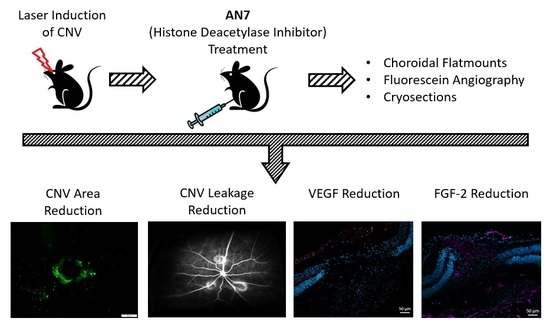
The Histone Deacetylase Inhibitor AN7, Attenuates Choroidal Neovascularization in a Mouse Model
Abstract
:1. Introduction
2. Results
2.1. AN7 Treatment Elevates Histone H3 Acetylation Levels in Laser-induced CNV Lesions
2.2. Systemic Administration of AN7 Reduces CNV Area in Choroidal Flatmounts
2.3. AN7 Reduces CD31, VEGF, and FGF-2 at the Laser Lesion Site
2.4. AN7 Reduces Vascular Leakage from CNV Lesions
2.5. AN7 Treatment Stabilizes Tight Junctions and Reduces Permeability of RPE Cells Exposed to Hypoxia
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Histology and Immunofluorescence Staining
4.3. Choroidal Flatmounts and CNV Area Quantification
4.4. Fluorescein Angiography
4.5. Cell Culture
4.6. Immunofluorescence for RPE Cell Culture
4.7. RPE Cell Culture Permeability Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Green, W.R.; Wilson, D.J. Choroidal Neovascularization. Ophthalmology 1986, 93, 1169–1176. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Green, W.R. Choroidal neovascularization. Am. J. Ophthalmol. 2004, 137, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Fine, S.L. Age-Related Macular Degeneration. N. Engl. J. Med. 2000, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Gunda, V.; Sudhakar, Y.A. Regulation of tumor angiogenesis and choroidal neovascularization by endogenous angioinhibitors. J. Cancer Sci. Ther. 2013, 5, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; McGuire, P.G. Retinal and choroidal angiogenesis: Pathophysiology and strategies for inhibition. Prog. Retin. Eye Res. 2003, 22, 721–748. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.; Li, W.W. Factors controlling ocular angiogenesis. Am. J. Ophthalmol. 1997, 124, 521–529. [Google Scholar] [CrossRef]
- Bressler, S.B. Introduction: Understanding the Role of Angiogenesis and Antiangiogenic Agents in Age-Related Macular Degeneration. Ophthalmology 2009, 116, S1–S7. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Gregory, P.D.; Wagner, K.; Hörz, W. Histone acetylation and chromatin remodeling. Exp. Cell Res. 2001, 265, 195–202. [Google Scholar] [CrossRef]
- Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 1997, 389, 349–352. [Google Scholar] [CrossRef]
- Hake, S.B.; Xiao, A.; Allis, C.D. Linking the epigenetic “language” of covalent histone modifications to cancer. Br. J. Cancer 2004, 90, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Rosato, R.R.; Grant, S. Histone deacetylase inhibitors in clinical development. Expert Opin. Investig. Drugs 2004, 13, 21–38. [Google Scholar] [CrossRef]
- Dokmanovic, M.; Clarke, C.; Marks, P.A. Histone Deacetylase Inhibitors: Overview and Perspectives. Mol. Cancer Res. 2007, 5, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Kelly, W.K.; O’Connor, O.A.; Marks, P.A.; Connor, O.A.O. Histone deacetylase inhibitors: From target to clinical trials. Expert Opin. Investig. Drugs 2002, 11, 1695–1713. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Alsarraf, O.; Dahrouj, M.; Platt, K.A.; Chou, C.J.; Rice, D.S.; Crosson, C.E. Inhibition of HDAC2 protects the retina from ischemic injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4072–4080. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cepko, C.L. Requirement of histone deacetylase activity for the expression of critical photoreceptor genes. BMC Dev. Biol. 2007, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Schwechter, B.R.; Millet, L.E.; Levin, L.A. Histone deacetylase inhibition-mediated differentiation of RGC-5 cells and interaction with survival. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2845–2857. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.W.; Chen, J.; Wang, M.; Mast, N.; Pikuleva, I.A.; Turko, I.V. Quantification of histone deacetylase isoforms in human frontal cortex, human retina, and mouse brain. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Chindasub, P.; Lindsey, J.D.; Duong-Polk, K.; Leung, C.K.; Weinreb, R.N. Inhibition of histone deacetylases 1 and 3 protects injured retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Alsarraf, O.; Fan, J.; Dahrouj, M.; Chou, C.J.; Menick, D.R.; Crosson, C.E. Acetylation: A lysine modification with neuroprotective effects in ischemic retinal degeneration. Exp. Eye Res. 2014, 127, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Biermann, J.; Grieshaber, P.; Goebel, U.; Martin, G.; Thanos, S.; Di Giovanni, S.; Lagrèze, W.A. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.S.; Wang, C.C.; Bortner, C.D.; Peng, G.S.; Wu, X.; Pang, H.; Lu, R.B.; Gean, P.W.; Chuang, D.M.; Hong, J.S. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience 2007, 149, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Crosson, C.E.; Mani, S.K.; Husain, S.; Alsarraf, O.; Menick, D.R. Inhibition of histone deacetylase protects the retina from ischemic injury. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3639–3645. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.; He, S.; Spee, C.K.; Ishikawa, K.; Hinton, D.R. Attenuation of choroidal neovascularization by histone deacetylase inhibitor. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.H.; Oh, M.; Yu, Y.S.; Kim, K.W.; Kwon, H.J. N-hydroxy-7-(2-naphthylthio) heptanomide inhibits retinal and choroidal angiogenesis. Mol. Pharm. 2009, 6, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Rephaeli, A.; Zhuk, R. Nudelman, a Prodrugs of butyric acid from bench to bedside: Synthetic design, mechanisms of action, and clinical applications. Drug Dev. Res. 2000, 50, 379–391. [Google Scholar] [CrossRef]
- Rephaeli, A.; Entin-Meer, M.; Angel, D.; Tarasenko, N.; Gruss-Fischer, T.; Bruachman, I.; Phillips, D.R.; Cutts, S.M.; Haas-Kogan, D.; Nudelman, A. The selectivty and anti-metastatic activity of oral bioavailable butyric acid prodrugs. Investig. New Drugs 2006, 24, 383–392. [Google Scholar] [CrossRef]
- Nudelman, A.; Gnizi, E.; Katz, Y.; Azulai, R.; Cohen-Ohana, M.; Zhuk, R.; Sampson, S.R.; Langzam, L.; Fibach, E.; Prus, E.; et al. Prodrugs of butyric acid. Novel derivatives possessing increased aqueous solubility and potential for treating cancer and blood diseases. Eur. J. Med. Chem. 2001, 36, 63–74. [Google Scholar] [CrossRef]
- Tarasenko, N.; Nudelman, A.; Tarasenko, I.; Entin-Meer, M.; Hass-Kogan, D.; Inbal, A.; Rephaeli, A. Histone deacetylase inhibitors: The anticancer, antimetastatic and antiangiogenic activities of AN-7 are superior to those of the clinically tested AN-9 (Pivanex). Clin. Exp. Metastasis 2008, 25, 703–716. [Google Scholar] [CrossRef]
- Nudelman, A.; Levovich, I.; Cutts, S.M.; Phillips, D.R.; Rephaeli, A. The role of intracellularly released formaldehyde and butyric acid in the anticancer activity of acyloxyalkyl esters. J. Med. Chem. 2005, 48, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Schaap-Fogler, M.; Bahar, I.; Rephaeli, A.; Dahbash, M.; Nudelman, A.; Livny, E.; Barliya, T.; Nisgav, Y.; Livnat, T. Effect of Histone Deacetylase Inhibitor, Butyroyloxymethyl-Diethyl Phosphate (AN-7), on Corneal Neovascularization in a Mouse Model. J. Ocul. Pharmacol. Ther. 2017, 33, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, Y.; Miller, J.; Kim, I. Age-Related Macular Degeneration: Advances in Management and Diagnosis. J. Clin. Med. 2015, 4, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Toma, H.S.; Barnett, J.M.; Penn, J.S. Ketorolac inhibits choroidal neovascularization by suppression of retinal VEGF. Exp. Eye Res. 2010, 91, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Hata, Y.; Yoshikawa, H.; Nakagawa, K.; Sueishi, K.; Inomata, H. Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Arjamaa, O.; Nikinmaa, M.; Salminen, A.; Kaarniranta, K. Regulatory role of HIF-1α in the pathogenesis of age-related macular degeneration (AMD). Ageing Res. Rev. 2009, 8, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Forooghian, F.; Cukras, C.; Meyerle, C.B.; Chew, E.Y.; Wong, W.T. Tachyphylaxis after intravitreal bevacizumab for exudative age-related macular degeneration. Retina 2009, 29, 723–731. [Google Scholar] [CrossRef]
- Kaiser, P.K. Antivascular Endothelial Growth Factor Agents and Their Development: Therapeutic Implications in Ocular Diseases. Am. J. Ophthalmol. 2006, 142, 660–668. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Harding, S.P.; Rogers, C.A.; Downes, S.M.; Lotery, A.J.; Culliford, L.A.; Reeves, B.C. IVAN study investigators Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013, 382, 1258–1267. [Google Scholar] [CrossRef]
- Gillies, M.C.; Campain, A.; Barthelmes, D.; Simpson, J.M.; Arnold, J.J.; Guymer, R.H.; McAllister, I.L.; Essex, R.W.; Morlet, N.; Hunyor, A.P. Long-Term Outcomes of Treatment of Neovascular Age-Related Macular Degeneration: Data from an Observational Study. Ophthalmology 2015, 122, 1837–1844. [Google Scholar] [CrossRef]
- Rephaeli, A.; Blank-Porat, D.; Tarasenko, N.; Entin-Meer, M.; Levovich, I.; Cutts, S.M.; Phillips, D.R.; Malik, Z.; Nudelman, A. In vivo and in vitro antitumor activity of butyroyloxymethyl-diethyl phosphate (AN-7), a histone deacetylase inhibitor, in human prostate cancer. Int. J. Cancer 2005, 116, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Tarasenko, N.; Chekroun-Setti, H.; Nudelman, A.; Rephaeli, A. Comparison of the anticancer properties of a novel valproic acid prodrug to leading histone deacetylase inhibitors. J. Cell. Biochem. 2018, 119, 3417–3428. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.M.; Bishton, M.J.; Harrison, S.J. Clinical studies of histone deacetylase inhibitors. Clin. Cancer Res. 2009, 15, 3958–3969. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, C.; Minucci, S.; Pelicci, P.G. Histone deacetylases and epigenetic therapies of hematological malignancies. Pharmacol. Res. 2010, 62, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Gryder, B.E.; Sodji, Q.H.; Oyelere, A.K. Targeted cancer therapy: Giving histone deacetylase inhibitors all they need to succeed. Future Med. Chem. 2012, 4, 1369–1370. [Google Scholar] [CrossRef] [PubMed]
- Mottamal, M.; Zheng, S.; Huang, T.L.; Wang, G. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules 2015, 20, 3898–3941. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Patel, D.; Zhang, X.; Veenstra, R.D. Changes in cardiac Na v 1.5 expression, function, and acetylation by pan-histone deacetylase inhibitors. Am. J. Physiol.—Hear. Circ. Physiol. 2016, 311, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, A.; O’Byrne, K.J.; Richard, D.J. Combination Therapy With Histone Deacetylase Inhibitors (HDACi) for the Treatment of Cancer: Achieving the Full Therapeutic Potential of HDACi. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, X.; Qi, Y.; He, Y.; Du, W.; Pang, J.J. Histone deacetylases inhibitors in the treatment of retinal degenerative diseases: Overview and perspectives. J. Ophthalmol. 2015. [Google Scholar] [CrossRef]
- Ratay, M.L.; Balmert, S.C.; Bassin, E.J.; Little, S.R. Controlled release of an HDAC inhibitor for reduction of inflammation in dry eye disease. Acta Biomater. 2018, 71, 261–270. [Google Scholar] [CrossRef]
- Sung, M.S.; Eom, G.H.; Kim, S.J.; Kim, S.Y.; Heo, H.; Woo, S. Trichostatin A Ameliorates Conjunctival Fibrosis in a Rat Trabeculectomy Model. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3115–3123. [Google Scholar] [CrossRef] [PubMed]
- Clemson, C.M.; Tzekov, R.; Krebs, M.; Checchi, J.M.; Bigelow, C.; Kaushal, S. Therapeutic potential of valproic acid for retinitis pigmentosa. Br. J. Ophthalmol. 2011, 95, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.A.; Breslow, R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat. Biotechnol. 2007, 25, 84–90. [Google Scholar] [CrossRef] [PubMed]
- De Ruijter, A.J.M.; van Gennip, A.H.; Caron, H.N.; Kemp, S.; van Kuilenburg, A.B.P. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, D.; Liu, Y.; Crosson, C.E.; Ablonczy, Z. Histone Deacetylase Inhibition Restores Retinal Pigment Epithelium Function in Hyperglycemia. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Blank-Porat, D.; Gruss-Fischer, T.; Tarasenko, N.; Malik, Z.; Nudelman, A.; Rephaeli, A. The anticancer prodrugs of butyric acid AN-7 and AN-9, possess antiangiogenic properties. Cancer Lett. 2007, 256, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Tarasenko, N.; Cutts, S.M.; Phillips, D.R.; Inbal, A.; Nudelman, A.; Kessler-Icekson, G.; Rephaeli, A. Disparate impact of butyroyloxymethyl diethylphosphate (AN-7), a histone deacetylase inhibitor, and doxorubicin in mice bearing a mammary tumor. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Tarasenko, N.; Kessler-Icekson, G.; Boer, P.; Inbal, A.; Schlesinger, H.; Phillips, D.R.; Cutts, S.M.; Nudelman, A.; Rephaeli, A. The histone deacetylase inhibitor butyroyloxymethyl diethylphosphate (AN-7) protects normal cells against toxicity of anticancer agents while augmenting their anticancer activity. Investig. New Drugs 2012, 30, 130–143. [Google Scholar] [CrossRef]
- Lehrmann, H.; Pritchard, L.L.; Harel-Bellan, A. Histone acetyltransferases and deacetylases in the control of cell proliferation and differentiation. Adv. Cancer Res. 2002, 86, 41–65. [Google Scholar]
- Mai, A.; Massa, S.; Rotili, D.; Cerbara, I.; Valente, S.; Pezzi, R.; Simeoni, S.; Ragna, R. Histone deacetylation in epigenetics: An attractive target for anticancer therapy. Med. Res. Rev. 2005, 25, 261–309. [Google Scholar] [CrossRef]
- Rusnati, M.; Presta, M. Fibroblast growth factors/fibroblast growth factor receptors as targets for the development of anti-angiogenesis strategies. Curr. Pharm. Des. 2007, 13, 2025–2044. [Google Scholar] [CrossRef] [PubMed]
- Zubilewicz, A.; Hecquet, C.; Jeanny, J.C.; Soubrane, G.; Courtois, Y.; Mascarelli, F. Two distinct signalling pathways are involved in FGF2-stimulated proliferation of choriocapillary endothelial cells: A comparative study with VEGF. Oncogene 2001, 20, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.A.; McLeod, D.S.; Hasegawa, T.; Kim, S.Y.; Merges, C.; Tong, P.; Lutty, G.A. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp. Eye Res. 2006, 82, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Puklin, J.E.; Frank, R.N. Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3178–3188. [Google Scholar]
- Weinberger, D.; Bor-Shavit, E.; Barliya, T.; Dahbash, M.; Kinrot, O.; Gaton, D.D.; Nisgav, Y.; Livnat, T. Mobile Laser Indirect Ophthalmoscope: For the Induction of Choroidal Neovascularization in a Mouse Model. Curr. Eye Res. 2017, 42, 1545–1551. [Google Scholar] [CrossRef] [PubMed]





| Name | Structure | Metabolites |
|---|---|---|
| AN7 (Butyroyloxymethyl-diethyl phosphate) |  | Butyric acid |
| Formaldehyde | ||
| Phosphoric acid ethanol |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahbash, M.; Sella, R.; Megiddo-Barnir, E.; Nisgav, Y.; Tarasenko, N.; Weinberger, D.; Rephaeli, A.; Livnat, T. The Histone Deacetylase Inhibitor AN7, Attenuates Choroidal Neovascularization in a Mouse Model. Int. J. Mol. Sci. 2019, 20, 714. https://doi.org/10.3390/ijms20030714
Dahbash M, Sella R, Megiddo-Barnir E, Nisgav Y, Tarasenko N, Weinberger D, Rephaeli A, Livnat T. The Histone Deacetylase Inhibitor AN7, Attenuates Choroidal Neovascularization in a Mouse Model. International Journal of Molecular Sciences. 2019; 20(3):714. https://doi.org/10.3390/ijms20030714
Chicago/Turabian StyleDahbash, Mor, Ruti Sella, Elinor Megiddo-Barnir, Yael Nisgav, Nataly Tarasenko, Dov Weinberger, Ada Rephaeli, and Tami Livnat. 2019. "The Histone Deacetylase Inhibitor AN7, Attenuates Choroidal Neovascularization in a Mouse Model" International Journal of Molecular Sciences 20, no. 3: 714. https://doi.org/10.3390/ijms20030714
APA StyleDahbash, M., Sella, R., Megiddo-Barnir, E., Nisgav, Y., Tarasenko, N., Weinberger, D., Rephaeli, A., & Livnat, T. (2019). The Histone Deacetylase Inhibitor AN7, Attenuates Choroidal Neovascularization in a Mouse Model. International Journal of Molecular Sciences, 20(3), 714. https://doi.org/10.3390/ijms20030714