Ascorbic Acid in Colon Cancer: From the Basic to the Clinical Applications
Abstract
:1. Background
2. Methods
3. Results
3.1. Glycolysis in Colorectal Cancer
3.2. Pharmacokinetics of Ascorbic Acid
3.3. Mechanism of Action of Ascorbic Acid in Cancer in General
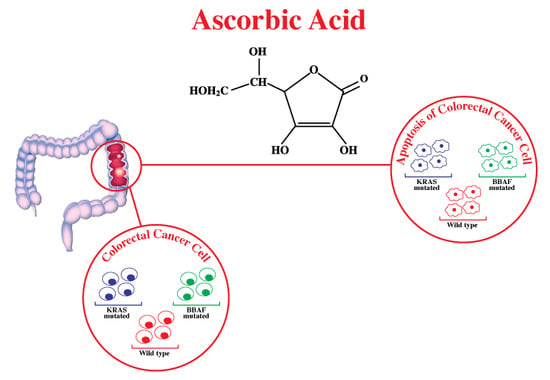
3.4. Mechanism of Action of Ascorbic Acid in Glycolysis, Particularly in CRC with Mutated BRAF/KRAS
3.5. Previous Studies on Intravenous Ascorbic Acid Administration in Cancer in General
3.6. Previous Studies on Intravenous Ascorbic Acid Administration in Colorectal Cancer
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| 6-bOcta-AA-2G | 2-O-α-d-glucopyranosyl-6-O-(2-pentylheptanoyl)-l-ascorbic acid |
| AA | Ascorbic acid |
| CRC | Colorectal cancer |
| CRP | C-reactive protein |
| DHA | Dehydroascorbate |
| EGFR | Epidermal growth factor receptor |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| GLUT | Glucose transporters |
| G6PD | Glucose-6-phosphate dehydrogenase |
| H2O2 | Hydrogen peroxide |
| HIF | Hypoxia-inducible transcription |
| HK-2 | Hexokinase-2 |
| IL | Interleukins |
| MAPK/ERK | Mitogen-Activated Protein Kinases/Extracellular Signal-Regulated Kinases |
| MEK/ERK/PKM2 | Phosphorylation cascade |
| NAD | Nicotinamide adenine dinucleotide |
| PDK1 | Pyruvate dehydrogenase kinase isozyme 1 |
| PPP | Pentose phosphate pathway |
| ROS | Reactive oxygen species |
| Sp | Specificity protein |
| STAT | Signal transducer and activator of transcription |
| SVCT1 | Sodium-dependent vitamin C transporters 1 |
| SVCT2 | Sodium-dependent vitamin C transporters 2 |
| TCA | Tricarboxylic acid cycle |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless-related integration site |
| Ψm | Mitochondrial membrane potential |
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, E.G.; Révész, D.; Tamminga, H.J.; Punt, C.J.; Koopman, M.; Onwuteaka-Philipsen, B.D.; Steyerberg, E.W.; Jansma, I.P.; De Vet, H.C.; Coupé, V.M. Clinical usefulness of tools to support decision-making for palliative treatment of metastatic colorectal cancer: A systematic review. Clin. Colorectal Cancer 2017, 17, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Sebolt-Leopold, J.S.; Herrera, R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer 2004, 4, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Bürzle, M.; Suzuki, Y.; Ackermann, D.; Miyazaki, H.; Maeda, N.; Clémençon, B.; Burrier, R.; Hediger, M.A. The sodium-dependent ascorbic acid transporter family slc23. Mol. Asp. Med. 2013, 34, 436–454. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Du, X. Kras mutation testing in metastatic colorectal cancer. World J. Gastroenterol. WJG 2012, 18, 5171. [Google Scholar] [PubMed]
- Lievre, A.; Bachet, J.-B.; Le Corre, D.; Boige, V.; Landi, B.; Emile, J.-F.; Côté, J.-F.; Tomasic, G.; Penna, C.; Ducreux, M. Kras mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006, 66, 3992–3995. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.; Flower, G.; Weeks, L.; Cooley, K.; Callachan, M.; McGowan, J.; Skidmore, B.; Kirchner, L.; Seely, D. Intravenous vitamin C and cancer: A systematic review. Integr. Cancer Ther. 2014, 13, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiang, F.; Huang, Y.; Shi, L.; Hu, C.; Yang, Y.; Wang, D.; He, N.; Tao, K.; Wu, K. Interleukin-22 promotes aerobic glycolysis associated with tumor progression via targeting hexokinase-2 in human colon cancer cells. Oncotarget 2017, 8, 25372. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Fang, X. Advances in glucose metabolism research in colorectal cancer. Biomed. Rep. 2016, 5, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Pate, K.T.; Stringari, C.; Sprowl-Tanio, S.; Wang, K.; TeSlaa, T.; Hoverter, N.P.; McQuade, M.M.; Garner, C.; Digman, M.A.; Teitell, M.A. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014, 33, 1454–1473. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Rago, C.; Cheong, I.; Pagliarini, R.; Angenendt, P.; Rajagopalan, H.; Schmidt, K.; Willson, J.K.; Markowitz, S.; Zhou, S. Glucose deprivation contributes to the development of kras pathway mutations in tumor cells. Science 2009, 325, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Duconge, J.; Miranda-Massari, J.R.; Gonzalez, M.J.; Jackson, J.A.; Warnock, W.; Riordan, N.H. Pharmacokinetics of vitamin C: Insights into the oral and intravenous administration of ascorbate. P. R. Health Sci. J. 2008, 27, 7–19. [Google Scholar] [PubMed]
- Mikirova, N.; Casciari, J.; Riordan, N.; Hunninghake, R. Clinical experience with intravenous administration of ascorbic acid: Achievable levels in blood for different states of inflammation and disease in cancer patients. J. Transl. Med. 2013, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin c pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer: A controlled trial. N. Engl. J. Med. 1979, 301, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padayatty, S.J.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Hoffer, L.J.; Levine, M. Intravenously administered vitamin C as cancer therapy: Three cases. Can. Med.Assoc. J. 2006, 174, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Drisko, J.A.; Chapman, J.; Hunter, V.J. The use of antioxidants with first-line chemotherapy in two cases of ovarian cancer. J. Am. Coll. Nutr. 2003, 22, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.E.; Falls, K.C.; Schoenfeld, J.D.; Rodman, S.N.; Gu, Z.; Zhan, F.; Cullen, J.J.; Wagner, B.A.; Buettner, G.R.; Allen, B.G.; et al. Augmentation of intracellular iron using iron sucrose enhances the toxicity of pharmacological ascorbate in colon cancer cells. Redox Biol. 2018, 14, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Haraguchi, M.; Ito, H.; Tai, A. Potential antitumor activity of 2-O-alpha-d-glucopyranosyl-6-O-(2-pentylheptanoyl)-l-ascorbic acid. Int. J. Mol. Sci. 2018, 19, 535. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Levine, M. New insights into the physiology and pharmacology of vitamin C. Can. Med. Assoc. J. 2001, 164, 353–355. [Google Scholar] [PubMed]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Martin, S.M.; Levine, M.; Wagner, B.A.; Buettner, G.R.; Wang, S.-H.; Taghiyev, A.F.; Du, C.; Knudson, C.M.; Cullen, J.J. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin. Cancer Res. 2010, 16, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Pathi, S.S.; Lei, P.; Sreevalsan, S.; Chadalapaka, G.; Jutooru, I.; Safe, S. Pharmacologic doses of ascorbic acid repress specificity protein (sp) transcription factors and sp-regulated genes in colon cancer cells. Nutr. Cancer 2011, 63, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kang, J.S.; Lee, W.J. Vitamin c induces apoptosis in human colon cancer cell line, hct-8 via the modulation of calcium influx in endoplasmic reticulum and the dissociation of bad from 14-3-3beta. Immune Netw. 2012, 12, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, C.; Dachs, G.U.; Currie, M.J.; Vissers, M.C. Intracellular ascorbate enhances hypoxia-inducible factor (hif)-hydroxylase activity and preferentially suppresses the hif-1 transcriptional response. Free Radic. Biol. Med. 2014, 69, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Uetaki, M.; Tabata, S.; Nakasuka, F.; Soga, T.; Tomita, M. Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress. Sci. Rep. 2015, 5, 13896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, A.S.; Marques, C.R.; Encarnação, J.C.; Abrantes, A.M.; Mamede, A.C.; Laranjo, M.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Botelho, M.F. Ascorbic acid and colon cancer: An oxidative stimulus to cell death depending on cell profile. Eur. J. Cell Biol. 2016, 95, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Expression of glucose transporters in cancers. Biochim. Biophys. Acta (BBA) Rev. Cancer 2013, 1835, 164–169. [Google Scholar] [CrossRef] [PubMed]
- McCain, J. The mapk (erk) pathway: Investigational combinations for the treatment of braf-mutated metastatic melanoma. Pharm. Ther. 2013, 38, 96–108. [Google Scholar]
- Hutton, J.E.; Wang, X.; Zimmerman, L.J.; Slebos, R.J.; Trenary, I.A.; Young, J.D.; Li, M.; Liebler, D.C. Oncogenic kras and braf drive metabolic reprogramming in colorectal cancer. Mol. Cell. Proteom. 2016, 15, 2924–2938. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, O.; Muñoz-Sagastibelza, M.; Torrejón, B.; Borrero-Palacios, A.; del Puerto-Nevado, L.; Martínez-Useros, J.; Rodriguez-Remirez, M.; Zazo, S.; García, E.; Fraga, M. Vitamin c uncouples the warburg metabolic switch in kras mutant colon cancer. Oncotarget 2016, 7, 47954. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C. Vitamin c selectively kills kras and braf mutant colorectal cancer cells by targeting gapdh. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.A.; Lee, D.H.; Moon, J.H.; Hong, S.W.; Shin, J.S.; Hwang, I.Y.; Shin, Y.J.; Kim, J.H.; Gong, E.Y.; Kim, S.M.; et al. L-ascorbic acid can abrogate svct-2-dependent cetuximab resistance mediated by mutant kras in human colon cancer cells. Free Radic. Biol. Med. 2016, 95, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976, 73, 3685–3689. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Fleming, T.R.; Creagan, E.T.; Rubin, J.; O’Connell, M.J.; Ames, M.M. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy: A randomized double-blind comparison. N. Engl. J. Med. 1985, 312, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Cassileth, B.R.; Deng, G. Complementary and alternative therapies for cancer. Oncologist 2004, 9, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Mitchell, E.; Bazzan, A.J.; Littman, S.; Zabrecky, G.; Yeo, C.J.; Pillai, M.V.; Newberg, A.B.; Deshmukh, S.; Levine, M. Phase i evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS ONE 2012, 7, e29794. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, A.Y.; Chen, Q.; Espey, M.G.; Drisko, J.; Levine, M. Vitamin c: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE 2010, 5, e11414. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014, 6, 222ra218. [Google Scholar] [CrossRef] [PubMed]
- Yeom, C.H.; Jung, G.C.; Song, K.J. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J. Korean Med. Sci. 2007, 22, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Mizuno, H.; Yanagisawa, A. High-dose intravenous vitamin C improves quality of life in cancer patients. Person. Med. Univ. 2012, 1, 49–53. [Google Scholar] [CrossRef]
- Carr, A.C.; Vissers, M.; Cook, J.S. The effect of intravenous vitamin C on cancer-and chemotherapy-related fatigue and quality of life. Front. Oncol. 2014, 4, 283. [Google Scholar] [CrossRef] [PubMed]
- Bigelsen, S. Evidence-based complementary treatment of pancreatic cancer: A review of adjunct therapies including paricalcitol, hydroxychloroquine, intravenous vitamin C, statins, metformin, curcumin, and aspirin. Cancer Manag. Res. 2018, 10, 2003. [Google Scholar] [CrossRef] [PubMed]
- Klimant, E.; Wright, H.; Rubin, D.; Seely, D.; Markman, M. Intravenous vitamin C in the supportive care of cancer patients: A review and rational approach. Curr. Oncol. 2018, 25, 139. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.A.; Riordan, H.D.; Hunninghake, R.E.; Riordan, N. High dose intravenous vitamin C and long time survival of a patient with cancer of the head of the pancreas. J. Orthomol. Med. 1995, 10, 87–88. [Google Scholar]
- Campbell, A.; Jack, T.; Cameron, E. Reticulum cell sarcoma: Two complete ‘spontaneous’ regressions, in reponse to high-dose ascorbic acid therapy. Oncology 1991, 48, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.; Campbell, A.; Jack, T. The orthomolecular treatment of cancer: III. Reticulum cell sarcoma: Double complete regression induced by high-dose ascorbic acid therapy. Chem. Biol. Interact. 1975, 11, 387–393. [Google Scholar] [CrossRef]
- Abou-Jawde, R.M.; Reed, J.; Kelly, M.; Walker, E.; Andresen, S.; Baz, R.; Karam, M.A.; Hussein, M. Efficacy and safety results with the combination therapy of arsenic trioxide, dexamethasone, and ascorbic acid in multiple myeloma patients. Med. Oncol. 2006, 23, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: Results of a retrospective, multicentre, epidemiological cohort study in germany. In Vivo 2011, 25, 983–990. [Google Scholar] [PubMed]
- Murata, A.; Morishige, F.; Yamaguchi, H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int. J. Vitam. Nutr. Res. Suppl. 1982, 23, 103–113. [Google Scholar] [PubMed]
- Kuiper, C.; Molenaar, I.G.; Dachs, G.U.; Currie, M.J.; Sykes, P.H.; Vissers, M.C. Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res. 2010, 70, 5749–5758. [Google Scholar] [CrossRef] [PubMed]
- Mayland, C.R.; Bennett, M.I.; Allan, K. Vitamin c deficiency in cancer patients. Palliat. Med. 2005, 19, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Pollard, H.B.; Levine, M.A.; Eidelman, O.; Pollard, M. Pharmacological ascorbic acid suppresses syngeneic tumor growth and metastases in hormone-refractory prostate cancer. In Vivo 2010, 24, 249–255. [Google Scholar] [PubMed]
- Riordan, H.D.; Riordan, N.H.; Jackson, J.A.; Casciari, J.J.; Hunninghake, R.; González, M.J.; Mora, E.M.; Miranda-Massari, J.R.; Rosario, N.; Rivera, A. Intravenous vitamin C as a chemotherapy agent: A report on clinical cases. P. R. Health Sci. J. 2004, 23. [Google Scholar]
- Ichim, T.E.; Minev, B.; Braciak, T.; Luna, B.; Hunninghake, R.; Mikirova, N.A.; Jackson, J.A.; Gonzalez, M.J.; Miranda-Massari, J.R.; Alexandrescu, D.T. Intravenous ascorbic acid to prevent and treat cancer-associated sepsis? J. Transl. Med. 2011, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drisko, J.A.; Serrano, O.K.; Spruce, L.R.; Chen, Q.; Levine, M. Treatment of pancreatic cancer with intravenous vitamin C: A case report. Anti-Cancer Drugs 2018, 29, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.; Wagner, B.; Van’t Erve, T.; Zehr, P.; Berg, D.; Halfdanarson, T.R.; Yee, N.; Bodeker, K.; Du, J.; Roberts, L. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (pacman): Results from a phase i clinical trial. Cancer Chemother. Pharmacol. 2013, 71, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Robitaille, L.; Zakarian, R.; Melnychuk, D.; Kavan, P.; Agulnik, J.; Cohen, V.; Small, D.; Miller, W.H., Jr. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: A phase i-ii clinical trial. PLoS ONE 2015, 10, e0120228. [Google Scholar] [CrossRef] [PubMed]
- Mikirova, N.; Casciari, J.; Rogers, A.; Taylor, P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J. Transl. Med. 2012, 10, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riordan, H.D.; Casciari, J.J.; González, M.J.; Riordan, N.H.; Miranda-Massari, J.R.; Taylor, P.; Jackson, J.A. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P. R. Health Sci. J. 2009, 24, 269–276. [Google Scholar]
- Littman, S.J.; Monti, D.; Newberg, A.; Bazzan, A.; Pillai, M.V.; Lewis, N.; Yeo, C.; Levine, M.; Mitchell, E.P. A phase i open-label, dose-escalation study of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2012, 30, 323. [Google Scholar] [CrossRef]
- Saygili, E.; Konukoglu, D.; Papila, C.; Akcay, T. Levels of plasma vitamin e, vitamin C, tbars, and cholesterol in male patients with colorectal tumors. Biochemistry 2003, 68, 325–328. [Google Scholar] [PubMed]
- Jeon, Y.; Park, J.S.; Moon, S.; Yeo, J. Effect of intravenous high dose vitamin C on postoperative pain and morphine use after laparoscopic colectomy: A randomized controlled trial. Pain Res. Manag. 2016, 2016, 9147279. [Google Scholar] [CrossRef] [PubMed]

| Condition | Type of Study | Study Sample | Concomitant Therapy | Finding | Reference |
|---|---|---|---|---|---|
| Stage IV pancreatic cancer | Phase I open-label, dose-escalating trial | 14 | Gemcitabine/Erlotinib |
| [39] |
| Stage IV pancreatic cancer | Case report | 1 | None | Objective regression of the disease | [58] |
| Stage IV pancreatic cancer | Phase I clinical trial | 9 | Concurrent Gemcitabine |
| [59] |
| Stages II–III breast cancer | Epidemiological, retrospective and observational study | 125 | Standard tumour therapy |
| [51] |
| Stage IV renal cell carcinoma | Case reports | 2 | None | Resolution of metastatic lesions | [56] |
| Terminal cancer | Nonrandomized clinical trials | 99 | None |
| [52] |
| Different types of cancer | Phase I–II clinical trial | 14 | Standard tumour therapy |
| [60] |
| Different types of cancer | Observational study | 45 | None | Reduction in pro-inflammatory cytokines | [61] |
| Condition | Aim of the Study | Finding | Reference |
|---|---|---|---|
| Colon cancer (Duke B–C stages) |
| Level of plasma vitamin C is lower in patients with CRC when compared to healthy subjects. | [64] |
| Resectable colon cancer | Effect of intravenous AA on post laparoscopic colectomy pain | High dose intravenous AA decreases pain within 24 h post-op. | [65] |
| Colon cancer (stage IV) and other cancer types | Case report on the effects of intravenous AA given alone or concomitantly with chemotherapy |
| [56] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Halabi, I.; Bejjany, R.; Nasr, R.; Mukherji, D.; Temraz, S.; Nassar, F.J.; El Darsa, H.; Shamseddine, A. Ascorbic Acid in Colon Cancer: From the Basic to the Clinical Applications. Int. J. Mol. Sci. 2018, 19, 2752. https://doi.org/10.3390/ijms19092752
El Halabi I, Bejjany R, Nasr R, Mukherji D, Temraz S, Nassar FJ, El Darsa H, Shamseddine A. Ascorbic Acid in Colon Cancer: From the Basic to the Clinical Applications. International Journal of Molecular Sciences. 2018; 19(9):2752. https://doi.org/10.3390/ijms19092752
Chicago/Turabian StyleEl Halabi, Ibrahim, Rachelle Bejjany, Rihab Nasr, Deborah Mukherji, Sally Temraz, Farah J. Nassar, Haidar El Darsa, and Ali Shamseddine. 2018. "Ascorbic Acid in Colon Cancer: From the Basic to the Clinical Applications" International Journal of Molecular Sciences 19, no. 9: 2752. https://doi.org/10.3390/ijms19092752
APA StyleEl Halabi, I., Bejjany, R., Nasr, R., Mukherji, D., Temraz, S., Nassar, F. J., El Darsa, H., & Shamseddine, A. (2018). Ascorbic Acid in Colon Cancer: From the Basic to the Clinical Applications. International Journal of Molecular Sciences, 19(9), 2752. https://doi.org/10.3390/ijms19092752