] [CrossRef] [PubMed]
- Schurmans, S.; Schiffmann, S.N.; Gurden, H.; Lemaire, M.; Lipp, H.P.; Schwam, V.; Pochet, R.; Imperato, A.; Bohme, G.A.; Parmentier, M. Impaired long-term potentiation induction in dentate gyrus of calretinin-deficient mice. Proc. Natl. Acad. Sci. USA 1997, 94, 10415–10420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwaller, B. Calretinin: From a “simple” Ca(2+) buffer to a multifunctional protein implicated in many biological processes. Front. Neuroanat. 2014, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Morita, Y.; Hironaka, T.; Emson, P.C.; Tohyama, M. Ontological study of calbindin-D28k-like and parvalbumin-like immunoreactivities in rat spinal cord and dorsal root ganglia. J. Comparat. Neurol. 1990, 302, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Maskey, D.; Pradhan, J.; Kim, H.J.; Park, K.S.; Ahn, S.C.; Kim, M.J. Immunohistochemical localization of calbindin D28-k, parvalbumin, and calretinin in the cerebellar cortex of the circling mouse. Neurosci. Lett. 2010, 483, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Rychlik, B.; Chu, C.; Christakos, S. Evidence for calcium-reducing and excito-protective roles for the calcium-binding protein calbindin-D28k in cultured hippocampal neurons. Neuron 1991, 6, 41–51. [Google Scholar] [CrossRef]
- Chard, P.S.; Bleakman, D.; Christakos, S.; Fullmer, C.S.; Miller, R.J. Calcium buffering properties of calbindin D28k and parvalbumin in rat sensory neurones. J. Physiol. 1993, 472, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Iacopino, A.; Christakos, S.; German, D.; Sonsalla, P.K.; Altar, C.A. Calbindin-D28K-containing neurons in animal models of neurodegeneration: Possible protection from excitotoxicity. Brain Res. Mol. Brain Res. 1992, 13, 251–261. [Google Scholar] [CrossRef]
- Bu, J.; Sathyendra, V.; Nagykery, N.; Geula, C. Age-related changes in calbindin-D28k, calretinin, and parvalbumin-immunoreactive neurons in the human cerebral cortex. Exp. Neurol. 2003, 182, 220–231. [Google Scholar] [CrossRef]
- Heizmann, C.W.; Braun, K. Changes in Ca(2+)-binding proteins in human neurodegenerative disorders. Trends Neurosci. 1992, 15, 259–264. [Google Scholar] [CrossRef]
- Ahmadian, S.S.; Rezvanian, A.; Peterson, M.; Weintraub, S.; Bigio, E.H.; Mesulam, M.M.; Geula, C. Loss of calbindin-D28K is associated with the full range of tangle pathology within basal forebrain cholinergic neurons in Alzheimer’s disease. Neurobiol. Aging 2015, 36, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Nagerl, U.V.; Mody, I.; Jeub, M.; Lie, A.A.; Elger, C.E.; Beck, H. Surviving granule cells of the sclerotic human hippocampus have reduced Ca(2+) influx because of a loss of calbindin-D(28k) in temporal lobe epilepsy. J. Neurosci. 2000, 20, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Yanez, M.; Gil-Longo, J.; Campos-Toimil, M. Calcium binding proteins. Adv. Exp. Med. Biol. 2012, 740, 461–482. [Google Scholar] [PubMed]
- Nairn, A.C.; Picciotto, M.R. Calcium/calmodulin-dependent protein kinases. Semin. Cancer Biol. 1994, 5, 295–303. [Google Scholar] [PubMed]
- Hani, A.J.; Mikati, H.M.; Mikati, M.A. Genetics of pediatric epilepsy. Pediatr. Clin. N. Am. 2015, 62, 703–722. [Google Scholar] [CrossRef] [PubMed]
- Asai, M.; Benitez-King, G. Variations of rat brain calmodulin content in dark and light phases: Effect of pentylenetetrazol-induced kindling. Neurochem. Res. 1998, 23, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.S.; Silva, A.J.; Abeliovich, A.; Watanabe, Y.; Tonegawa, S.; McNamara, J.O. Limbic epilepsy in transgenic mice carrying a Ca2+/calmodulin-dependent kinase II alpha-subunit mutation. Proc. Natl. Acad. Sci. USA 1995, 92, 6852–6855. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Jones, E.G. Differential and time-dependent changes in gene expression for type II calcium/calmodulin-dependent protein kinase, 67 kDa glutamic acid decarboxylase, and glutamate receptor subunits in tetanus toxin-induced focal epilepsy. J. Neurosci. 1997, 17, 2168–2180. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, Y.; Imoto, K.; Obata, K. A mechanism for the inactivation of Ca2+/calmodulin-dependent protein kinase II during prolonged seizure activity and its consequence after the recovery from seizure activity in rats in vivo. Neuroscience 2006, 140, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, J.M.; Micevych, P.; Popper, P.; Huez, G.; Farber, D.B.; Wasterlain, C.G. Long-lasting decreases of type II calmodulin kinase expression in kindled rat brains. Brain Res. 1992, 584, 257–260. [Google Scholar] [CrossRef]
- Murray, K.D.; Isackson, P.J.; Eskin, T.A.; King, M.A.; Montesinos, S.P.; Abraham, L.A.; Roper, S.N. Altered mRNA expression for brain-derived neurotrophic factor and type II calcium/calmodulin-dependent protein kinase in the hippocampus of patients with intractable temporal lobe epilepsy. J. Comparat. Neurol. 2000, 418, 411–422. [Google Scholar] [CrossRef]
- Lee, M.C.; Ban, S.S.; Woo, Y.J.; Kim, S.U. Calcium/calmodulin kinase II activity of hippocampus in kainate-induced epilepsy. J. Korean Med. Sci. 2001, 16, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Lie, A.A.; Blumcke, I.; Beck, H.; Schramm, J.; Wiestler, O.D.; Elger, C.E. Altered patterns of Ca2+/calmodulin-dependent protein kinase II and calcineurin immunoreactivity in the hippocampus of patients with temporal lobe epilepsy. J. Neuropathol. Exp. Neurol. 1998, 57, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.R.; Suzuki, T.; Shimizu, H.; Nishino, H. Amygdala kindling activates the phosphorylation of Ca2+/calmodulin-dependent protein kinase II in rat hippocampus. Neurosci. Lett. 1994, 171, 45–48. [Google Scholar] [CrossRef]
- Wu, K.; Wasterlain, C.; Sachs, L.; Siekevitz, P. Effect of septal kindling on glutamate binding and calcium/calmodulin-dependent phosphorylation in a postsynaptic density fraction isolated from rat cerebral cortex. Proc. Natl. Acad. Sci. USA 1990, 87, 5298–5302. [Google Scholar] [CrossRef] [PubMed]
- Wasterlain, C.G.; Farber, D.B. Kindling alters the calcium/calmodulin-dependent phosphorylation of synaptic plasma membrane proteins in rat hippocampus. Proc. Natl. Acad. Sci. USA 1984, 81, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Rosenberg, H.C. Brief seizure activity alters Ca2+/calmodulin dependent protein kinase II dephosphorylation and subcellular distribution in rat brain for several hours. Neurosci. Lett. 2004, 357, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, J.M.; Farber, D.B.; Micevych, P.E.; Lasher, R.; Wasterlain, C.G. Kindling induced changes in calmodulin kinase II immunoreactivity. Brain Res. 1990, 524, 49–53. [Google Scholar] [CrossRef]
- Savina, T.A.; Shchipakina, T.G.; Godukhin, O.V. Effect of seizure activity on subunit composition of Ca2+/calmodulin-dependent protein kinase II in hippocampus of Krushinskii-Molodkina rats. Rossiiskii Fiziologicheskii Zhurnal Imeni I.M. Sechenova 2011, 97, 590–600. [Google Scholar] [PubMed]
- Matsu-ura, T.; Nakadai, T.; Oda, Y.; Nagasu, T.; Mikoshiba, K.; Tamura, T.A. Seizure-mediated accumulation of the beta subunit of Ca2+/calmodulin-dependent protein kinase II in nuclei of mouse brain cells. Neurosci. Lett. 2002, 322, 149–152. [Google Scholar] [CrossRef]
- Gary, D.S.; Sooy, K.; Chan, S.L.; Christakos, S.; Mattson, M.P. Concentration- and cell type-specific effects of calbindin D28k on vulnerability of hippocampal neurons to seizure-induced injury. Brain Res. Mol. Brain Res. 2000, 75, 89–95. [Google Scholar] [CrossRef]
- Montpied, P.; Winsky, L.; Dailey, J.W.; Jobe, P.C.; Jacobowitz, D.M. Alteration in levels of expression of brain calbindin D-28k and calretinin mRNA in genetically epilepsy-prone rats. Epilepsia 1995, 36, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Valencia, I.; Legido, A.; Yelin, K.; Khurana, D.; Kothare, S.V.; Katsetos, C.D. Anomalous inhibitory circuits in cortical tubers of human tuberous sclerosis complex associated with refractory epilepsy: Aberrant expression of parvalbumin and calbindin-D28k in dysplastic cortex. J. Child Neurol. 2006, 21, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.K.; Lee, H.Y.; Seong, N.S.; Chung, H.G.; Kim, J.H.; Lee, H.J.; Kim, J.D.; Kang, T.C.; Won, M.H. Changes of calbindin D-28k immunoreactivity in the hippocampus after adrenalectomy in the seizure sensitive gerbil. Anat. Histol. Embryol. 2004, 33, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Magloczky, Z.; Halasz, P.; Vajda, J.; Czirjak, S.; Freund, T.F. Loss of Calbindin-D28K immunoreactivity from dentate granule cells in human temporal lobe epilepsy. Neuroscience 1997, 76, 377–385. [Google Scholar] [CrossRef]
- Karadi, K.; Janszky, J.; Gyimesi, C.; Horvath, Z.; Lucza, T.; Doczi, T.; Kallai, J.; Abraham, H. Correlation between calbindin expression in granule cells of the resected hippocampal dentate gyrus and verbal memory in temporal lobe epilepsy. Epilepsy Behav. 2012, 25, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Thom, M.; Holton, J.L.; D’Arrigo, C.; Griffin, B.; Beckett, A.; Sisodiya, S.; Alexiou, D.; Sander, J.W. Microdysgenesis with abnormal cortical myelinated fibres in temporal lobe epilepsy: A histopathological study with calbindin D-28-K immunohistochemistry. Neuropathol. Appl. Neurobiol. 2000, 26, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bouilleret, V.; Schwaller, B.; Schurmans, S.; Celio, M.R.; Fritschy, J.M. Neurodegenerative and morphogenic changes in a mouse model of temporal lobe epilepsy do not depend on the expression of the calcium-binding proteins parvalbumin, calbindin, or calretinin. Neuroscience 2000, 97, 47–58. [Google Scholar] [CrossRef]
- Wittner, L.; Eross, L.; Szabo, Z.; Toth, S.; Czirjak, S.; Halasz, P.; Freund, T.F.; Magloczky, Z.S. Synaptic reorganization of calbindin-positive neurons in the human hippocampal CA1 region in temporal lobe epilepsy. Neuroscience 2002, 115, 961–978. [Google Scholar] [CrossRef]
- Schiffmann, S.N.; Cheron, G.; Lohof, A.; d’Alcantara, P.; Meyer, M.; Parmentier, M.; Schurmans, S. Impaired motor coordination and Purkinje cell excitability in mice lacking calretinin. Proc. Natl. Acad. Sci. USA 1999, 96, 5257–5262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gall, D.; Roussel, C.; Nieus, T.; Cheron, G.; Servais, L.; D’Angelo, E.; Schiffmann, S.N. Role of calcium binding proteins in the control of cerebellar granule cell neuronal excitability: Experimental and modeling studies. Prog. Brain Res. 2005, 148, 321–328. [Google Scholar] [PubMed]
- Miettinen, R.; Gulyas, A.I.; Baimbridge, K.G.; Jacobowitz, D.M.; Freund, T.F. Calretinin is present in non-pyramidal cells of the rat hippocampus—II. Co-existence with other calcium binding proteins and GABA. Neuroscience 1992, 48, 29–43. [Google Scholar] [CrossRef]
- Liu, Y.; Fujise, N.; Kosaka, T. Distribution of calretinin immunoreactivity in the mouse dentate gyrus. I. General description. Exp. Brain Res. 1996, 108, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Gulyas, A.I.; Hajos, N.; Freund, T.F. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J. Neurosci. 1996, 16, 3397–3411. [Google Scholar] [CrossRef] [PubMed]
- Hajos, N.; Acsady, L.; Freund, T.F. Target selectivity and neurochemical characteristics of VIP-immunoreactive interneurons in the rat dentate gyrus. Eur. J. Neurosci. 1996, 8, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Urban, Z.; Magloczky, Z.; Freund, T.F. Calretinin-containing interneurons innervate both principal cells and interneurons in the CA1 region of the human hippocampus. Acta Biol. Hung. 2002, 53, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Magloczky, Z.; Wittner, L.; Borhegyi, Z.; Halasz, P.; Vajda, J.; Czirjak, S.; Freund, T.F. Changes in the distribution and connectivity of interneurons in the epileptic human dentate gyrus. Neuroscience 2000, 96, 7–25. [Google Scholar] [CrossRef]
- Toth, K.; Eross, L.; Vajda, J.; Halasz, P.; Freund, T.F.; Magloczky, Z. Loss and reorganization of calretinin-containing interneurons in the epileptic human hippocampus. Brain 2010, 133, 2763–2777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumcke, I.; Beck, H.; Suter, B.; Hoffmann, D.; Fodisch, H.J.; Wolf, H.K.; Schramm, J.; Elger, C.E.; Wiestler, O.D. An increase of hippocampal calretinin-immunoreactive neurons correlates with early febrile seizures in temporal lobe epilepsy. Acta Neuropathol. 1999, 97, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Blumcke, I.; Beck, H.; Nitsch, R.; Eickhoff, C.; Scheffler, B.; Celio, M.R.; Schramm, J.; Elger, C.E.; Wolf, H.K.; Wiestler, O.D. Preservation of calretinin-immunoreactive neurons in the hippocampus of epilepsy patients with Ammon’s horn sclerosis. J. Neuropathol. Exp. Neurol. 1996, 55, 329–341. [Google Scholar] [PubMed]
- Toth, K.; Magloczky, Z. The vulnerability of calretinin-containing hippocampal interneurons to temporal lobe epilepsy. Front. Neuroanat. 2014, 8, 100. [Google Scholar] [PubMed]
- Barinka, F.; Druga, R.; Marusic, P.; Krsek, P.; Zamecnik, J. Calretinin immunoreactivity in focal cortical dysplasias and in non-malformed epileptic cortex. Epilepsy Res. 2010, 88, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Magloczky, Z.; Freund, T.F. Selective neuronal death in the contralateral hippocampus following unilateral kainate injections into the CA3 subfield. Neuroscience 1993, 56, 317–335. [Google Scholar] [CrossRef]
- Magloczky, Z.; Freund, T.F. Delayed cell death in the contralateral hippocampus following kainate injection into the CA3 subfield. Neuroscience 1995, 66, 847–860. [Google Scholar] [CrossRef]
- Bouilleret, V.; Loup, F.; Kiener, T.; Marescaux, C.; Fritschy, J.M. Early loss of interneurons and delayed subunit-specific changes in GABA(A)-receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus 2000, 10, 305–324. [Google Scholar] [CrossRef]
- Andre, V.; Marescaux, C.; Nehlig, A.; Fritschy, J.M. Alterations of hippocampal GAbaergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus 2001, 11, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.I.; Blasco-Ibanez, J.M.; Crespo, C.; Marques-Mari, A.I.; Martinez-Guijarro, F.J. Calretinin/PSA-NCAM immunoreactive granule cells after hippocampal damage produced by kainic acid and DEDTC treatment in mouse. Brain Res. 2003, 966, 206–217. [Google Scholar] [CrossRef]
- Van Vliet, E.A.; Aronica, E.; Tolner, E.A.; Lopes da Silva, F.H.; Gorter, J.A. Progression of temporal lobe epilepsy in the rat is associated with immunocytochemical changes in inhibitory interneurons in specific regions of the hippocampal formation. Exp. Neurol. 2004, 187, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Cobos, I.; Calcagnotto, M.E.; Vilaythong, A.J.; Thwin, M.T.; Noebels, J.L.; Baraban, S.C.; Rubenstein, J.L. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat. Neurosci. 2005, 8, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Tang, F. Survival of calbindin, calretinin and parvalbumin positive neurons in mouse hippocampal CA area at chronic stage of pilocarpine-induced epilepsy. Zhong Nan Da Xue Xue Bao 2013, 38, 437–442. [Google Scholar] [PubMed]
- Tang, F.R.; Chia, S.C.; Jiang, F.L.; Ma, D.L.; Chen, P.M.; Tang, Y.C. Calcium binding protein containing neurons in the gliotic mouse hippocampus with special reference to their afferents from the medial septum and the entorhinal cortex. Neuroscience 2006, 140, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Huusko, N.; Romer, C.; Ndode-Ekane, X.E.; Lukasiuk, K.; Pitkanen, A. Loss of hippocampal interneurons and epileptogenesis: A comparison of two animal models of acquired epilepsy. Brain Struct. Funct. 2015, 220, 153–191. [Google Scholar] [CrossRef] [PubMed]
- Drexel, M.; Preidt, A.P.; Kirchmair, E.; Sperk, G. Parvalbumin interneurons and calretinin fibers arising from the thalamic nucleus reuniens degenerate in the subiculum after kainic acid-induced seizures. Neuroscience 2011, 189, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Thom, M.; Sisodiya, S.M.; Beckett, A.; Martinian, L.; Lin, W.R.; Harkness, W.; Mitchell, T.N.; Craig, J.; Duncan, J.; Scaravilli, F. Cytoarchitectural abnormalities in hippocampal sclerosis. J. Neuropathol. Exp. Neurol. 2002, 61, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Volz, F.; Bock, H.H.; Gierthmuehlen, M.; Zentner, J.; Haas, C.A.; Freiman, T.M. Stereologic estimation of hippocampal GluR2/3- and calretinin-immunoreactive hilar neurons (presumptive mossy cells) in two mouse models of temporal lobe epilepsy. Epilepsia 2011, 52, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, M.; Losi, G.; Chiavegato, A.; Zonta, M.; Carmignoto, G. Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. J. Physiol. 2013, 591, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, C.; Kohler, J.; Bartos, M. Persistent discharges in dentate gyrus perisoma-inhibiting interneurons require hyperpolarization-activated cyclic nucleotide-gated channel activation. J. Neurosci. 2015, 35, 4131–4139. [Google Scholar] [CrossRef] [PubMed]
- Schwaller, B.; Tetko, I.V.; Tandon, P.; Silveira, D.C.; Vreugdenhil, M.; Henzi, T.; Potier, M.C.; Celio, M.R.; Villa, A.E. Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol. Cell. Neurosci. 2004, 25, 650–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloviter, R.S. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: Localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J. Comparat. Neurol. 1989, 280, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Sloviter, R.S.; Sollas, A.L.; Barbaro, N.M.; Laxer, K.D. Calcium-binding protein (calbindin-D28K) and parvalbumin immunocytochemistry in the normal and epileptic human hippocampus. J. Comparat. Neurol. 1991, 308, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Scotti, A.L.; Nitsch, C. The perforant path in the seizure sensitive gerbil contains the Ca(2+)-binding protein parvalbumin. Exp. Brain Res. 1991, 85, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Scotti, A.L.; Frank, C.; Sagratella, S.; Scotti de Carolis, A.; Nitsch, C. Absence of calcium-induced LTP-like response in the dentate area of seizure-prone gerbils and its relation to parvalbumin in the entorhinal perforant path synapse of this species. Brain Res. Bull. 1993, 31, 501–507. [Google Scholar] [CrossRef]
- Houser, C.R.; Swartz, B.E.; Walsh, G.O.; Delgado-Escueta, A.V. Granule cell disorganization in the dentate gyrus: Possible alterations of neuronal migration in human temporal lobe epilepsy. Epilepsy Res. Suppl. 1992, 9, 41–48, discussion 48–49. [Google Scholar] [PubMed]
- Curia, G.; Lucchi, C.; Vinet, J.; Gualtieri, F.; Marinelli, C.; Torsello, A.; Costantino, L.; Biagini, G. Pathophysiogenesis of mesial temporal lobe epilepsy: Is prevention of damage antiepileptogenic? Curr. Med. Chem. 2014, 21, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Soukupova, M.; Binaschi, A.; Falcicchia, C.; Zucchini, S.; Roncon, P.; Palma, E.; Magri, E.; Grandi, E.; Simonato, M. Impairment of GABA release in the hippocampus at the time of the first spontaneous seizure in the pilocarpine model of temporal lobe epilepsy. Exp. Neurol. 2014, 257, 39–49. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, P.; Inaba, Y.; Biagini, G.; Baldelli, E.; Mollinari, C.; Merlo, D.; Avoli, M. Subiculum network excitability is increased in a rodent model of temporal lobe epilepsy. Hippocampus 2006, 16, 843–860. [Google Scholar] [CrossRef] [PubMed]
- Knopp, A.; Frahm, C.; Fidzinski, P.; Witte, O.W.; Behr, J. Loss of GABAergic neurons in the subiculum and its functional implications in temporal lobe epilepsy. Brain 2008, 131, 1516–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Guzman, P.; Inaba, Y.; Baldelli, E.; de Curtis, M.; Biagini, G.; Avoli, M. Network hyperexcitability within the deep layers of the pilocarpine-treated rat entorhinal cortex. J. Physiol. 2008, 586, 1867–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benini, R.; Longo, D.; Biagini, G.; Avoli, M. Perirhinal cortex hyperexcitability in pilocarpine-treated epileptic rats. Hippocampus 2011, 21, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Vizi, S.; Bagosi, A.; Krisztin-Peva, B.; Gulya, K.; Mihaly, A. Repeated 4-aminopyridine seizures reduce parvalbumin content in the medial mammillary nucleus of the rat brain. Brain Res. Mol. Brain Res. 2004, 131, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortel, A.; Levesque, M.; Biagini, G.; Gotman, J.; Avoli, M. Convulsive status epilepticus duration as determinant for epileptogenesis and interictal discharge generation in the rat limbic system. Neurobiol. Dis. 2010, 40, 478–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagini, G.; D’Antuono, M.; Benini, R.; de Guzman, P.; Longo, D.; Avoli, M. Perirhinal cortex and temporal lobe epilepsy. Front. Cell. Neurosci. 2013, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Represa, A. Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends Neurosci. 1990, 13, 312–318. [Google Scholar] [CrossRef]
- Andrioli, A.; Alonso-Nanclares, L.; Arellano, J.I.; DeFelipe, J. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience 2007, 149, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Wittner, L.; Magloczky, Z.; Borhegyi, Z.; Halasz, P.; Toth, S.; Eross, L.; Szabo, Z.; Freund, T.F. Preservation of perisomatic inhibitory input of granule cells in the epileptic human dentate gyrus. Neuroscience 2001, 108, 587–600. [Google Scholar] [CrossRef]
- Arellano, J.I.; Munoz, A.; Ballesteros-Yanez, I.; Sola, R.G.; DeFelipe, J. Histopathology and reorganization of chandelier cells in the human epileptic sclerotic hippocampus. Brain 2004, 127, 45–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.Q.; Armstrong, D.L.; Hamilton, W.J.; Grossman, R.G. Disproportionate loss of CA4 parvalbumin-immunoreactive interneurons in patients with Ammon’s horn sclerosis. J. Neuropathol. Exp. Neurol. 1997, 56, 988–998. [Google Scholar] [CrossRef]
- Gualtieri, F.; Marinelli, C.; Longo, D.; Pugnaghi, M.; Nichelli, P.F.; Meletti, S.; Biagini, G. Hypoxia markers are expressed in interneurons exposed to recurrent seizures. Neuromol. Med. 2013, 15, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kang, T.C. p47Phox/CDK5/DRP1-Mediated Mitochondrial Fission Evokes PV Cell Degeneration in the Rat Dentate Gyrus Following Status Epilepticus. Front. Cell. Neurosci. 2017, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Adotevi, N.K.; Leitch, B. Synaptic Changes in AMPA Receptor Subunit Expression in Cortical Parvalbumin Interneurons in the Stargazer Model of Absence Epilepsy. Front. Cell. Neurosci. 2017, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Neves, G.; Shah, M.M.; Liodis, P.; Achimastou, A.; Denaxa, M.; Roalfe, G.; Sesay, A.; Walker, M.C.; Pachnis, V. The LIM homeodomain protein Lhx6 regulates maturation of interneurons and network excitability in the mammalian cortex. Cerebral Cortex 2013, 23, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Batista-Brito, R.; Rossignol, E.; Hjerling-Leffler, J.; Denaxa, M.; Wegner, M.; Lefebvre, V.; Pachnis, V.; Fishell, G. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron 2009, 63, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dye, C.A.; Sohal, V.; Long, J.E.; Estrada, R.C.; Roztocil, T.; Lufkin, T.; Deisseroth, K.; Baraban, S.C.; Rubenstein, J.L. Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons. J. Neurosci. 2010, 30, 5334–5345. [Google Scholar] [CrossRef] [PubMed]
- Gant, J.C.; Thibault, O.; Blalock, E.M.; Yang, J.; Bachstetter, A.; Kotick, J.; Schauwecker, P.E.; Hauser, K.F.; Smith, G.M.; Mervis, R.; et al. Decreased number of interneurons and increased seizures in neuropilin 2 deficient mice: Implications for autism and epilepsy. Epilepsia 2009, 50, 629–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canty, A.J.; Dietze, J.; Harvey, M.; Enomoto, H.; Milbrandt, J.; Ibanez, C.F. Regionalized loss of parvalbumin interneurons in the cerebral cortex of mice with deficits in GFRalpha1 signaling. J. Neurosci. 2009, 29, 10695–10705. [Google Scholar] [CrossRef] [PubMed]
- Yutsudo, N.; Kitagawa, H. Involvement of chondroitin 6-sulfation in temporal lobe epilepsy. Exp. Neurol. 2015, 274, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.H.; Mantegazza, M.; Westenbroek, R.E.; Robbins, C.A.; Kalume, F.; Burton, K.A.; Spain, W.J.; McKnight, G.S.; Scheuer, T.; Catterall, W.A. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat. Neurosci. 2006, 9, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; DeCan, E.; Stoll, K.; Marceau, E.; Deisseroth, K.; Lawrence, J.J. Muscarinic excitation of parvalbumin-positive interneurons contributes to the severity of pilocarpine-induced seizures. Epilepsia 2015, 56, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Ball, J.; Stoll, K.E.; Satpute, V.C.; Mitchell, S.M.; Pauli, J.L.; Holloway, B.B.; Johnston, A.D.; Nathanson, N.M.; Deisseroth, K.; et al. Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: Roles in cellular excitability, inhibitory transmission and cognition. J. Physiol. 2014, 592, 3463–3494. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, E.; Kruglikov, I.; van den Maagdenberg, A.M.; Rudy, B.; Fishell, G. CaV 2.1 ablation in cortical interneurons selectively impairs fast-spiking basket cells and causes generalized seizures. Ann. Neurol. 2013, 74, 209–222. [Google Scholar] [PubMed]
- Liu, X.; Yang, P.S.; Yang, W.; Yue, D.T. Enzyme-inhibitor-like tuning of Ca(2+) channel connectivity with calmodulin. Nature 2010, 463, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.X.; Erickson, M.G.; Yue, D.T. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science 2004, 304, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Westenbroek, R.E.; Yu, F.H.; Clark, J.P., 3rd; Marshall, M.R.; Scheuer, T.; Catterall, W.A. Deletion of the distal C terminus of CaV1.2 channels leads to loss of beta-adrenergic regulation and heart failure in vivo. J. Biol. Chem. 2011, 286, 12617–12626. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, C.D.; Soong, T.W.; Alseikhan, B.A.; Alvania, R.S.; Yue, D.T. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature 2001, 411, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.J.; Rungta, R.L.; Garcia, E.; van den Maagdenberg, A.M.; MacVicar, B.A.; Snutch, T.P. Contribution of calcium-dependent facilitation to synaptic plasticity revealed by migraine mutations in the P/Q-type calcium channel. Proc. Natl. Acad. Sci. USA 2010, 107, 18694–18699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaitsev, A.V.; Povysheva, N.V.; Lewis, D.A.; Krimer, L.S. P/Q-type, but not N-type, calcium channels mediate GABA release from fast-spiking interneurons to pyramidal cells in rat prefrontal cortex. J. Neurophysiol. 2007, 97, 3567–3573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gupta, A.; Toledo-Rodriguez, M.; Wu, C.Z.; Markram, H. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cerebral Cortex 2002, 12, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, E. Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast. 2011, 2011, 649325. [Google Scholar] [CrossRef] [PubMed]
- Rudy, B.; Fishell, G.; Lee, S.; Hjerling-Leffler, J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 2011, 71, 45–61. [Google Scholar] [CrossRef] [PubMed]

| VDCCs | Physiological Function | Possible Roles in Pathological Process of Epilepsy |
|---|---|---|
| L-Type | Shape neuronal firing and activate Ca2+-dependent pathways involved in the control of gene expression, and support neuronal plasticity [47] | Control neuronal excitability and likely provide the gene basis of epileptogenesis through regulation of gene expression |
| P/Q-Type | Regulate neurotransmitter release [49,50] | Inhibit epileptogenesis based on the fact that its null mutation can cause the occurrence of absence epilepsy |
| N-Type | Regulate neurotransmitter release [51,52] | Inhibit neuronal excitability through fast redistribution in the subcellullar organs of neurons |
| R-Type | Regulate long-term potentiation (LTP) and neurotransmitter release [53,54] | Trigger epileptiform activity in neuronal populations and promote epileptogenesis |
| T-Type | Regulate rhythmic firing of neurons [45] | Control burst firing of action potentials of neurons, and the plasticity of neurons induced by epiletogenic factors and promote the formation of epileptogenic focus |
| VDCCs | Regional and Cellular Distribution | Subcellular Distribution |
|---|---|---|
| L-Type | Cav1.1 and Cav1.4 are expressed in a limited subset of neurons in the brain [55]; 90% of the L-type VDCCs in the brain are Cav1.2, and only 10% are Cav1.3 [55,56] | Located postsynaptically, predominantly in the soma, dendritic spines, and shafts of dendrites [16,19,56,57,58,59,60,61,62] |
| P/Q-Type | Expressed in hippocampal principal cells of the human [16] and rat [19,56,60,61,62,63], and in both hippocampal principal cells and interneurons of mice [35] | Widely expressed at the presynaptic terminals [49,50] |
| N-Type | Expressed in the dorsal cortex and the hippocampal formation of rats [64,65] and in both the neuron and astrocyte of the mouse brain [66] | Localized in the dendrites, presynaptic membrane, and nucleus |
| R-Type | Expressed in the most basal ganglia regions, the thalamus, hypothalamus, amygdala, hippocampus, and cortex [67,68,69] | Localized in the presynaptic membrane [70] |
| T-Type | Present in neurons in both the central and peripheral nerve system | Localized in both soma and dendrites [71] |
| L-Type VDCCs | Epileptic Animal Model | Patients with TLE |
|---|---|---|
| Cav1.2 | Increased in the somata of the pyramidal cells and granule cells in the KA rat model [62]; in the granule cells of the mouse pilocarpine model [35] | Increased in the astrocytes in Ammon’s horn (or hippocampal) sclerosis (AHS) specimens [16] |
| Decreased in the neuropil of the CA3 stratum pyramidale and the part of CA1 regions in the KA rat model [62], in the hilar neurons of the mouse pilocarpine model [35]. | Decreased in the dentate gyrus granule cells and in the residual CA3 pyramidal neurons [16] | |
| No changes in the hippocampal subareas in the kindling model [19] | ||
| Cav1.3 | Increased in the hippocampal subareas in the kindling model [19], and in the granule cells of the dentate gyrus in the mouse pilocarpine model [35] | Increased in the neuropil of molecular layer of the dentate gyrus [16] |
| Decreased in CA3 and the hilus of the dentate gyrus of the KA rat model [62]; in the hippocampal neurons of the kindling model [76] |
| VDCCs | Epileptic Animal Model | Patients with TLE | Gene Knockout Outcomes |
|---|---|---|---|
| Cav2.2 | Increased in the dendritic fields of CA1 and CA3 areas of hippocampus in the rat kindling model [77,78], in the dentate granular cells of the animal KA model [79], and in the stratum lucidum of CA3 in the mouse pilocarpine model [66]. | Increased in the molecular layer [16] and granular cells of the dentate gyrus [81]; | Knockout mice displayed hyperactivity and vigilance state [82] |
| Decreased in the stratum lucidum of CA3 of the KA rat model [62], and in the stratum pyramidale of CA3 in the mouse pilocarpine model [66]. | |||
| No changes in CA1 neurons in the mouse pilocarpine model [80] | |||
| Cav2.3 | Increased in the inferior colliculus neurons of seizure-naïve rats [83]; | Increased in the molecular layer of the dentate gyrus [16]; | Knockout mice show hippocampal seizure resistance and reduced neuronal excitotoxicity [86,87,88] |
| Decreased in both cerebellum and medulla of genetic absence epilepsy rats from Strasbourg (GAERS) [84,85]; |
| VDCCs | Alterations in the Pathological Process of Epilepsy | Gene Interference, Mutation and Knockout Outcomes |
|---|---|---|
| Cav2.1 | Increased in the molecular layer of the dentate gyrus of patient with TLE [16]; different hippocampal subareas of kindling model [19] | Gene null mice exhibit ataxia and absence seizures [89]; point mutation (including tottering (tg), rocker (rkr), tottering leaner (tgla), and rolling Nagoya (tgrol)) mice exhibit reminiscent of tonic-clonic seizure, as well as electrographic and behavioral hallmarks of absence epilepsy [90]; isolated deletion in layer VI corticothalamic neurons generated absence epilepsy [91]; loss of function result in absence epilepsy [92] |
| Decreased in CA3 and the hilus of dentate gyrus of the rat KA model [62]; hippocampus and neocortex of KA rat model (6 h, 24 h and 7 days after KA treatment) [61] | ||
| No change in the cerebellum of the rat KA model (6 h, 24 h and 7 days after KA treatment) [61] | ||
| Cav3.1 | Increased in the reticular thalamic neurons of GAERS rats [93] and in neurons of the ventral posterior thalamic relay nuclei of adult GAERS [94] | Knockout mice did not show the burst firing of action potentials and were resistant to baclofen-induced seizures [95]; overexpression resulted in absence epilepsy [96] |
| Cav3.2 | Increased in both messenger RNA (mRNA) and protein level in the hippocampal CA1 area in the mouse pilocarpine model [97] | Mutation has been associated with seizure disorders, autism, and hyperaldosteronism [98]; single nucleotide mutation has been reported in patients with childhood absence epilepsy and other types of idiopathic generalized epilepsies [99,100,101,102,103,104] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.-H.; Tang, F.-R. Voltage-Dependent Calcium Channels, Calcium Binding Proteins, and Their Interaction in the Pathological Process of Epilepsy. Int. J. Mol. Sci. 2018, 19, 2735. https://doi.org/10.3390/ijms19092735
Xu J-H, Tang F-R. Voltage-Dependent Calcium Channels, Calcium Binding Proteins, and Their Interaction in the Pathological Process of Epilepsy. International Journal of Molecular Sciences. 2018; 19(9):2735. https://doi.org/10.3390/ijms19092735
Chicago/Turabian StyleXu, Jie-Hua, and Feng-Ru Tang. 2018. "Voltage-Dependent Calcium Channels, Calcium Binding Proteins, and Their Interaction in the Pathological Process of Epilepsy" International Journal of Molecular Sciences 19, no. 9: 2735. https://doi.org/10.3390/ijms19092735
APA StyleXu, J. -H., & Tang, F. -R. (2018). Voltage-Dependent Calcium Channels, Calcium Binding Proteins, and Their Interaction in the Pathological Process of Epilepsy. International Journal of Molecular Sciences, 19(9), 2735. https://doi.org/10.3390/ijms19092735