OsMGT1 Confers Resistance to Magnesium Deficiency By Enhancing the Import of Mg in Rice
Abstract
:1. Introduction
2. Results
2.1. OsMGT1 Was Up-Regulated by Mg Deficiency
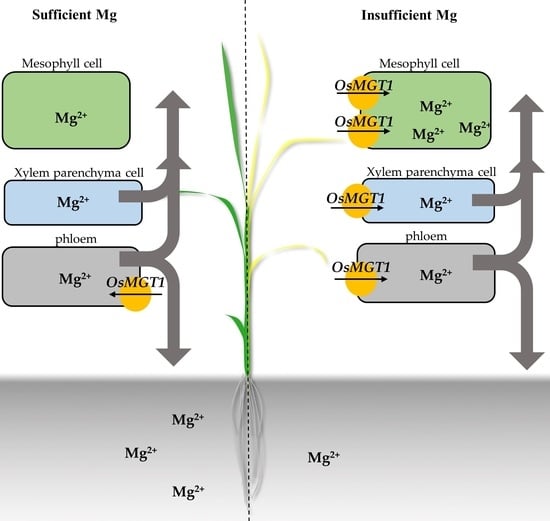
2.2. Mg Deficiency Altered the Tissue Expression Pattern of OsMGT1
2.3. Knockout of OsMGT1 Resulted in Higher Sensitivity to Mg Deficiency
2.4. Excessive Ca Aggravated Mg Deficiency in osmgt1 Mutants
2.5. Overexpression of OsMGT1 Promoted Rice Growth Under Low Mg Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. RNA Isolation and Gene Expression Analysis
4.3. Immunohistological Analysis of OsMGT1
4.4. Phenotypic Analysis
4.5. Mg Determination in Plant Tissues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Williams, L.; Salt, D.E. The plant ionome coming into focus. Curr. Opin. Plant Biol. 2009, 12, 247–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: New York, NY, USA, 2012; pp. 165–170. ISBN 978-0-12-384905-2. [Google Scholar]
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.C.; Nobel, P.S. Control of photosynthesis by Mg2+. Arch. Biochem. Biophys. 1971, 145, 622–632. [Google Scholar] [CrossRef]
- Sperrazza, J.M.; Spremulli, L.L. Quantitation of cation binding to wheat germ ribosomes: Influences on submit association equilibria and ribosome activity. Nucleic Acids Res. 1983, 11, 2665–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, J.; Lorimer, G.H.; Reddy, G.S. Kinetic mechanism of ribulosebisphosphate carboxylase: Evidence for an ordered, sequential reaction. Biochemistry 1986, 25, 1636–1644. [Google Scholar] [CrossRef]
- Rissler, H.M.; Collakova, E.; DellaPenna, D.; Whelan, J.; Pogson, B.J. Chlorophyll biosynthesis. Expression of a second Chl I gene of magnesium chelatase in Arabidopsis supports only limited chlorophyll synthesis. Plant Physiol. 2002, 128, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I. Magnesium in crop production, food quality and human health. Plant Soil 2013, 368, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Welch, R.M.; Mayland, H.F.; Grunes, D.L. Magnesium in plants: Uptake, distribution, function and utilization by man and animals. Met. Ions Biol. Syst. 1990, 26, 33–56. [Google Scholar]
- Maguire, M.E.; Cowan, J.A. Magnesium chemistry and biochemistry. Biometals 2002, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Grzebisz, W. Magnesium-food and human health. J. Elemntol. 2011, 16, 299–323. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Rosanoff, A. Changing crop magnesium concentrations: Impact on human health. Plant Soil 2013, 368, 139–153. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Li, L.G.; Sokolov, L.N.; Yang, Y.H.; Li, D.P.; Ting, J.; Pandy, G.K.; Luan, S. A mitochondrial magnesium transporter functions in Arabidopsis pollen development. Mol. Plant 2008, 1, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Conn, S.J.; Chen, J.G.; Xiao, Q.Y.; Verbruggen, N. An update on magnesium homeostasis mechanisms in plants. Metallomics 2013, 5, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Shaul, O.; Hilgemann, D.W.; de-Almeida-Engler, J.; Montagu, M.V.; Inze´, D.; Galili, G. Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J. 1999, 18, 3973–3980. [Google Scholar] [CrossRef]
- Li, L.G.; Tutone, A.F.; Drummond, R.S.M.; Gardner, R.C.; Luan, S. A novel family of magnesium transport genes in Arabidopsis. Plant Cell 2001, 13, 2761–2775. [Google Scholar] [CrossRef]
- Gebert, M.; Meschenmoser, K.; Svidova´, S.; Weghuber, J.; Schweyen, R.; Eifler, K.; Lenz, H.; Weyand, K.; Knoop, V. A root-expressed magnesium transporter of the MRS2/MGT gene family in Arabidopsis thaliana allows for growth in low-Mg2+ environments. Plant Cell 2009, 21, 4018–4030. [Google Scholar] [CrossRef]
- Tang, R.J.; Luan, S. Regulation of calcium and magnesium homeostasis in plants: From transporters to signaling network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, H.; Wang, J.; Zhang, B.; Wang, W.; Lin, H.; Luan, S.; Gao, J.; Lan, W. The rice high-affinity k+ transporter OsHKT2;4 mediates Mg2+ homeostasis under high-Mg2+ conditions in transgenic Arabidopsis. Front. Plant Sci. 2017, 8, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.W.M.; Schorrak, L.M.; Smith, R.K., Jr.; Bent, A.F.; Sussman, M.R. A cyclic nucleotide-gated ion channel, CNGC2, is crucial for plant development and adaptation to calcium stress. Plant Physiol. 2003, 132, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.M.; Babourina, O.; Christopher, D.A.; Borsic, T.; Rengel, Z. The cyclic nucleotide-gated channel AtCNGC10 transports Ca2+ and Mg2+ in Arabidopsis. Physiol. Plant. 2010, 139, 303–312. [Google Scholar] [PubMed]
- Saito, T.; Kobayashi, N.I.; Tanoi, K.; Iwata, N.; Suzuki, H.; Iwata, R.; Nakanishi, T.M. Expression and functional analysis of the CorA-MRS2-ALR-type magnesium transporter family in rice. Plant Cell Physiol. 2013, 54, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Graschopf, A.; Stadler, J.A.; Hoellerer, M.K.; Eder, S.; Sieghardt, M.; Kohlwein, S.D.; Schweyen, R.J. The yeast plasma membrane protein Alr1 controls Mg2+ homeostasis and is subject to Mg2+ dependent control of its synthesis and degradation. J. Biol. Chem. 2001, 276, 16216. [Google Scholar] [CrossRef]
- Niegowski, D.; Eshaghi, S. The CorA family: Structure and function revisited. Cell. Mol. Life Sci. 2007, 64, 2564–2574. [Google Scholar] [CrossRef] [PubMed]
- Moomaw, A.S.; Maguire, M.E. The unique nature of Mg2+ channels. Physiology 2008, 23, 275–285. [Google Scholar] [CrossRef]
- Knoop, V.; Groth-Malonek, M.; Gebert, M.; Eifler, K.; Weyand, K. Transport of magnesium and other divalent cations: Evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol. Genet. Genom. 2005, 274, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Lunin, V.V.; Dobrovetsky, E.; Khutoreskaya, G.; Zhang, R.G.; Joachimiak, A.; Doyle, D.A.; Bochkarev, A.; Maguire, M.E.; Edwards, A.M.; Koth, C.M. Crystal structure of the CorA Mg2+ transporter. Nature 2006, 440, 833–837. [Google Scholar] [CrossRef]
- Dalmas, O.; Sompornpisut, P.; Bezanilla, F.; Perozo, E. Molecular mechanism of Mg2+-dependent gating in CorA. Nat. Commun. 2014, 5, 3590. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Du, H.M.; Huang, K.F.; Chen, X.; Liu, T.Y.; Gao, S.B.; Liu, H.L.; Tang, Q.L.; Rong, T.Z.; Zhang, S.Z. Identification, and functional and expression analyses of the CorA/MRS2/MGT-type magnesium transporter family in maize. Plant Cell Physiol. 2016, 57, 1153–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, B.M. Moving magnesium in plant cells. New Phytol. 2011, 190, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Yamaji, N.; Motoyama, R.; Nagamura, Y.; Ma, J.F. Up-regulation of a magnesium transporter gene OsMGT1 is required for conferring aluminum tolerance in rice. Plant Physiol. 2012, 159, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.D.; Chen, J.; Tian, L.F.; Liu, Z.; Yang, L.; Tang, R.; Li, J.; Lu, C.Q.; Yang, Y.H.; Shi, J.S.; et al. Arabidopsis transporter MGT6 mediates magnesium uptake and is required for growth under magnesium limitation. Plant Cell 2014, 26, 2234–2248. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.W.; Mao, D.D.; Yang, L.; Qi, J.L.; Zhang, X.X.; Tang, Q.L.; Li, Y.P.; Tang, R.J.; Luan, S. Magnesium transporter MGT6 plays an essential role in maintaining magnesium homeostasis and regulating high magnesium tolerance in Arabidopsis. Front. Plant Sci. 2018, 9, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Yamaji, N.; Horie, T.; Che, J.; Li, J.; An, G.; Ma, J.F. A magnesium transporter OsMGT1 plays a critical role in salt tolerance in rice. Plant Physiol. 2017, 174, 1837–1849. [Google Scholar] [CrossRef]
- Drummond, R.S.M.; Tutone, A.; Li, Y.C.; Gardner, R.C. A putative magnesium transporter AtMRS2-11 is localized to the plant chloroplast envelope membrane system. Plant Sci. 2006, 170, 78–89. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, R.N.; Li, L.G.; Huang, J.R. The magnesium transporter MGT10 is essential for chloroplast development and photosynthesis in Arabidopsis thaliana. Mol. Plant 2017, 10, 1584–1587. [Google Scholar] [CrossRef]
- Liang, S.; Qi, Y.; Zhao, J.; Li, Y.; Wang, R.; Shao, J.; Liu, X.; An, L.; Yu, F. Mutations in the Arabidopsis AtMRS2-11/AtMGT10/VAR5 gene cause leaf reticulation. Front. Plant Sci. 2017, 8, 2007–2019. [Google Scholar] [CrossRef]
- Conn, S.J.; Conn, V.; Tyerman, S.D.; Kaiser, B.N.; Leigh, R.A.; Gilliham, M. Magnesium transporters, MGT2/MRS2-1 and MGT3/MRS2-5, are important for magnesium partitioning within Arabidopsis thaliana mesophyll vacuoles. New Phytol. 2011, 190, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.G.; Liu, Z.H.; Yuan, Y.J.; Guo, L.L.; Mao, D.D.; Tian, L.F.; Chen, L.B.; Luan, S.; Li, D.P. Magnesium transporter AtMGT9 is essential for pollen development in Arabidopsis. Cell Res. 2009, 19, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Y.; Tan, H.; Yang, X.; Tian, L.; Luan, S.; Chen, L.B.; Li, D.P. An endoplasmic reticulum magnesium transporter is essential for pollen development in Arabidopsis. Plant Sci. 2015, 231, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Wang, B.; Lou, Y.; Han, W.J.; Lu, J.Y.; Li, D.D.; Li, L.G.; Zhu, J.; Yang, Z.N. Magnesium Transporter 5 plays an important role in Mg transport for male gametophyte development in Arabidopsis. Plant J. 2015, 84, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Hmiel, S.P.; Snavely, M.D.; Miller, C.G.; Maguire, M.E. Magnesium transport in Salmonella typhimurium: Characterization of magnesium influx and cloning of a transport gene. J. Bacteriol. 1986, 168, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Vuylsteke, M.; Coppens, F.; Craciun, A.; Inzé, D.; Verbruggen, N. Early transcriptomic changes induced by magnesium deficiency in Arabidopsis thaliana reveal the alteration of circadian clock gene expression in roots and the triggering of abscisic acid-responsive genes. New Phytol. 2010, 187, 119–131. [Google Scholar] [CrossRef]
- Hermans, C.; Vuylsteke, M.; Coppens, F.; Cristescu, S.M.; Harren, F.J.M.; Inzé, D.; Verbruggen, N. Systems analysis of the responses to long-term magnesium deficiency and restoration in Arabidopsis thaliana. New Phytol. 2010, 187, 132–144. [Google Scholar] [CrossRef]
- Chen, Z.C.; Ma, J.F. Magnesium transporters and their role in Al tolerance in plants. Plant Soil 2013, 368, 51–56. [Google Scholar] [CrossRef]
- Kinraide, T.B.; Parker, D.R. Cation amelioration of aluminum toxicity in wheat. Plant Physiol. 1987, 83, 546–551. [Google Scholar] [CrossRef]
- Thomas, K.J.; Rice, C.V. Revised model of calcium and magnesium binding to the bacterial cell wall. BioMetals 2014, 27, 1361–1370. [Google Scholar] [CrossRef] [Green Version]
- Yermiyahu, U.; Nir, S.; Ben-Hayyim, G.; Kafkafi, U. Quantitative competition of calcium with sodium or magnesium for sorption sites on plasma membrane vesicles of melon (Cucumis melo L.) root cells. J. Membr. Biol. 1994, 138, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hmiel, S.P.; Snavely, M.D.; Florer, J.B.; Maguire, M.E.; Miller, C.G. Magnesium transport in Salmonella typhimurium: Genetic characterization and cloning of three magnesium transport loci. J. Bacteriol. 1987, 171, 4742–4751. [Google Scholar] [CrossRef]
- Tao, T.; Snavely, M.D.; Farr, S.G.; Maguire, M.E. Magnesium transport in Salmonella typhimurium: mgtA encodes a Ptype ATPase and is regulated by Mg2+ in a manner similar to that of the mgtB P-type ATPase. J. Bacteriol. 1995, 177, 2654–2662. [Google Scholar] [CrossRef] [PubMed]
- Soncini, F.C.; García, V.E.; Solomon, F.; Groisman, E.A. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: Identification of PhoP-regulated genes. J. Bacteriol. 1996, 178, 5092–5099. [Google Scholar] [CrossRef] [PubMed]
- Hiei, Y.; Komari, T.; Kubo, T. Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol. Biol. 1997, 35, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Ma, J.F. Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol. 2007, 143, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Yamaji, N.; Fujii-Kashino, M.; Ma, J.F. A cation-chloride cotransporter gene is required for cell elongation and osmoregulation in rice. Plant Physiol. 2016, 171, 494–507. [Google Scholar] [CrossRef] [Green Version]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Peng, Y.; Li, J.; Tian, X.; Chen, Z. OsMGT1 Confers Resistance to Magnesium Deficiency By Enhancing the Import of Mg in Rice. Int. J. Mol. Sci. 2019, 20, 207. https://doi.org/10.3390/ijms20010207
Zhang L, Peng Y, Li J, Tian X, Chen Z. OsMGT1 Confers Resistance to Magnesium Deficiency By Enhancing the Import of Mg in Rice. International Journal of Molecular Sciences. 2019; 20(1):207. https://doi.org/10.3390/ijms20010207
Chicago/Turabian StyleZhang, Ludan, Yuyang Peng, Jian Li, Xinyue Tian, and Zhichang Chen. 2019. "OsMGT1 Confers Resistance to Magnesium Deficiency By Enhancing the Import of Mg in Rice" International Journal of Molecular Sciences 20, no. 1: 207. https://doi.org/10.3390/ijms20010207
APA StyleZhang, L., Peng, Y., Li, J., Tian, X., & Chen, Z. (2019). OsMGT1 Confers Resistance to Magnesium Deficiency By Enhancing the Import of Mg in Rice. International Journal of Molecular Sciences, 20(1), 207. https://doi.org/10.3390/ijms20010207