Protective Actions of Anserine Under Diabetic Conditions
Abstract
:1. Introduction
2. Results
2.1. Effect of Anserine in Glucose-Stressed Tubular Cells
2.2. Effect of Anserine in H2O2-Stressed Tubular Cells
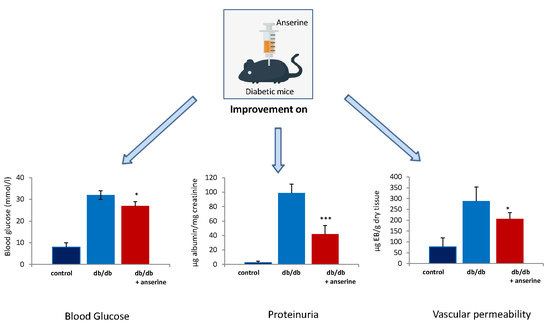
2.3. Treatment of Diabetic Mice with Anserine
3. Discussion
4. Materials and Methods
4.1. Total Antioxidant Capacity
4.2. Dipeptide Concentrations and CN1 Activity
4.3. Cell Culture
4.4. Western Immunoblotting
4.5. Western Blot of Carbonylated Proteins
4.6. Expression of Heat Shock Protein 70
4.7. Db/db Mice
4.8. Anserine Treatment
4.9. Animal Rights
4.10. Proteinuria
4.11. Tissue and Blood Sampling
4.12. Vascular Permeability Assay
4.13. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Kiersztan, A.; Drozak, J. Biosynthesis of carnosine and related dipeptides in vertebrates. Curr. Protein Pep. Sci. 2018, 19, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Teufel, M.; Saudek, V.; Ledig, J.P.; Bernhardt, A.; Boularand, S.; Carreau, A.; Cairns, N.J.; Carter, C.; Cowley, D.J.; Duverger, D.; et al. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J. Biol. Chem. 2003, 278, 6251–6531. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Jansen, E.E.; Jakobs, C.; Riedl, E.; Janssen, B.; Yard, B.A.; Wedel, J.; Hoffmann, G.F.; Zschocke, J.; Gotthardt, D.; et al. Anserine inhibits carnosine degradation but in human serum carnosinase (CN1) is not correlated with histidine dipeptide concentration. Clin. Chim. Acta 2011, 412, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.; Hohenadel, D.; Brinkkoetter, P.; Peters, V.; Rind, N.; Fischer, C.; Rychlik, I.; Cerna, M.; Romzova, M.; de Heer, E.; et al. Carnosine as a protective factor in diabetic nephropathy: Association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 2005, 54, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Zhang, S.; Braun, J.D.; Xia, L.; Rodriquez, A.; Qiu, J.; Peters, V.; Schmitt, C.P.; van den Born, J.; Bakker, S.J.L.; et al. The CNDP1 (CTG)5 Polymorphism Is Associated with Biopsy-Proven Diabetic Nephropathy, Time on Hemodialysis, and Diabetes Duration. J. Diabetes Res. 2017, 2017, 9506730. [Google Scholar] [CrossRef] [PubMed]
- Mooyaart, A.; van Valkengoed, I.G.; Shaw, P.K.; Peters, V.; Baelde, H.J.; Rabelink, T.J.; Bruijn, J.A.; Stronks, K.; de Heer, E. Lower frequency of the 5/5 homozygous CNDP1 genotype in South Asian Surinamese. Diabetes Res. Clin. Pract. 2009, 85, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, T.S.; Lindholm, E.; Groop, L.C. Common variants in CNDP1 and CNDP2, and risk of nephropathy in type 2 diabetes. Diabetologia 2011, 54, 2295–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhalaf, A.; Landman, G.W.; van Hateren, K.J.; Groenier, K.H.; Mooyaart, A.L.; De Heer, E.; Gans, R.O.; Navis, G.J.; Bakker, S.J.; Kleefstra, N.; et al. Sex specific association between carnosinase gene CNDP1 and cardiovascular mortality in patients with type 2 diabetes (ZODIAC-22). J. Nephrol. 2015, 28, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.I.; Hicks, P.J.; Sale, M.M.; Pierson, E.D.; Langefeld, C.D.; Rich, S.S.; Xu, J.; McDonough, C.; Janssen, B.; Yard, B.A.; et al. A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol. Dial. Transpl. 2007, 22, 1131–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, V.; Zschocke, J.; Schmitt, C.P. Carnosinase, diabetes mellitus and the potential relevance of carnosinase deficiency. J. Inherit. Metab. Dis. 2018, 41, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Alhamdani, M.; Al-Azzawie, H.F.; Abbas, F.K. Decreased formation of advanced glycation end-products in peritoneal fluid by carnosine and related peptides. Perit. Dial. Int. 2007, 27, 86–89. [Google Scholar] [PubMed]
- Hou, W.; Chen, H.J.; Lin, Y.H. Antioxidant peptides with Angiotensin converting enzyme inhibitory activities and applications for Angiotensin converting enzyme purification. J. Agric. Food Chem. 2003, 51, 1706–1709. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Ueno, A.; Nishikawa, Y. Interactions between carnosine and captopril on free radical scavenging activity and angiotensin-converting enzyme activity in vitro. Yakugaku Zasshi 2006, 126, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Livisay, S.A.; Zhou, S. A re-evaluation of the antioxidant activity of purified carnosine. Biochemistry 2000, 65, 766–770. [Google Scholar] [PubMed]
- Velez, S.; Nair, N.G.; Reddy, V.P. Transition metal ion binding studies of carnosine and histidine: Biologically relevant antioxidants. Colloids Surf B Biointerfaces 2008, 66, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R. Energy metabolism, proteotoxic stress and age-related dysfunction—Protection by carnosine. Mol. Aspects Med. 2011, 32, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Lankin, V.Z.; Savel’Yeva, E.L.; Deyev, A.I.; Yegorov, Y.E. Diabetes mellitus: Novel insights, analysis and interpretation of pathophysiology and complications management with imidazole-containing peptidomimetic antioxidants. Recent Pat. Drug Deliv. Formul. 2013, 7, 216–256. [Google Scholar] [CrossRef] [PubMed]
- Barski, O.A.; Xie, Z.; Baba, S.P.; Sithu, S.D.; Agarwal, A.; Cai, J.; Bhatnagar, A.; Srivastava, S. Dietary carnosine prevents early atherosclerotic lesion formation in apolipoprotein E.-null mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Fleming, T.; De Buhr, S.; Beijer, B.; Lindner, T.; Wischnjow, A.; Kender, Z.; Peters, V.; Kopf, S.; Haberkorn, U.; et al. A scavenger peptide prevents methylglyoxal induced pain in mice. Biochim. Biophys. Acta 2017, 1863, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; Colzani, M.; Mazzolari, A.; Gilardoni, E.; Rivaletto, C.; Carini, M.; Aldini, G. Quenching activity of carnosine derivatives towards reactive carbonyl species: Focus on alpha-(methylglyoxal) and beta-(malondialdehyde) dicarbonyls. Biochem. Biophys. Res. Commun. 2017, 492, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Colzani, M.; De Maddis, D.; Casali, G.; Carini, M.; Vistoli, G.; Aldini, G. Reactivity, Selectivity, and Reaction Mechanisms of Aminoguanidine, Hydralazine, Pyridoxamine, and Carnosine as Sequestering Agents of Reactive Carbonyl Species: A. Comparative Study. Chem. Med. Chem. 2016, 19, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Orioli, M.; Rossoni, G.; Savi, F.; Braidotti, P.; Vistoli, G.; Yeum, K.J.; Negrisoli, G.; Carini, M. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J. Cell Mol. Med. 2011, 15, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Ansurudeen, I.; Sunkari, V.G.; Grunler, J.; Peters, V.; Schmitt, C.P.; Catrina, S.B.; Brismar, K.; Forsberg, E.A. Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino Acids 2012, 43, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Menini, S.; Blasetti Fantauzzi, C.; Pesce, C.M.; Giaccari, A.; Salomone, E.; Lapolla, A.; Orioli, M.; Aldini, G.; Pugliese, G. FL-926-16, a novel bioavailable carnosinase-resistant carnosine derivative, prevents onset and stops progression of diabetic nephropathy in db/db mice. Br. J. Pharmacol. 2018, 175, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Riedl, E.; Pfister, F.; Braunagel, M.; Brinkkotter, P.; Sternik, P.; Deinzer, M.; Bakker, S.J.; Henning, R.H.; van den Born, J.; Kramer, B.K.; et al. Carnosine prevents apoptosis of glomerular cells and podocyte loss in STZ diabetic rats. Cell Physiol. Biochem 2011, 28, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Riedl, E.; Braunagel, M.; Hoger, S.; Hauske, S.; Pfister, F.; Zschocke, J.; Lanthaler, B.; Benck, U.; Hammes, H.P.; et al. Carnosine treatment in combination with ACE inhibition in diabetic rats. Regul. Pept. 2014, 194–195, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Bellia, F.; Calabrese, V.; Guarino, F.; Cavallaro, M.; Cornelius, C.; De Pinto, V.; Rizzarelli, E. Carnosinase levels in aging brain: Redox state induction and cellular stress response. Antioxid. Redox. Signal. 2009, 11, 2759–2775. [Google Scholar] [CrossRef] [PubMed]
- Pfister, F.; Riedl, E.; Wang, Q.; vom Hagen, F.; Deinzer, M.; Harmsen, M.C.; Molema, G.; Yard, B.; Feng, Y.; Hammes, H.P. Oral carnosine supplementation prevents vascular damage in experimental diabetic retinopathy. Cell Physiol. Biochem. 2011, 28, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; Colzani, M.; Mazzolari, A.; Maddis, D.D.; Grazioso, G.; Pedretti, A.; Carini, M.; Aldini, G. Computational approaches in the rational design of improved carbonyl quenchers: Focus on histidine containing dipeptides. Future Med. Chem. 2016, 8, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Hobart, L.J.; Seibel, I.; Yeargans, G.S.; Seidler, N.W. Anti-crosslinking properties of carnosine: Significance of histidine. Life Sci. 2004, 75, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.; Bulygina, E.; Leinsoo, T.; Petrushanko, I.; Tsubone, S.; Abe, H. Protection of neuronal cells against reactive oxygen species by carnosine and related compounds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 137, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Klessens, C.Q.; Baelde, H.J.; Singler, B.; Veraar, K.A.; Zutinic, A.; Drozak, J.; Zschocke, J.; Schmitt, C.P.; de Heer, E. Intrinsic carnosine metabolism in the human kidney. Amino Acids 2015, 47, 2541–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauerhofer, S.; Yuan, G.; Braun, G.S.; Deinzer, M.; Neumaier, M.; Gretz, N.; Floege, J.; Kriz, W.; van der Woude, F.; Moeller, M.J. L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 2007, 56, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Schilperoort, M.; Zhang, S.; Braun, J.D.; Qiu, J.; Rodriguez, A.; Pastene, D.O.; Kramer, B.K.; Koppel, H.; Baelde, H.; et al. Carnosine Attenuates the Development of both Type 2 Diabetes and Diabetic Nephropathy in BTBR ob/ob Mice. Sci. Rep. 2017, 7, 44492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, K.; Tanida, M.; Niijima, A.; Tsuruoka, N.; Kiso, Y.; Horii, Y.; Shen, J.; Okumura, N. Role of L-carnosine in the control of blood glucose, blood pressure, thermogenesis, and lipolysis by autonomic nerves in rats: Involvement of the circadian clock and histamine. Amino Acids 2012, 43, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Hsu, C.C.; Lin, M.H.; Liu, K.S.; Yin, M.C. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005, 513, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Ricci, C.; Scipioni, A.; Blasetti Fantauzzi, C.; Giaccari, A.; Salomone, E.; Canevotti, R.; Lapolla, A.; Orioli, M.; et al. D-Carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. Br. J. Pharmacol. 2012, 166, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Schmitt, C.P.; Zschocke, J.; Gross, M.L.; Brismar, K.; Forsberg, E. Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino Acids 2012, 42, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Weigand, T.; Singler, B.; Fleming, T.; Nawroth, P.; Klika, K.D.; Thiel, C.; Baelde, H.; Garbade, S.F.; Wagner, A.H.; Hecker, M.; et al. Carnosine Catalyzes the Formation of the Oligo/Polymeric Products of Methylglyoxal. Cell Physiol. Biochem. 2018, 46, 713–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.X.; Neuhofer, W.; Muller, E. Molecular chaperones in the kidney: Distribution, putative roles, and regulation. Am. J. Physiol. Renal. Physiol. 2000, 279, F203–F215. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, N.; Bobkova, I.; Shilov, E. Heat shock proteins and kidney disease: Perspectives of HSP therapy. Cell Stress Chaperones 2017, 22, 319–343. [Google Scholar] [CrossRef] [PubMed]
- Bellia, F.; Vecchio, G.; Cuzzocrea, S.; Calabrese, V.; Rizzarelli, E. Neuroprotective features of carnosine in oxidative driven diseases. Mol. Aspects Med. 2011, 32, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Pinach, S.; Giunti, S.; Vittone, F.; Forbes, J.M.; Chiarle, R.; Arnstein, M.; Perin, P.C.; Camussi, G.; Cooper, M.E.; et al. Heat shock protein expression in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2008, 295, F1817–F1824. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Lanthaler, B.; Amberger, A.; Fleming, T.; Forsberg, E.; Hecker, M.; Wagner, A.H.; Yue, W.W.; Hoffmann, G.F.; Nawroth, P.; et al. Carnosine metabolism in diabetes is altered by reactive metabolites. Amino Acids 2015, 47, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Ibla, J.C.; Khoury, J. Methods to assess tissue permeability. Methods Mol. Biol. 2006, 341, 111–117. [Google Scholar] [PubMed]
- Matthew, C.B.; Sils, I.V.; Bastille, A.M. Tissue-specific extravasation of albumin-bound Evans blue in hypothermic and rewarmed rats. Can. J. Physiol. Pharmacol. 2002, 80, 233–243. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, V.; Calabrese, V.; Forsberg, E.; Volk, N.; Fleming, T.; Baelde, H.; Weigand, T.; Thiel, C.; Trovato, A.; Scuto, M.; et al. Protective Actions of Anserine Under Diabetic Conditions. Int. J. Mol. Sci. 2018, 19, 2751. https://doi.org/10.3390/ijms19092751
Peters V, Calabrese V, Forsberg E, Volk N, Fleming T, Baelde H, Weigand T, Thiel C, Trovato A, Scuto M, et al. Protective Actions of Anserine Under Diabetic Conditions. International Journal of Molecular Sciences. 2018; 19(9):2751. https://doi.org/10.3390/ijms19092751
Chicago/Turabian StylePeters, Verena, Vittorio Calabrese, Elisabete Forsberg, Nadine Volk, Thomas Fleming, Hans Baelde, Tim Weigand, Christian Thiel, Angela Trovato, Maria Scuto, and et al. 2018. "Protective Actions of Anserine Under Diabetic Conditions" International Journal of Molecular Sciences 19, no. 9: 2751. https://doi.org/10.3390/ijms19092751
APA StylePeters, V., Calabrese, V., Forsberg, E., Volk, N., Fleming, T., Baelde, H., Weigand, T., Thiel, C., Trovato, A., Scuto, M., Modafferi, S., & Schmitt, C. P. (2018). Protective Actions of Anserine Under Diabetic Conditions. International Journal of Molecular Sciences, 19(9), 2751. https://doi.org/10.3390/ijms19092751