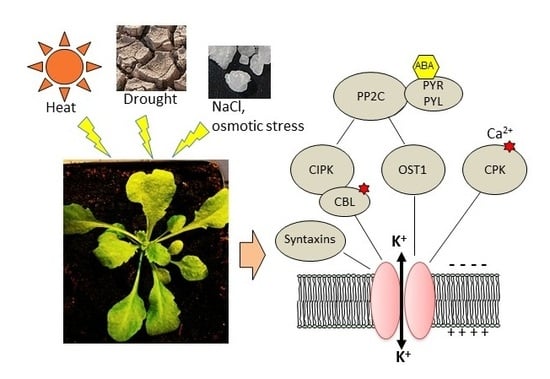
The Complex Fine-Tuning of K+ Fluxes in Plants in Relation to Osmotic and Ionic Abiotic Stresses
Abstract
:1. Introduction
2. K+ Transport in Plants
2.1. Structure and Function of K+ Transport Systems
2.1.1. K+-Selective Channels
2.1.2. K+-Selective KUP/HAK/KT Transporters (K+ Uptake Permease, High Affinity K+, K+ Transporters)
2.1.3. Cation Transporters
2.2. Role of Potassium Transport Systems in Adaptation to Stress
2.2.1. K+ Uptake and Transfer to the Xylem in Roots
2.2.2. K+ Loading and Unloading in the Phloem
2.2.3. K+ Fluxes in Guard Cells
2.2.4. K+ Fluxes in Reproductive Organs
3. Post-Translational Regulations Linked to Osmotic, Salt, or Water Stress and Heat
3.1. Regulation by Kinases, Phosphatases, and Associated Proteins
3.1.1. Regulation of K+ Uptake and Release at the Root/Soil Interface
3.1.2. AKT2 and Its Orthologs: Regulation of Phloem K+ Potential
3.1.3. Stomatal Aperture Control in Case of Abiotic Stress
3.1.4. Regulation of K+ Transport in Grape Berry by CIPK/CBL Complexes and SnRK2s
3.1.5. Vacuolar K+ Uptake and Release in Response to Salt Stress
3.2. Regulation by Vesicle-Associated Proteins
4. Cross-Talks with Anion Transport
Funding
Conflicts of Interest
References
- De Boeck, H.J.; Dreesen, F.E.; Janssens, I.A.; Nijs, I. Whole-system responses of experimental plant communities to climate extremes imposed in different seasons. New Phytol. 2011, 189, 806–817. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohalb, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Tuteja, N. Abscisic Acid and abiotic stress signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef]
- Hong, J.H.; Seah, S.W.; Xu, J. The root of ABA action in environmental stress response. Plant Cell Rep. 2013, 32, 971–983. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Lacombe, B.; Achard, P. Long-distance transport of phytohormones through the plant vascular system. Curr. Opin. Plant Biol. 2016, 34, 1–8. [Google Scholar] [CrossRef]
- Geilfus, C.M. The pH of the Apoplast: Dynamic Factor with Functional Impact Under Stress. Mol. Plant 2017, 10, 1371–1386. [Google Scholar] [CrossRef]
- Luan, M.; Tang, R.J.; Tang, Y.; Tian, W.; Hou, C.; Zhao, F.; Lan, W.; Luan, S. Transport and homeostasis of potassium and phosphate: Limiting factors for sustainable crop production. J. Exp. Bot. 2017, 68, 3091–3105. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, Y.; Jahan, N.; Chen, G.; Ren, D.; Guo, L. Sensing of Abiotic Stress and Ionic Stress Responses in Plants. Int. J. Mol. Sci. 2018, 19, 3298. [Google Scholar] [CrossRef]
- Sharma, T.; Dreyer, I.; Riedelsberger, J. The role of K(+) channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Front. Plant Sci. 2013, 4, 224. [Google Scholar] [CrossRef]
- Collins, K.D. Charge density-dependent strength of hydration and biological structure. Biophys. J. 1997, 72, 65–76. [Google Scholar] [CrossRef]
- Leigh, R.A.; Wyn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distributionj of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Trankner, M.; Tavakol, E.; Jakli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.K. Regulation of K+ channels in maize roots by water stress and abscisic acid. Plant Physiol. 1998, 116, 145–153. [Google Scholar] [CrossRef]
- Anschutz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Maser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.; Sanders, D.; et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef] [PubMed]
- Very, A.A.; Nieves-Cordones, M.; Daly, M.; Khan, I.; Fizames, C.; Sentenac, H. Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species? J. Plant Physiol. 2014, 171, 748–769. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Antunes, S.; Hoshi, T.; Muller-Rober, B.; Palme, K.; Pongs, O.; Reintanz, B.; Hedrich, R. Plant K+ channel alpha-subunits assemble indiscriminately. Biophys. J. 1997, 72, 2143–2150. [Google Scholar] [CrossRef]
- Xicluna, J.; Lacombe, B.; Dreyer, I.; Alcon, C.; Jeanguenin, L.; Sentenac, H.; Thibaud, J.B.; Cherel, I. Increased functional diversity of plant K+ channels by preferential heteromerization of the shaker-like subunits AKT2 and KAT2. J. Biol. Chem. 2007, 282, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Lebaudy, A.; Pascaud, F.; Very, A.A.; Alcon, C.; Dreyer, I.; Thibaud, J.B.; Lacombe, B. Preferential KAT1-KAT2 heteromerization determines inward K+ current properties in Arabidopsis guard cells. J. Biol. Chem. 2010, 285, 6265–6274. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Michard, E.; Lacombe, B.; Thibaud, J.B. A plant Shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or “leak” current. FEBS Lett. 2001, 505, 233–239. [Google Scholar] [CrossRef]
- Duby, G.; Hosy, E.; Fizames, C.; Alcon, C.; Costa, A.; Sentenac, H.; Thibaud, J.B. AtKC1, a conditionally targeted Shaker-type subunit, regulates the activity of plant K+ channels. Plant J. 2008, 53, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Geiger, D.; Becker, D.; Vosloh, D.; Gambale, F.; Palme, K.; Rehers, M.; Anschuetz, U.; Dreyer, I.; Kudla, J.; Hedrich, R. Heteromeric AtKC1/AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J. Biol. Chem. 2009, 284, 21288–21295. [Google Scholar] [CrossRef] [PubMed]
- Jeanguenin, L.; Alcon, C.; Duby, G.; Boeglin, M.; Cherel, I.; Gaillard, I.; Zimmermann, S.; Sentenac, H.; Very, A.A. AtKC1 is a general modulator of Arabidopsis inward Shaker channel activity. Plant J. 2011, 67, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Reintanz, B.; Szyroki, A.; Ivashikina, N.; Ache, P.; Godde, M.; Becker, D.; Palme, K.; Hedrich, R. AtKC1, a silent Arabidopsis potassium channel alpha -subunit modulates root hair K+ influx. Proc. Natl. Acad. Sci. USA 2002, 99, 4079–4084. [Google Scholar] [CrossRef] [PubMed]
- Lebaudy, A.; Very, A.A.; Sentenac, H. K+ channel activity in plants: Genes, regulations and functions. FEBS Lett. 2007, 581, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Voelker, C.; Gomez-Porras, J.L.; Becker, D.; Hamamoto, S.; Uozumi, N.; Gambale, F.; Mueller-Roeber, B.; Czempinski, K.; Dreyer, I. Roles of tandem-pore K+ channels in plants - a puzzle still to be solved. Plant Biol. 2010, 12 (Suppl. 1), 56–63. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Geiger, D.; Dunkel, M.; Roller, A.; Bertl, A.; Latz, A.; Carpaneto, A.; Dietrich, P.; Roelfsema, M.R.; Voelker, C.; et al. AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proc. Natl. Acad. Sci. USA 2004, 101, 15621–15626. [Google Scholar] [CrossRef] [PubMed]
- Carraretto, L.; Formentin, E.; Teardo, E.; Checchetto, V.; Tomizioli, M.; Morosinotto, T.; Giacometti, G.M.; Finazzi, G.; Szabo, I. A thylakoid-located two-pore K+ channel controls photosynthetic light utilization in plants. Science 2013, 342, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Bihler, H.; Eing, C.; Hebeisen, S.; Roller, A.; Czempinski, K.; Bertl, A. TPK1 is a vacuolar ion channel different from the slow-vacuolar cation channel. Plant Physiol. 2005, 139, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Isayenkov, S.; Voelker, C.; Czempinski, K.; Maathuis, F.J. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Natl. Acad. Sci. USA 2007, 104, 10726–10731. [Google Scholar] [CrossRef] [PubMed]
- Sierla, M.; Waszczak, C.; Vahisalu, T.; Kangasjarvi, J. Reactive Oxygen Species in the Regulation of Stomatal Movements. Plant Physiol. 2016, 171, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Caballero, F.; Martinez, V.; Rubio, F. Disruption of the Arabidopsis thaliana inward-rectifier K+ channel AKT1 improves plant responses to water stress. Plant Cell Physiol. 2012, 53, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Pilot, G.; Gaymard, F.; Mouline, K.; Cherel, I.; Sentenac, H. Regulated expression of Arabidopsis shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 2003, 51, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Long, Y.; Qi, G.N.; Li, J.; Xu, Z.J.; Wu, W.H.; Wang, Y. The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex. Plant Cell 2014, 26, 3387–3402. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Mian, A.; Maathuis, F.J. Overexpression of the rice AKT1 potassium channel affects potassium nutrition and rice drought tolerance. J. Exp. Bot. 2016, 67, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, I.; Stolzle, S.; Ivashikina, N.; Hedrich, R. Rice K+ uptake channel OsAKT1 is sensitive to salt stress. Planta 2005, 221, 212–221. [Google Scholar] [CrossRef]
- Golldack, D.; Quigley, F.; Michalowski, C.B.; Kamasani, U.R.; Bohnert, H.J. Salinity stress-tolerant and -sensitive rice (Oryza sativa L.) regulate AKT1-type potassium channel transcripts differently. Plant Mol. Biol. 2003, 51, 71–81. [Google Scholar] [CrossRef]
- Cuellar, T.; Pascaud, F.; Verdeil, J.L.; Torregrosa, L.; Adam-Blondon, A.F.; Thibaud, J.B.; Sentenac, H.; Gaillard, I. A grapevine Shaker inward K(+) channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J. 2010, 61, 58–69. [Google Scholar] [CrossRef]
- Cuellar, T.; Azeem, F.; Andrianteranagna, M.; Pascaud, F.; Verdeil, J.L.; Sentenac, H.; Zimmermann, S.; Gaillard, I. Potassium transport in developing fleshy fruits: The grapevine inward K(+) channel VvK1.2 is activated by CIPK-CBL complexes and induced in ripening berry flesh cells. Plant J. 2013, 73, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Deeken, R.; Geiger, D.; Fromm, J.; Koroleva, O.; Ache, P.; Langenfeld-Heyser, R.; Sauer, N.; May, S.T.; Hedrich, R. Loss of the AKT2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta 2002, 216, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Gomez-Porras, J.L.; Riedelsberger, J. The potassium battery: A mobile energy source for transport processes in plant vascular tissues. New Phytol. 2017, 216, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, B.; Pilot, G.; Michard, E.; Gaymard, F.; Sentenac, H.; Thibaud, J.B. A shaker-like K(+) channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 2000, 12, 837–851. [Google Scholar] [PubMed]
- Leonhardt, N.; Kwak, J.M.; Robert, N.; Waner, D.; Leonhardt, G.; Schroeder, J.I. Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 2004, 16, 596–615. [Google Scholar] [CrossRef] [PubMed]
- Kilian, J.; Whitehead, D.; Horak, J.; Wanke, D.; Weinl, S.; Batistic, O.; D’Angelo, C.; Bornberg-Bauer, E.; Kudla, J.; Harter, K. The AtGenExpress global stress expression data set: Protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007, 50, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J. The role of monovalent cation transporters in plant responses to salinity. J. Exp. Bot. 2006, 57, 1137–1147. [Google Scholar] [CrossRef]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA Is Required for Plant Acclimation to a Combination of Salt and Heat Stress. PLoS ONE 2016, 11, e0147625. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Andrianteranagna, M.; Cuéllar, T.; Chérel, I.; Gibrat, R.; Boeglin, M.; Moreau, B.; Paris, N.; Verdeil, J.L.; Zimmermann, S.; et al. Characterization of the grapevine Shaker K+ channel VvK3.1 supports its function in massive potassium fluxes necessary for berry potassium loading and pulvinus-actuated leaf movements. New Phytol. 2019, in press. [Google Scholar] [CrossRef]
- Pilot, G.; Lacombe, B.; Gaymard, F.; Cherel, I.; Boucherez, J.; Thibaud, J.B.; Sentenac, H. Guard cell inward K+ channel activity in arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J. Biol. Chem. 2001, 276, 3215–3221. [Google Scholar] [CrossRef]
- Kwak, J.M.; Murata, Y.; Baizabal-Aguirre, V.M.; Merrill, J.; Wang, M.; Kemper, A.; Hawke, S.D.; Tallman, G.; Schroeder, J.I. Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in arabidopsis. Plant Physiol. 2001, 127, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Ren, H.M.; Tan, Y.Q.; Qi, G.N.; Yao, F.Y.; Wu, G.L.; Yang, L.W.; Hussain, J.; Sun, S.J.; Wang, Y.F. S-type Anion Channels SLAC1 and SLAH3 Function as Essential Negative Regulators of Inward K+ Channels and Stomatal Opening in Arabidopsis. Plant Cell 2016, 28, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Gaymard, F.; Pilot, G.; Lacombe, B.; Bouchez, D.; Bruneau, D.; Boucherez, J.; Michaux-Ferriere, N.; Thibaud, J.B.; Sentenac, H. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 1998, 94, 647–655. [Google Scholar] [CrossRef]
- Hosy, E.; Vavasseur, A.; Mouline, K.; Dreyer, I.; Gaymard, F.; Poree, F.; Boucherez, J.; Lebaudy, A.; Bouchez, D.; Very, A.A.; et al. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 2003, 100, 5549–5554. [Google Scholar] [CrossRef]
- Becker, D.; Hoth, S.; Ache, P.; Wenkel, S.; Roelfsema, M.R.; Meyerhoff, O.; Hartung, W.; Hedrich, R. Regulation of the ABA-sensitive Arabidopsis potassium channel gene GORK in response to water stress. FEBS Lett. 2003, 554, 119–126. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Rodenas, R.; Chavanieu, A.; Rivero, R.M.; Martinez, V.; Gaillard, I.; Rubio, F. Uneven HAK/KUP/KT Protein Diversity Among Angiosperms: Species Distribution and Perspectives. Front. Plant Sci. 2016, 7, 127. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Aleman, F.; Martinez, V.; Rubio, F. The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol. Plant 2010, 3, 326–333. [Google Scholar] [CrossRef]
- Osakabe, Y.; Arinaga, N.; Umezawa, T.; Katsura, S.; Nagamachi, K.; Tanaka, H.; Ohiraki, H.; Yamada, K.; Seo, S.U.; Abo, M.; et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 2013, 25, 609–624. [Google Scholar] [CrossRef]
- Chen, G.; Liu, C.; Gao, Z.; Zhang, Y.; Jiang, H.; Zhu, L.; Ren, D.; Yu, L.; Xu, G.; Qian, Q. OsHAK1, a High-Affinity Potassium Transporter, Positively Regulates Responses to Drought Stress in Rice. Front. Plant Sci. 2017, 8, 1885. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, S.; Hu, Y.; Wu, F.; Hu, Q.; Chen, G.; Cai, J.; Wu, T.; Moran, N.; Yu, L.; et al. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 2014, 166, 945–959. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, L.; Shen, Z.; Jing, W.; Ge, H.; Zhao, J.; Zhang, W. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Environ. 2015, 38, 2766–2779. [Google Scholar] [CrossRef] [PubMed]
- Latz, A.; Mehlmer, N.; Zapf, S.; Mueller, T.D.; Wurzinger, B.; Pfister, B.; Csaszar, E.; Hedrich, R.; Teige, M.; Becker, D. Salt stress triggers phosphorylation of the Arabidopsis vacuolar K+ channel TPK1 by calcium-dependent protein kinases (CDPKs). Mol. Plant 2013, 6, 1274–1289. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Costa, A.; Leonhardt, N.; Siegel, R.S.; Schroeder, J.I. Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 2008, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Vahisalu, T.; Kollist, H.; Wang, Y.F.; Nishimura, N.; Chan, W.Y.; Valerio, G.; Lamminmaki, A.; Brosche, M.; Moldau, H.; Desikan, R.; et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 2008, 452, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Negi, J.; Matsuda, O.; Nagasawa, T.; Oba, Y.; Takahashi, H.; Kawai-Yamada, M.; Uchimiya, H.; Hashimoto, M.; Iba, K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 2008, 452, 483–486. [Google Scholar] [CrossRef]
- Pandey, S.; Wang, R.S.; Wilson, L.; Li, S.; Zhao, Z.; Gookin, T.E.; Assmann, S.M.; Albert, R. Boolean modeling of transcriptome data reveals novel modes of heterotrimeric G-protein action. Mol. Syst. Biol. 2010, 6, 372. [Google Scholar] [CrossRef] [PubMed]
- Cubero-Font, P.; Maierhofer, T.; Jaslan, J.; Rosales, M.A.; Espartero, J.; Diaz-Rueda, P.; Muller, H.M.; Hurter, A.L.; Al-Rasheid, K.A.; Marten, I.; et al. Silent S-Type Anion Channel Subunit SLAH1 Gates SLAH3 Open for Chloride Root-to-Shoot Translocation. Curr. Biol. 2016, 26, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Geiger, D.; Maierhofer, T.; Al-Rasheid, K.A.; Scherzer, S.; Mumm, P.; Liese, A.; Ache, P.; Wellmann, C.; Marten, I.; Grill, E.; et al. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci. Signal. 2011, 4, ra32. [Google Scholar] [CrossRef]
- Gutermuth, T.; Lassig, R.; Portes, M.T.; Maierhofer, T.; Romeis, T.; Borst, J.W.; Hedrich, R.; Feijo, J.A.; Konrad, K.R. Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20. Plant Cell 2013, 25, 4525–4543. [Google Scholar] [CrossRef] [PubMed]
- Tsay, Y.F.; Schroeder, J.I.; Feldmann, K.A.; Crawford, N.M. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 1993, 72, 705–713. [Google Scholar] [CrossRef]
- Guo, F.Q.; Young, J.; Crawford, N.M. The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant Cell 2003, 15, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and Transport of Sodium and Potassium in Plants. Met. Ions Life Sci. 2016, 16, 291–324. [Google Scholar] [PubMed]
- Santa-Maria, G.E.; Oliferuk, S.; Moriconi, J.I. KT-HAK-KUP transporters in major terrestrial photosynthetic organisms: A twenty years tale. J. Plant Physiol. 2018, 226, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, G.; Alli, A.; Yu, L. Plant HAK/KUP/KT K(+) transporters: Function and regulation. Semin. Cell Dev. Biol. 2018, 74, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.H.; Luan, S. AtKuP1: A dual-affinity K+ transporter from Arabidopsis. Plant Cell 1998, 10, 63–73. [Google Scholar] [PubMed]
- Sze, H.; Chanroj, S. Plant Endomembrane Dynamics: Studies of K(+)/H(+) Antiporters Provide Insights on the Effects of pH and Ion Homeostasis. Plant Physiol. 2018, 177, 875–895. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.; Nieves-Cordones, M.; Aleman, F.; Martinez, V. Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol. Plant. 2008, 134, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Aleman, F.; Nieves-Cordones, M.; Martinez, V.; Rubio, F. Root K(+) acquisition in plants: The Arabidopsis thaliana model. Plant Cell Physiol. 2011, 52, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123 Pt 9, 1468–1479. [Google Scholar] [CrossRef]
- Han, M.; Wu, W.; Wu, W.H.; Wang, Y. Potassium Transporter KUP7 Is Involved in K(+) Acquisition and Translocation in Arabidopsis Root under K(+)-Limited Conditions. Mol. Plant 2016, 9, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 2013, 64, 2255–2268. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Mechanisms and physiological roles of K+ efflux from root cells. J. Plant Physiol. 2014, 171, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Shabala, L.; Pottosin, I.; Zeng, F.; Velarde-Buendia, A.M.; Massart, A.; Poschenrieder, C.; Hariadi, Y.; Shabala, S. Kinetics of xylem loading, membrane potential maintenance, and sensitivity of K(+) -permeable channels to reactive oxygen species: Physiological traits that differentiate salinity tolerance between pea and barley. Plant Cell Environ. 2014, 37, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Benlloch-Gonzalez, M.; Sanchez-Lucas, R.; Benlloch, M. Effects of olive root warming on potassium transport and plant growth. J. Plant Physiol. 2017, 218, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Ivashikina, N.; Becker, D.; Ache, P.; Meyerhoff, O.; Felle, H.H.; Hedrich, R. K(+) channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett. 2001, 508, 463–469. [Google Scholar] [CrossRef]
- Hetherington, A.M. Plant physiology: Spreading a drought warning. Curr. Biol. 1998, 8, R911–R913. [Google Scholar] [CrossRef]
- Chen, G.; Hu, Q.; Luo, L.; Yang, T.; Zhang, S.; Hu, Y.; Yu, L.; Xu, G. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 2015, 38, 2747–2765. [Google Scholar] [CrossRef]
- Marten, I.; Hoth, S.; Deeken, R.; Ache, P.; Ketchum, K.A.; Hoshi, T.; Hedrich, R. AKT3, a phloem-localized K+ channel, is blocked by protons. Proc. Natl. Acad. Sci. USA 1999, 96, 7581–7586. [Google Scholar] [CrossRef]
- Ivashikina, N.; Deeken, R.; Ache, P.; Kranz, E.; Pommerrenig, B.; Sauer, N.; Hedrich, R. Isolation of AtSUC2 promoter-GFP-marked companion cells for patch-clamp studies and expression profiling. Plant J. 2003, 36, 931–945. [Google Scholar] [CrossRef]
- Deeken, R.; Sanders, C.; Ache, P.; Hedrich, R. Developmental and light-dependent regulation of a phloem-localised K+ channel of Arabidopsis thaliana. Plant J. 2000, 23, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Ache, P.; Becker, D.; Deeken, R.; Dreyer, I.; Weber, H.; Fromm, J.; Hedrich, R. VFK1, a Vicia faba K(+) channel involved in phloem unloading. Plant J. 2001, 27, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Philippar, K.; Buchsenschutz, K.; Abshagen, M.; Fuchs, I.; Geiger, D.; Lacombe, B.; Hedrich, R. The K+ channel KZM1 mediates potassium uptake into the phloem and guard cells of the C4 grass Zea mays. J. Biol. Chem. 2003, 278, 16973–16981. [Google Scholar] [CrossRef] [PubMed]
- Hafke, J.B.; Furch, A.C.; Reitz, M.U.; van Bel, A.J. Functional sieve element protoplasts. Plant Physiol. 2007, 145, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, K.E.; Nishimura, N.; Hitomi, K.; Getzoff, E.D.; Schroeder, J.I. Early abscisic acid signal transduction mechanisms: Newly discovered components and newly emerging questions. Genes Dev. 2010, 24, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Saha, A.; Valon, C.; Leung, J. A brand new START: Abscisic acid perception and transduction in the guard cell. Sci. Signal. 2011, 4, re4. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [PubMed]
- Assmann, S.M.; Jegla, T. Guard cell sensory systems: Recent insights on stomatal responses to light, abscisic acid, and CO2. Curr. Opin. Plant Biol. 2016, 33, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Blatt, M.R. The Membrane Transport System of the Guard Cell and Its Integration for Stomatal Dynamics. Plant Physiol. 2017, 174, 487–519. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Costa, J.M.; Zarrouk, O.; Pinheiro, C.; Lopes, C.M.; Pereira, J.S. Controlling stomatal aperture in semi-arid regions-The dilemma of saving water or being cool? Plant Sci. 2016, 251, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Gayatri, G.; Agurla, S.; Raghavendra, A.S. Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Front. Plant Sci. 2013, 4, 425. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Mori, I.C.; Munemasa, S. Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 2015, 66, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Farnese, F.S.; Menezes-Silva, P.E.; Gusman, G.S.; Oliveira, J.A. When Bad Guys Become Good Ones: The Key Role of Reactive Oxygen Species and Nitric Oxide in the Plant Responses to Abiotic Stress. Front. Plant Sci. 2016, 7, 471. [Google Scholar] [CrossRef] [PubMed]
- Kollist, H.; Jossier, M.; Laanemets, K.; Thomine, S. Anion channels in plant cells. FEBS J. 2011, 278, 4277–4292. [Google Scholar] [CrossRef] [PubMed]
- Ache, P.; Becker, D.; Ivashikina, N.; Dietrich, P.; Roelfsema, M.R.; Hedrich, R. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K(+)-selective, K(+)-sensing ion channel. FEBS Lett. 2000, 486, 93–98. [Google Scholar] [CrossRef]
- Szyroki, A.; Ivashikina, N.; Dietrich, P.; Roelfsema, M.R.; Ache, P.; Reintanz, B.; Deeken, R.; Godde, M.; Felle, H.; Steinmeyer, R.; et al. KAT1 is not essential for stomatal opening. Proc. Natl. Acad. Sci. USA 2001, 98, 2917–2921. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Ebisu, Y.; Kinoshita, T.; Doi, M.; Okuma, E.; Murata, Y.; Shimazaki, K. bHLH transcription factors that facilitate K(+) uptake during stomatal opening are repressed by abscisic acid through phosphorylation. Sci. Signal. 2013, 6, ra48. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.E.; Lewis, B.D.; Spalding, E.P.; Sussman, M.R. A role for the AKT1 potassium channel in plant nutrition. Science 1998, 280, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Isner, J.C.; Begum, A.; Nuehse, T.; Hetherington, A.M.; Maathuis, F.J.M. KIN7 Kinase Regulates the Vacuolar TPK1 K(+) Channel during Stomatal Closure. Curr. Biol. 2018, 28, 466–472.e4. [Google Scholar] [CrossRef]
- Mouline, K.; Very, A.A.; Gaymard, F.; Boucherez, J.; Pilot, G.; Devic, M.; Bouchez, D.; Thibaud, J.B.; Sentenac, H. Pollen tube development and competitive ability are impaired by disruption of a Shaker K(+) channel in Arabidopsis. Genes Dev. 2002, 16, 339–350. [Google Scholar] [CrossRef]
- Mira de Orduna, R. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the Grape (Vitis vinifera L.) Berry: Transport and Function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef]
- Kodur, S. Effects of juice pH and potassium on juice and wine quality, and regulation of potassium in grapevines through rootstocks (Vitis): A short review. Vitis 2011, 50, 1–6. [Google Scholar]
- Bondada, B.R.; Matthews, M.A.; Shackel, K.A. Functional xylem in the post-veraison grape berry. J. Exp. Bot. 2005, 56, 2949–2957. [Google Scholar] [CrossRef]
- Davies, C.; Shin, R.; Liu, W.; Thomas, M.R.; Schachtman, D.P. Transporters expressed during grape berry (Vitis vinifera L.) development are associated with an increase in berry size and berry potassium accumulation. J. Exp. Bot. 2006, 57, 3209–3216. [Google Scholar] [CrossRef]
- Mustilli, A.C.; Merlot, S.; Vavasseur, A.; Fenzi, F.; Giraudat, G. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 2002, 14, 3089–3099. [Google Scholar] [CrossRef]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef]
- Kleist, T.J.; Luan, S. Constant change: Dynamic regulation of membrane transport by calcium signalling networks keeps plants in tune with their environment. Plant Cell Environ. 2016, 39, 467–481. [Google Scholar] [CrossRef]
- Halford, N.G.; Hey, S.J. Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem. J. 2009, 419, 247–259. [Google Scholar] [CrossRef]
- Coello, P.; Hey, S.J.; Halford, N.G. The sucrose non-fermenting-1-related (SnRK) family of protein kinases: Potential for manipulation to improve stress tolerance and increase yield. J. Exp. Bot. 2011, 62, 883–893. [Google Scholar] [CrossRef]
- Boudsocq, M.; Sheen, J. CDPKs in immune and stress signaling. Trends Plant. Sci. 2013, 18, 30–40. [Google Scholar] [CrossRef]
- Chaves-Sanjuan, A.; Sanchez-Barrena, M.J.; Gonzalez-Rubio, J.M.; Moreno, M.; Ragel, P.; Jimenez, M.; Pardo, J.M.; Martinez-Ripoll, M.; Quintero, F.J.; Albert, A. Structural basis of the regulatory mechanism of the plant CIPK family of protein kinases controlling ion homeostasis and abiotic stress. Proc. Natl. Acad. Sci. USA 2014, 111, E4532–E4541. [Google Scholar] [CrossRef]
- Hashimoto, K.; Eckert, C.; Anschutz, U.; Scholz, M.; Held, K.; Waadt, R.; Reyer, A.; Hippler, M.; Becker, D.; Kudla, J. Phosphorylation of calcineurin B-like (CBL) calcium sensor proteins by their CBL-interacting protein kinases (CIPKs) is required for full activity of CBL-CIPK complexes toward their target proteins. J. Biol. Chem. 2012, 287, 7956–7968. [Google Scholar] [CrossRef]
- Mao, J.; Manik, S.M.; Shi, S.; Chao, J.; Jin, Y.; Wang, Q.; Liu, H. Mechanisms and Physiological Roles of the CBL-CIPK Networking System in Arabidopsis thaliana. Genes 2016, 7, 62. [Google Scholar] [CrossRef]
- Lee, S.C.; Lan, W.Z.; Kim, B.G.; Li, L.; Cheong, Y.H.; Pandey, G.K.; Lu, G.; Buchanan, B.B.; Luan, S. A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc. Natl. Acad. Sci. USA 2007, 104, 15959–15964. [Google Scholar] [CrossRef]
- Lee, S.C.; Lan, W.; Buchanan, B.B.; Luan, S. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. USA 2009, 106, 21419–21424. [Google Scholar] [CrossRef]
- Lee, S.C.; Luan, S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012, 35, 53–60. [Google Scholar] [CrossRef]
- Umezawa, T.; Sugiyama, N.; Mizoguchi, M.; Hayashi, S.; Myouga, F.; Yamaguchi-Shinozaki, K.; Ishihama, Y.; Hirayama, T.; Shinozaki, K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 17588–17593. [Google Scholar] [CrossRef]
- Geiger, D.; Scherzer, S.; Mumm, P.; Marten, I.; Ache, P.; Matschi, S.; Liese, A.; Wellmann, C.; Al-Rasheid, K.A.; Grill, E.; et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA 2010, 107, 8023–8028. [Google Scholar] [CrossRef]
- Lan, W.Z.; Lee, S.C.; Che, Y.F.; Jiang, Y.Q.; Luan, S. Mechanistic analysis of AKT1 regulation by the CBL-CIPK-PP2CA interactions. Mol. Plant 2011, 4, 527–536. [Google Scholar] [CrossRef]
- Scherzer, S.; Maierhofer, T.; Al-Rasheid, K.A.; Geiger, D.; Hedrich, R. Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol. Plant 2012, 5, 1409–1412. [Google Scholar] [CrossRef]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Geiger, D.; Scherzer, S.; Mumm, P.; Stange, A.; Marten, I.; Bauer, H.; Ache, P.; Matschi, S.; Liese, A.; Al-Rasheid, K.A.; et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA 2009, 106, 21425–21430. [Google Scholar] [CrossRef]
- Soon, F.F.; Ng, L.M.; Zhou, X.E.; West, G.M.; Kovach, A.; Tan, M.H.; Suino-Powell, K.M.; He, Y.; Xu, Y.; Chalmers, M.J.; et al. Molecular mimicry regulates ABA signaling by SnRK2 kinases and PP2C phosphatases. Science 2012, 335, 85–88. [Google Scholar] [CrossRef]
- Acharya, B.R.; Jeon, B.W.; Zhang, W.; Assmann, S.M. Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol. 2013, 200, 1049–1063. [Google Scholar] [CrossRef]
- Sato, A.; Sato, Y.; Fukao, Y.; Fujiwara, M.; Umezawa, T.; Shinozaki, K.; Hibi, T.; Taniguchi, M.; Miyake, H.; Goto, D.B.; et al. Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 2009, 424, 439–448. [Google Scholar] [CrossRef]
- Yoshida, R.; Umezawa, T.; Mizoguchi, T.; Takahashi, S.; Takahashi, F.; Shinozaki, K. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J. Biol. Chem. 2006, 281, 5310–5318. [Google Scholar] [CrossRef]
- Liu, S.; Lv, Z.; Liu, Y.; Li, L.; Zhang, L. Network analysis of ABA-dependent and ABA-independent drought responsive genes in Arabidopsis thaliana. Genet. Mol. Biol. 2018, 41, 624–637. [Google Scholar] [CrossRef]
- Held, K.; Pascaud, F.; Eckert, C.; Gajdanowicz, P.; Hashimoto, K.; Corratge-Faillie, C.; Offenborn, J.N.; Lacombe, B.; Dreyer, I.; Thibaud, J.B.; et al. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 2011, 21, 1116–1130. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.Q.; Zhou, L.; Ren, F.; Li, D.D.; Li, X.B. Arabidopsis CBL-interacting protein kinase (CIPK6) is involved in plant response to salt/osmotic stress and ABA. Mol. Biol. Rep. 2013, 40, 4759–4767. [Google Scholar] [CrossRef]
- Roy, S.J.; Huang, W.; Wang, X.J.; Evrard, A.; Schmockel, S.M.; Zafar, Z.U.; Tester, M. A novel protein kinase involved in Na(+) exclusion revealed from positional cloning. Plant Cell Environ. 2013, 36, 553–568. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef]
- Li, L.; Kim, B.G.; Cheong, Y.H.; Pandey, G.K.; Luan, S. A Ca(2)+ signaling pathway regulates a K(+) channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 12625–12630. [Google Scholar] [CrossRef]
- Ragel, P.; Rodenas, R.; Garcia-Martin, E.; Andres, Z.; Villalta, I.; Nieves-Cordones, M.; Rivero, R.M.; Martinez, V.; Pardo, J.M.; Quintero, F.J.; et al. The CBL-Interacting Protein Kinase CIPK23 Regulates HAK5-Mediated High-Affinity K+ Uptake in Arabidopsis Roots. Plant Physiol. 2015, 169, 2863–2873. [Google Scholar]
- Maierhofer, T.; Diekmann, M.; Offenborn, J.N.; Lind, C.; Bauer, H.; Hashimoto, K.; S Al-Rasheid, K.A.; Luan, S.; Kudla, J.; Geiger, D.; et al. Site- and kinase-specific phosphorylation-mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Sci. Signal. 2014, 7, ra86. [Google Scholar] [CrossRef]
- Ho, C.H.; Lin, S.H.; Hu, H.C.; Tsay, Y.F. CHL1 functions as a nitrate sensor in plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef]
- Grefen, C.; Karnik, R.; Larson, E.; Lefoulon, C.; Wang, Y.; Waghmare, S.; Zhang, B.; Hills, A.; Blatt, M.R. A vesicle-trafficking protein commandeers Kv channel voltage sensors for voltage-dependent secretion. Nat. Plants 2015, 1, 15108. [Google Scholar] [CrossRef]
- Kudla, J.; Xu, Q.; Harter, K.; Gruissem, W.; Luan, S. Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc. Natl. Acad. Sci. USA 1999, 96, 4718–4723. [Google Scholar] [CrossRef]
- Albrecht, V.; Weinl, S.; Blazevic, D.; D’Angelo, C.; Batistic, O.; Kolukisaoglu, U.; Bock, R.; Schulz, B.; Harter, K.; Kudla, J. The calcium sensor CBL1 integrates plant responses to abiotic stresses. Plant J. 2003, 36, 457–470. [Google Scholar] [CrossRef]
- Guo, Y.; Xiong, L.; Song, C.P.; Gong, D.; Halfter, U.; Zhu, J.K. A calcium sensor and its interacting protein kinase are global regulators of abscisic acid signaling in Arabidopsis. Dev. Cell 2002, 3, 233–244. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Kim, K.N.; Pandey, G.K.; Gupta, R.; Grant, J.J.; Luan, S. CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell 2003, 15, 1833–1845. [Google Scholar] [CrossRef]
- Corratge-Faillie, C.; Ronzier, E.; Sanchez, F.; Prado, K.; Kim, J.H.; Lanciano, S.; Leonhardt, N.; Lacombe, B.; Xiong, T.C. The Arabidopsis guard cell outward potassium channel GORK is regulated by CPK33. FEBS Lett. 2017, 591, 1982–1992. [Google Scholar] [CrossRef]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef]
- Ren, X.L.; Qi, G.N.; Feng, H.Q.; Zhao, S.; Zhao, S.S.; Wang, Y.; Wu, W.H. Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K+ homeostasis in Arabidopsis. Plant J. 2013, 74, 258–266. [Google Scholar] [CrossRef]
- Mori, I.C.; Murata, Y.; Yang, Y.; Munemasa, S.; Wang, Y.F.; Andreoli, S.; Tiriac, H.; Alonso, J.M.; Harper, J.F.; Ecker, J.R.; et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca(2+)-permeable channels and stomatal closure. PLoS Biol. 2006, 4, e327. [Google Scholar] [CrossRef]
- Brandt, B.; Brodsky, D.E.; Xue, S.; Negi, J.; Iba, K.; Kangasjarvi, J.; Ghassemian, M.; Stephan, A.B.; Hu, H.; Schroeder, J.I. Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. USA 2012, 109, 10593–10598. [Google Scholar] [CrossRef]
- Brandt, B.; Munemasa, S.; Wang, C.; Nguyen, D.; Yong, T.; Yang, P.G.; Poretsky, E.; Belknap, T.F.; Waadt, R.; Aleman, F.; et al. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife 2015, 4. [Google Scholar] [CrossRef]
- Xu, J.; Tian, Y.S.; Peng, R.H.; Xiong, A.S.; Zhu, B.; Jin, X.F.; Gao, F.; Fu, X.Y.; Hou, X.L.; Yao, Q.H. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta 2010, 231, 1251–1260. [Google Scholar] [CrossRef]
- Ronzier, E.; Corratge-Faillie, C.; Sanchez, F.; Prado, K.; Briere, C.; Leonhardt, N.; Thibaud, J.B.; Xiong, T.C. CPK13, a noncanonical Ca2+-dependent protein kinase, specifically inhibits KAT2 and KAT1 shaker K+ channels and reduces stomatal opening. Plant Physiol. 2014, 166, 314–326. [Google Scholar] [CrossRef]
- Van Kleeff, P.J.M.; Gao, J.; Mol, S.; Zwart, N.; Zhang, H.; Li, K.W.; de Boer, A.H. The Arabidopsis GORK K(+)-channel is phosphorylated by calcium-dependent protein kinase 21 (CPK21), which in turn is activated by 14-3-3 proteins. Plant Physiol. Biochem. 2018, 125, 219–231. [Google Scholar] [CrossRef]
- Franz, S.; Ehlert, B.; Liese, A.; Kurth, J.; Cazale, A.C.; Romeis, T. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol. Plant 2011, 4, 83–96. [Google Scholar] [CrossRef]
- Li, C.L.; Wang, M.; Wu, X.M.; Chen, D.H.; Lv, H.J.; Shen, J.L.; Qiao, Z.; Zhang, W. THI1, a Thiamine Thiazole Synthase, Interacts with Ca2+-Dependent Protein Kinase CPK33 and Modulates the S-Type Anion Channels and Stomatal Closure in Arabidopsis. Plant Physiol. 2016, 170, 1090–1104. [Google Scholar] [CrossRef]
- Lefoulon, C.; Boeglin, M.; Moreau, B.; Very, A.A.; Szponarski, W.; Dauzat, M.; Michard, E.; Gaillard, I.; Cherel, I. The Arabidopsis AtPP2CA Protein Phosphatase Inhibits the GORK K+ Efflux Channel and Exerts a Dominant Suppressive Effect on Phosphomimetic-activating Mutations. J. Biol. Chem. 2016, 291, 6521–6533. [Google Scholar] [CrossRef]
- Vranova, E.; Tahtiharju, S.; Sriprang, R.; Willekens, H.; Heino, P.; Palva, E.T.; Inze, D.; Van Camp, W. The AKT3 potassium channel protein interacts with the AtPP2CA protein phosphatase 2C. J. Exp. Bot. 2001, 52, 181–182. [Google Scholar] [CrossRef]
- Cherel, I.; Michard, E.; Platet, N.; Mouline, K.; Alcon, C.; Sentenac, H.; Thibaud, J.B. Physical and functional interaction of the Arabidopsis K(+) channel AKT2 and phosphatase AtPP2CA. Plant Cell 2002, 14, 1133–1146. [Google Scholar] [CrossRef]
- Kuhn, J.M.; Boisson-Dernier, A.; Dizon, M.B.; Maktabi, M.H.; Schroeder, J.I. The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiol. 2006, 140, 127–139. [Google Scholar] [CrossRef]
- Yoshida, T.; Nishimura, N.; Kitahata, N.; Kuromori, T.; Ito, T.; Asami, T.; Shinozaki, K.; Hirayama, T. ABA-hypersensitive germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol. 2006, 140, 115–126. [Google Scholar] [CrossRef]
- Leyman, B.; Geelen, D.; Quintero, F.J.; Blatt, M.R. A tobacco syntaxin with a role in hormonal control of guard cell ion channels. Science 1999, 283, 537–540. [Google Scholar] [CrossRef]
- Leyman, B.; Geelen, D.; Blatt, M.R. Localization and control of expression of Nt-Syr1, a tobacco SNARE protein. Plant J. 2000, 24, 369–381. [Google Scholar] [CrossRef]
- Honsbein, A.; Sokolovski, S.; Grefen, C.; Campanoni, P.; Pratelli, R.; Paneque, M.; Chen, Z.; Johansson, I.; Blatt, M.R. A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell 2009, 21, 2859–2877. [Google Scholar] [CrossRef]
- Eisenach, C.; Chen, Z.H.; Grefen, C.; Blatt, M.R. The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K(+) channel activity with vegetative growth. Plant J. 2012, 69, 241–251. [Google Scholar] [CrossRef]
- Zhang, B.; Karnik, R.; Wang, Y.; Wallmeroth, N.; Blatt, M.R.; Grefen, C. The Arabidopsis R-SNARE VAMP721 Interacts with KAT1 and KC1 K+ Channels to Moderate K+ Current at the Plasma Membrane. Plant Cell 2015, 27, 1697–1717. [Google Scholar] [CrossRef]
- Latz, A.; Becker, D.; Hekman, M.; Muller, T.; Beyhl, D.; Marten, I.; Eing, C.; Fischer, A.; Dunkel, M.; Bertl, A.; et al. TPK1, a Ca(2+)-regulated Arabidopsis vacuole two-pore K(+) channel is activated by 14-3-3 proteins. Plant J. 2007, 52, 449–459. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Pandey, G.K.; Grant, J.J.; Batistic, O.; Li, L.; Kim, B.G.; Lee, S.C.; Kudla, J.; Luan, S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 2007, 52, 223–239. [Google Scholar] [CrossRef]
- Pandey, G.K.; Cheong, Y.H.; Kim, K.N.; Grant, J.J.; Li, L.; Hung, W.; D’Angelo, C.; Weinl, S.; Kudla, J.; Luan, S. The calcium sensor calcineurin B-like 9 modulates abscisic acid sensitivity and biosynthesis in Arabidopsis. Plant Cell 2004, 16, 1912–1924. [Google Scholar] [CrossRef]
- Yang, W.; Kong, Z.; Omo-Ikerodah, E.; Xu, W.; Li, Q.; Xue, Y. Calcineurin B-like interacting protein kinase OsCIPK23 functions in pollination and drought stress responses in rice (Oryza sativa L.). J. Genet. Genom. 2008, 35, 531–543. [Google Scholar] [CrossRef]
- Cui, X.Y.; Du, Y.T.; Fu, J.D.; Yu, T.F.; Wang, C.T.; Chen, M.; Chen, J.; Ma, Y.Z.; Xu, Z.S. Wheat CBL-interacting protein kinase 23 positively regulates drought stress and ABA responses. BMC Plant Biol. 2018, 18, 93. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, F.; Liu, J.; Chen, X.; Hewezi, T.; Cheng, Z.M. Evolution of an intron-poor cluster of the CIPK gene family and expression in response to drought stress in soybean. Sci. Rep. 2016, 6, 28225. [Google Scholar] [CrossRef]
- Chen, L.; Ren, F.; Zhou, L.; Wang, Q.Q.; Zhong, H.; Li, X.B. The Brassica napus calcineurin B-Like 1/CBL-interacting protein kinase 6 (CBL1/CIPK6) component is involved in the plant response to abiotic stress and ABA signalling. J. Exp. Bot. 2012, 63, 6211–6222. [Google Scholar] [CrossRef]
- Ishitani, M.; Liu, J.; Halfter, U.; Kim, C.S.; Shi, W.; Zhu, J.K. SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 2000, 12, 1667–1678. [Google Scholar] [CrossRef]
- Leran, S.; Edel, K.H.; Pervent, M.; Hashimoto, K.; Corratge-Faillie, C.; Offenborn, J.N.; Tillard, P.; Gojon, A.; Kudla, J.; Lacombe, B. Nitrate sensing and uptake in Arabidopsis are enhanced by ABI2, a phosphatase inactivated by the stress hormone abscisic acid. Sci. Signal. 2015, 8, ra43. [Google Scholar] [CrossRef]
- Grefen, C.; Blatt, M.R. Do calcineurin B-like proteins interact independently of the serine threonine kinase CIPK23 with the K+ channel AKT1? Lessons learned from a menage a trois. Plant Physiol. 2012, 159, 915–919. [Google Scholar] [CrossRef]
- Antoni, R.; Gonzalez-Guzman, M.; Rodriguez, L.; Rodrigues, A.; Pizzio, G.A.; Rodriguez, P.L. Selective inhibition of clade A phosphatases type 2C by PYR/PYL/RCAR abscisic acid receptors. Plant Physiol. 2012, 158, 970–980. [Google Scholar] [CrossRef]
- Michard, E.; Dreyer, I.; Lacombe, B.; Sentenac, H.; Thibaud, J.B. Inward rectification of the AKT2 channel abolished by voltage-dependent phosphorylation. Plant J. 2005, 44, 783–797. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.K. An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc. Natl. Acad. Sci. USA 1997, 94, 14960–14964. [Google Scholar] [CrossRef]
- Sklodowski, K.; Riedelsberger, J.; Raddatz, N.; Riadi, G.; Caballero, J.; Cherel, I.; Schulze, W.; Graf, A.; Dreyer, I. The receptor-like pseudokinase MRH1 interacts with the voltage-gated potassium channel AKT2. Sci. Rep. 2017, 7, 44611. [Google Scholar] [CrossRef]
- Yin, Y.; Adachi, Y.; Ye, W.; Hayashi, M.; Nakamura, Y.; Kinoshita, T.; Mori, I.C.; Murata, Y. Difference in abscisic acid perception mechanisms between closure induction and opening inhibition of stomata. Plant Physiol. 2013, 163, 600–610. [Google Scholar] [CrossRef]
- Li, J.; Lee, Y.R.; Assmann, S.M. Guard cells possess a calcium-dependent protein kinase that phosphorylates the KAT1 potassium channel. Plant Physiol. 1998, 116, 785–795. [Google Scholar] [CrossRef]
- Mori, I.C.; Uozumi, N.; Muto, S. Phosphorylation of the inward-rectifying potassium channel KAT1 by ABR kinase in Vicia guard cells. Plant Cell Physiol. 2000, 41, 850–856. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.Q.; Watson, M.B.; Assmann, S.M. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 2000, 287, 300–303. [Google Scholar] [CrossRef]
- Sottocornola, B.; Visconti, S.; Orsi, S.; Gazzarrini, S.; Giacometti, S.; Olivari, C.; Camoni, L.; Aducci, P.; Marra, M.; Abenavoli, A.; et al. The potassium channel KAT1 is activated by plant and animal 14-3-3 proteins. J. Biol. Chem. 2006, 281, 35735–35741. [Google Scholar] [CrossRef]
- Kim, W.; Lee, Y.; Park, J.; Lee, N.; Choi, G. HONSU, a protein phosphatase 2C, regulates seed dormancy by inhibiting ABA signaling in Arabidopsis. Plant Cell Physiol. 2013, 54, 555–572. [Google Scholar] [CrossRef]
- Lim, C.W.; Kim, J.H.; Baek, W.; Kim, B.S.; Lee, S.C. Functional roles of the protein phosphatase 2C, AtAIP1, in abscisic acid signaling and sugar tolerance in Arabidopsis. Plant Sci. 2012, 187, 83–88. [Google Scholar] [CrossRef]
- Eisenach, C.; Papanatsiou, M.; Hillert, E.K.; Blatt, M.R. Clustering of the K+ channel GORK of Arabidopsis parallels its gating by extracellular K+. Plant J. 2014, 78, 203–214. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, J.; Dong, C.; Cheng, Z.M. The CBL and CIPK Gene Family in Grapevine (Vitis vinifera): Genome-Wide Analysis and Expression Profiles in Response to Various Abiotic Stresses. Front. Plant Sci. 2017, 8, 978. [Google Scholar] [CrossRef]
- Boneh, U.; Biton, I.; Schwartz, A.; Ben-Ari, G. Characterization of the ABA signal transduction pathway in Vitis vinifera. Plant Sci. 2012, 187, 89–96. [Google Scholar] [CrossRef]
- Wheeler, S.; Loveys, B.; Ford, C.; Davies, C. The relationship betwwen the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust. J. Grape Wine Res. 2009, 15, 195–204. [Google Scholar] [CrossRef]
- Carbonell-Bejerano, P.; Santa Maria, E.; Torres-Perez, R.; Royo, C.; Lijavetzky, D.; Bravo, G.; Aguirreolea, J.; Sanchez-Diaz, M.; Antolin, M.C.; Martinez-Zapater, J.M. Thermotolerance responses in ripening berries of Vitis vinifera L. cv Muscat Hamburg. Plant Cell Physiol. 2013, 54, 1200–1216. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Luchaire, N.; Chatbanyong, R.; Lecourieux, D.; Kelly, M.T.; Romieu, C. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (vitis vinifera) fruit. BMC Plant Biol. 2014, 14, 108. [Google Scholar] [CrossRef]
- Pottosin, I.; Shabala, S. Polyamines control of cation transport across plant membranes: Implications for ion homeostasis and abiotic stress signaling. Front. Plant Sci. 2014, 5, 154. [Google Scholar] [CrossRef]
- Wang, F.; Deng, S.; Ding, M.; Sun, J.; Wang, M.; Zhu, H.; Han, Y.; Shen, Z.; Jing, X.; Zhang, F.; et al. Overexpression of a poplar two-pore K+ channel enhances salinity tolerance in tobacco cells. Plant Cell Tissue Organ Cult. 2013, 112, 19–31. [Google Scholar] [CrossRef]
- Sutter, J.U.; Campanoni, P.; Tyrrell, M.; Blatt, M.R. Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. Plant Cell 2006, 18, 935–954. [Google Scholar] [CrossRef]
- Sutter, J.U.; Sieben, C.; Hartel, A.; Eisenach, C.; Thiel, G.; Blatt, M.R. Abscisic acid triggers the endocytosis of the arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 2007, 17, 1396–1402. [Google Scholar] [CrossRef]
- Hedrich, R.; Geiger, D. Biology of SLAC1-type anion channels - from nutrient uptake to stomatal closure. New Phytol. 2017, 216, 46–61. [Google Scholar] [CrossRef]
- Singh, A.; Pandey, A.; Srivastava, A.K.; Tran, L.S.; Pandey, G.K. Plant protein phosphatases 2C: From genomic diversity to functional multiplicity and importance in stress management. Crit. Rev. Biotechnol. 2016, 36, 1023–1035. [Google Scholar] [CrossRef]





| Gene Name 1 | ID | Protein Family | Function | Type of Stress | Transcriptional Regulation (Drought, ABA, Heat Stress, NaCl) | Protein Level/Activity |
|---|---|---|---|---|---|---|
| AKT1 | At2g26650 | Shaker | K+ uptake by roots [42], inhibition of stomatal closure in response to drought [43] | ABA | No significant change in roots, downregulation in shoots [44] and seedlings [45] | |
| NaCl | No significant change in roots and shoots [44] | Inhibition of currents by intracellular Na+ [43] | ||||
| OsAKT1 | LOC4326245 | Shaker | K+ uptake by roots [46,47] | NaCl | Downregulation [48], downregulation in the exodermis in salt-tolerant lines [49] | |
| VvK1.1 | VIT_11s0016 g04750 | Shaker | K+ uptake in roots and phloem [50] | Drought | Induction in leaves and berries, downregulation in roots [50] | |
| ABA | Induction in leaves, not in roots [50] | |||||
| VvK1.2 | VIT_04s0008 g04990 | Shaker | K+ uptake by flesh cells [51] | Drought | Strong induction in berries from véraison [51] | |
| AKT2 | At4g22200 | Shaker | Regulation of phloem membrane potential [52,53] | ABA | Induction in leaves [44,54], not in guard cells [55] | |
| Mannitol, NaCl, drought | Moderate induction in leaves [56] | |||||
| NaCl | Induction [57] | |||||
| Heat | Induction [58] | |||||
| VvK3.1 | VIT_12s0034 g02240 | Shaker | Phloem transport, leaf movements [59] | Drought | Induction in leaves and (moderately) in berries, not in roots [59] | |
| KAT1 | At5g46240 | Shaker | Stomatal opening [60,61] | ABA | Down-regulation in guard cells [55] | |
| Drought | No change in guard cells [62] | |||||
| NaCl | No significant change [44] | |||||
| KAT2 | At4g18290 | Shaker | Stomatal opening [60,61] | ABA | Down-regulation in guard cells [55] | |
| AtKC1 | At4g32650 | Shaker | K+ uptake in root hairs together with AKT1 [35] | ABA | Transient down-regulation [44] | |
| NaCl | High induction in leaves, not in roots [44] | |||||
| SKOR | At3g02850 | Shaker | K+ loading of the xylem in roots [63] | ABA | Downregulation [63] | |
| NaCl | No significant change [44], strong induction [57] | |||||
| GORK | At5g37500 | Shaker | Stomatal closure [64] | ABA | Induction in seedlings, cultured cells, root hair protoplasts, not in guard cells [65] | |
| Heat | Induction in roots [56] | |||||
| Water stress | Induction in leaves [65] | |||||
| NaCl | Induction in roots [56] | |||||
| HAK5 | At4g13420 | KUP | High-affinity K+ uptake from the soil [66] | NaCl | Strong downregulation [67] | |
| NaCl, drought, mannitol | Unclear [56] | |||||
| KUP6 | At1g70300 | KUP | K+ efflux from roots and stomata, ABA- and auxin-dependent inhibition of lateral root formation [68] | NaCl | Induction [57] | |
| Heat | Induction (Genevestigator, https://genevestigator.com, [58]) | |||||
| Mannitol | Strong induction [56] | |||||
| KUP8 | At5g14880 | KUP | K+ efflux from roots and stomata, ABA- and auxin-dependent inhibition of lateral root formation [68] | ABA, NaCl, mannitol | Slight downregulation [56], especially in roots (NaCl, mannitol) | |
| OsHAK1 | LOC4335729 | KUP | K+ acquisition [69] | Water stress | Transient induction in roots and shoots [69] | |
| OsHAK5 | LOC4326945 | KUP | K+ acquisition, root to shoot transport [70] | NaCl | Induction in roots and shoots [70] | |
| OsHAK21 | LOC9269115 | KUP | K+ uptake and distribution [71] | NaCl | Induction in roots and shoots [71] | |
| TPK1 | At1g02880 | Two-pore channels | K+ homeostasis, guard cell vacuolar K+ release [41] | NaCl | No significant change [72] | Induction of protein phosphorylation [72] |
| ABA | No change [45,73] | |||||
| SLAC1 | At1g12480 | C4 dicarboxylate transporters | Stomatal closure [74,75] | Drought | Short treatment: no change in roots and shoots [66], strong induction in guard cell protoplasts after a few days [62] | |
| ABA | No change [45,73,76] | |||||
| NaCl | Moderate induction in shoots [56] | |||||
| Mannitol | No significant change in roots and shoots [56] | |||||
| SLAH3 | At5g24030 | C4 dicarboxylate transporters | Cl− root to shoot translocation [77], NO3−-mediated stomatal closure [78], pollen tube growth [79] | Drought | Short treatment: downregulation in roots [56]; strong induction in guard cell protoplasts after a few days [62] | |
| ABA | No change [45] | |||||
| NaCl | Downregulation in roots [56,77] | |||||
| Mannitol | Downregulation in roots [56] | |||||
| PEG | Strong downregulation in roots [77] | |||||
| CHL1 /NRT1.1 /NPF6.3 | At1g12110 | NPF | Nitrate uptake [80], stomatal opening [81], nitrate sensing, auxin transport [82] | ABA | Unclear, depends on tissue/experimental conditions [45] | |
| NaCl, mannitol | Unclear, transient down-regulation in roots? [56] |
| Gene Name 1 | ID | Protein Family | Plasma Membrane Targets | Type of Stress | Transcriptional Regulation (Drought, ABA, Heat Stress, NaCl) | Protein Level/Activity |
|---|---|---|---|---|---|---|
| OST1/ SnRK2.6/ SRK2E | At4g33950 | SnRK2 kinases | KAT1 [146,147], SLAC1 [136,144], KUP6 [68] | ABA | No change in transcript level in whole plants [126], induction in guard cells [55] | Induction of kinase activity in roots and guard cell protoplasts [126] |
| Low air humidity | Induction of kinase activity in leaves [148] | |||||
| NaCl, sorbitol | Induction of kinase activity in roots [148] | |||||
| NaCl | Slow induction [56] | |||||
| Drought | Moderate induction [149] | |||||
| CIPK6 | At4g30960 | SnRK3 kinases | AKT1 [135,140], AKT2 [150] | ABA, mannitol | Induction in seedlings (including roots) [151] | |
| NaCl | Induction in shoots [56] and in seedlings (including roots) [151] | |||||
| CIPK16 | At2g25090 | SnRK3 kinases | AKT1 [135] | NaCl | Induction in root stele [152] | |
| CIPK23 | At1g30270 | SnRK3 kinases | AKT1 [135,153,154], HAK5 [155], SLAC1 [156], CHL1 [157] | ABA, drought, mannitol, NaCl | No change [45] | |
| CBL1 | At4g17615 | CBL | AKT1 [158] | Drought | Short air treatment: transient induction in roots only [56]; strong and transient induction after 1-3h [159,160]; long-term induction in soil [149] | |
| ABA | No significant change in mesophyll and guard cells [73] and in seedlings [160,161] | Protein accumulation in leaves [162] | ||||
| NaCl | Transient induction in roots [163] | Protein accumulation in leaves [162] | ||||
| CBL4/ SOS3 | At5g24270 | CBL | AKT1 [158] | NaCl, mannitol | Induction in roots [56], no significant change in seedlings [164] | |
| ABA | Down-regulation in guard cells [73] | |||||
| CBL9 | At5g47100 | CBL | AKT1 [158] | ABA, NaCl, mannitol, drought | No significant change [45] | |
| CBL10 | At4g33000 | CBL | AKT1 [165] | Mannitol | Down-regulation in shoots [56] | |
| NaCl | Transient and moderate induction [72] | |||||
| CPK3/ CDPK6 | At4g23650 | CPK/CDPK | KAT1/KAT2, GORK [163], TPK1 [72] | NaCl | No change in seedlings [72] | |
| ABA | Slight down-regulation in guard cells [73,76] | |||||
| CPK6/ CDPK3 | At2g17290 | CPK/CDPK | GORK [163], SLAC1 [139,166,167,168] | ABA, mannitol, NaCl | No change [45,73] | |
| NaCl, PEG | Transient induction [169] | |||||
| CPK13 | At3g51850 | CPK/CDPK | KAT1/KAT2 [170], GORK [163] | NaCl, drought | No significant change [56] | |
| Mannitol | Downregulation [56] | |||||
| ABA | No change in guard cells [73,76] | |||||
| CPK21 | At4g04720 | CPK/CDPK | GORK [171], SLAC1 [139], SLAH3 [78] | ABA, drought, mannitol, NaCl | No significant change, except perhaps a slight down-regulation in response to ABA in guard cells [45] | |
| Mannitol | Enhancement of kinase activity [172] | |||||
| CPK33 | At1g50700 | CPK/CDPK | GORK [163], slow anion channels? [173] | ABA, drought | Moderate up-regulation [173] | |
| ABI1 | At4g26080 | Clade A PP2C phosphatases | SLAC1 [167] | ABA | Induction in guard cells [55,73,76] and seedlings [45] | |
| NaCl, mannitol | Induction in roots and shoots [56] | |||||
| Heat | Twofold induction in leaves [58] | |||||
| ABI2 | At5g57050 | Clade A PP2Cs | GORK [174] | ABA | Induction in guard cells [55,73,76] and seedlings [45] | |
| NaCl, mannitol | Induction in roots and shoots [56] | |||||
| AtPP2CA | At3g11410 | Clade A PP2Cs | AKT2 [175,176], GORK [174], SLAC1 [136] | ABA | Strong induction in guard cells [55,73,76] and whole plants [56,176,177,178] | |
| NaCl, mannitol | Induction in roots and shoots [56] | |||||
| Heat | Fourfold induction in leaves [58] | |||||
| AIP1 | At1g07430 | Clade A PP2Cs | AKT1 [135,140] | ABA | Induction in seedlings [56] and guard cells [55] | |
| NaCl, mannitol | Strong induction in roots (NaCl) or shoots (mannitol) after 3 hours [56] | |||||
| Heat | Strong induction in leaves [58] | |||||
| NtSYR1 | LOC107768839 | Syntaxins | Nicotiana K and Cl channels [179] | NaCl | Induction of transcripts in leaves [180] | Protein accumulation in leaves [180] |
| ABA | Transient induction of transcript in leaves [179] | Protein accumulation in leaves, but not in roots [180] | ||||
| SYP121 | At3g11820 | Syntaxins | AtKC1, AKT1-AtKC1 [181], KAT1 [182] | NaCl | Induction in roots, not in leaves [56] | |
| ABA | No induction in seedlings and guard cells [45] | |||||
| VAMP721 | At1g04750 | Synaptobrevin-like | KAT1, AtKC1 [183] | ABA, NaCl, drought, mannitol | No significant change [45] | |
| VAMP722 | At2g33120 | Synaptobrevin-like | KAT1, AtKC1 [183] | ABA, NaCl, drought, mannitol | No significant change [45] | |
| GRF6 | At2g06200 | 14-3-3 | TPK1 [184] | Low expression |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chérel, I.; Gaillard, I. The Complex Fine-Tuning of K+ Fluxes in Plants in Relation to Osmotic and Ionic Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 715. https://doi.org/10.3390/ijms20030715
Chérel I, Gaillard I. The Complex Fine-Tuning of K+ Fluxes in Plants in Relation to Osmotic and Ionic Abiotic Stresses. International Journal of Molecular Sciences. 2019; 20(3):715. https://doi.org/10.3390/ijms20030715
Chicago/Turabian StyleChérel, Isabelle, and Isabelle Gaillard. 2019. "The Complex Fine-Tuning of K+ Fluxes in Plants in Relation to Osmotic and Ionic Abiotic Stresses" International Journal of Molecular Sciences 20, no. 3: 715. https://doi.org/10.3390/ijms20030715
APA StyleChérel, I., & Gaillard, I. (2019). The Complex Fine-Tuning of K+ Fluxes in Plants in Relation to Osmotic and Ionic Abiotic Stresses. International Journal of Molecular Sciences, 20(3), 715. https://doi.org/10.3390/ijms20030715