The Oxidative Pathway to Dopamine–Protein Conjugates and Their Pro-Oxidant Activities: Implications for the Neurodegeneration of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
2.1. Spectroscopic Examination of the Tyrosinase-Catalyzed Conjugation of 4-Methylcatechol (MeCA) with Non-Protein and Protein Thiols and Related Compounds
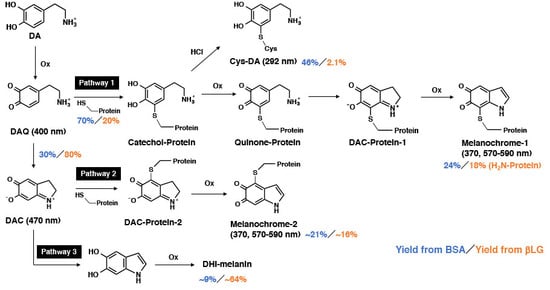
2.2. Spectroscopic Examination of Tyrosinase-Catalyzed Conjugation of DA with Non-Protein and Protein Thiols and Related Compounds
2.3. High-Performance Liquid Chromatography (HPLC) Analysis of Tyrosinase-Catalyzed Conjugation of 4-Methylcatechol (MeCA) and l-Dopamine (DA) with Bovine Serum Albumin (BSA) and β-Lactoglobulin (βLG)
2.4. Time Course of Conjugation of DA with BSA Catalyzed by Fe(III) and Cu(II)
2.5. Pro-Oxidant Activities of Various Melanins Derived Oxidatively from DA-Thiol Conjugates
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Instruments
4.3. Tyrosinase-Catalyzed Oxidation of DA in the Presence of BSA or βLG and HPLC Estimates of Binding through the Cys Residue
4.4. Fe (III) or Cu (II)-Catalyzed Oxidation of DA in the Presence of BSA or βLG and HPLC Estimates of Binding through the Cys Residue
4.5. Pro-Oxidant Activity of Various Melanins Derived Oxidatively from DA-Thiol Conjugates
4.6. Preparation of 4-S-Cysteinyl-5-Methylcatechol
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NAC | N-Acetyl-l-cysteine |
| NAcHis | N-Acetyl-l-histidine |
| NAcLeu | N-Acetyl-l-leucine |
| NAcLys | Nα-Acetyl-l-lysine |
| BSA | Bovine serum albumin |
| Cys | l-Cysteine |
| DBBQ | 3,5-di-tert-Butyl-1,2-benzoquinone |
| DA | l-Dopamine |
| DAC | DAchrome |
| DAQ | Dopamine quinone |
| DMSO | Dimethyl sulfoxide |
| DPRA | Direct peptide reactivity assay (peptide) |
| DTNB | 5,5′-dithiobis(2-nitrobenzoic acid) |
| EDTA·2Na | Ethylenediaminetetraacetic acid disodium salt dihydrate |
| fLG | Fibrillated β-lactoglobulin |
| GSH | (Reduced) Glutathione |
| GSSG | Oxidized glutathione |
| His | l-Histidine |
| βLG | β-Lactoglobulin |
| LC | Locus coeruleus |
| Lys | l-Lysine |
| MeBQ | 4-Methyl-1,2-benzoquinone |
| MeCA | 4-Methylcatechol |
| NM | Neuromelanin |
| NE | l-Norepinephrine |
| PD | Parkinson’s disease |
| ROS | Reactive oxygen species |
| SN | Substantia nigra |
References
- DeMattei, M.; Levi, A.C.; Fariello, R.G. Neuromelanic pigment in substantia nigra neurons of rats and dogs. Neurosci. Lett. 1986, 72, 37–42. [Google Scholar] [PubMed]
- Kemali, M.; Gioffre, D. Anatomical localisation of neuromelanin in the brains of the frog and tadpole. Ultrastructural comparison of neuromelanin with other melanins. J. Anat. 1985, 142, 73–83. [Google Scholar] [PubMed]
- Zecca, L.; Stroppolo, A.; Gatti, A.; Tampellini, D.; Toscani, M.; Gallorini, M.; Giaveri, G.; Arosio, P.; Santambrogio, P.; Fariello, R.G.; et al. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl. Acad. Sci. USA 2004, 101, 9843–9848. [Google Scholar] [CrossRef] [Green Version]
- Zecca, L.; Bellei, C.; Costi, P.; Albertini, A.; Monzani, E.; Casella, L.; Gallorini, M.; Bergamaschi, L.; Moscatelli, A.; Turro, N.J.; et al. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc. Natl. Acad. Sci. USA 2008, 105, 17567–17572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakamatsu, K.; Tabuchi, K.; Ojika, M.; Zucca, F.A.; Zecca, L.; Ito, S. Norepinephrine and its metabolites are involved in the synthesis of neuromelanin derived from the locus coeruleus. J. Neurochem. 2015, 135, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Zucca, F.A.; Basso, E.; Cupaioli, F.A.; Ferrari, E.; Sulzer, D.; Casella, L.; Zecca, L. Neuromelanin of the human substantia nigra: An update. Neurotox. Res. 2014, 25, 13–23. [Google Scholar] [CrossRef]
- Zecca, L.; Fariello, R.; Riederer, P.; Sulzer, D.; Gatti, A.; Tampellini, D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett. 2002, 510, 216–220. [Google Scholar] [CrossRef]
- Zecca, L.; Zucca, F.A.; Albertini, A.; Rizzio, E.; Fariello, R.G. A proposed dual role of neuromelanin in the pathogenesis of Parkinson’s disease. Neurology 2006, 67, S8–S11. [Google Scholar] [CrossRef] [PubMed]
- Bohic, S.; Murphy, K.; Paulus, W.; Cloetens, P.; Salomé, M.; Susini, J.; Double, K. Intracellular chemical imaging of the developmental phases of human neuromelanin using synchrotron X-ray microspectroscopy. Anal. Chem. 2008, 80, 9557–9566. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Bogulavsky, J.; Larsen, K.E.; Behr, G.; Karatekin, E.; Kleinman, M.H.; Turro, N.; Krantz, D.; Edwards, R.H.; Greene, L.A.; Zecca, L. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. USA 2000, 97, 11869–11874. [Google Scholar] [CrossRef] [Green Version]
- Cadet, J.L.; Brannock, C. Free radicals and the pathobiology of brain dopamine systems. Neurochem. Int. 1998, 32, 117–131. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M. Dopaminergic neuron specific oxidative stress caused by dopamine itself. Acta Med. Okayama 2008, 62, 141–150. [Google Scholar]
- Cheng, J.; Moss, S.C.; Eisner, M. X-ray characterization of melanins–II. Pigment Cell Res. 1994, 7, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Crippa, R.; Wang, Q.J.; Eisner, M.; Moss, S.C.; Zecca, L.; Zschack, P.; Gog, T. Structure of human neuromelanin by X-ray diffraction: comparison with synthetics. Abstract, XVIth International Pigment Cell Conference, Anaheim. Pigment Cell Res. 1996, 9. [Google Scholar] [CrossRef]
- Engelen, M.; Vanna, R.; Bellei, C.; Zucca, F.A.; Wakamatsu, K.; Monzani, E.; Ito, S.; Casella, L.; Zecca, L. Neuromelanins of human brain have soluble and insoluble components with dolichol attached to the melanic structure. PLoS ONE 2012, 7, e48490. [Google Scholar] [CrossRef] [PubMed]
- Fedorow, H.; Pickford, R.; Hook, J.M.; Double, K.L.; Halliday, G.M.; Gerlach, M.; Riederer, P.; Garner, B. Dolichol is the major lipid component of human substantia nigra neuromelanin. J. Neurochem. 2005, 92, 990–995. [Google Scholar] [CrossRef]
- Ward, W.C.; Guan, Z.; Zucca, F.A.; Fariello, R.G.; Kordestani, R.; Zecca, L.; Raetz, C.R.; Simon, J.D. Identification and quantification of dolichol and dolichoic acid in neuromelanin from substantia nigra of the human brain. J. Lipid Res. 2007, 48, 1457–1462. [Google Scholar] [CrossRef] [Green Version]
- Zecca, L.; Costi, P.; Mecacci, C.; Ito, S.; Terreni, M.; Sonnino, S. Interaction of human substantia nigra neuromelanin with lipids and peptides. J. Neurochem. 2000, 74, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, R.; Wang, G. Impact of dopamine oxidation on dopaminergic neurodegeneration. ACS Chem. Neurosci. 2019, 10, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Zecca, L. Intraneuronal dopamine-quinone synthesis: A review. Neurotox. Res. 2000, 1, 181–195. [Google Scholar] [CrossRef]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Tribl, F.; Arzberger, T.; Riederer, P.; Gerlach, M. Tyrosinase is not detected in human catecholaminergic neurons by immunohistochemistry and Western blot analysis. J. Neural Transm. Suppl. 2007, 72, 51–55. [Google Scholar]
- Ikemoto, K.; Nagatsu, I.; Ito, S.; King, R.A.; Nishimura, A.; Nagatsu, T. Does tyrosinase exist in neuromelanin-pigmented neurons in the human substantia nigra? Neurosci. Lett. 1998, 253, 198–200. [Google Scholar] [CrossRef]
- Carballo-Carbajal, I.; Laguna, A.; Romero-Giménez, J.; Cuadros, T.; Bové, J.; Martinez-Vicente, M.; Parent, A.; Gonzalez-Sepulveda, M.; Peñuelas, N.; Torra, A.; et al. Brain tyrosinase overexpression implicates age-dependent neuromelanin production in Parkinson’s disease pathogenesis. Nat. Commun. 2019, 10, 973. [Google Scholar] [CrossRef] [PubMed]
- Zucca, F.A.; Segura-Aguilar, J.; Ferrari, E.; Muñoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Capucciati, A.; Prada, I.; Zucca, F.A.; D’Arrigo, G.; Pontiroli, D.; Bridelli, M.G.; Sturini, M.; Bubacco, L.; Monzani, E.; et al. Synthesis, structure characterization, and evaluation in microglia cultures of neuromelanin analogues suitable for modeling Parkinson’s disease. ACS Chem. Neurosci. 2017, 8, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Kontopidis, G.; Holt, C.; Sawyer, L. Invited review: β-Lactoglobulin: Binding properties, structure, and function. J. Dairy Sci. 2004, 87, 785–796. [Google Scholar] [CrossRef]
- Makin, O.S.; Serpell, L.C. Structures for amyloid fibrils. FEBS J. 2005, 272, 5950–5961. [Google Scholar] [CrossRef] [PubMed]
- Dave, A.C.; Loveday, S.M.; Anema, S.G.; Loo, T.S.; Norris, G.E.; Jameson, G.B.; Singh, H. β-Lactoglobulin self-assembly: Structural changes in early stages and disulfide bonding in fibrils. J. Agric. Food Chem. 2013, 61, 7817–7828. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jongberg, S.; Andersen, M.L.; Davies, M.J.; Lund, M.N. Quinone-induced protein modifications: kinetic preference for reaction of 1,2-benzoquinones with thiol groups in proteins. Free Rad. Biol. Med. 2016, 97, 148–157. [Google Scholar] [CrossRef]
- Ito, S.; Yamanaka, Y.; Ojika, M.; Wakamatsu, K. The metabolic fate of ortho-quinones derived from catecholamine metabolites. Int. J. Mol. Sci. 2016, 17, 164. [Google Scholar] [CrossRef]
- Ito, S.; Prota, G. A facile one-step synthesis of cysteinyldopas using mushroom tyrosinase. Experientia 1977, 33, 1118–1119. [Google Scholar] [CrossRef] [PubMed]
- Gerken, T.A. Amino group environments and metal binding properties of carbon-13 reductivity methylated bovine alpha-lactalbumin. Biochemistry 1984, 23, 4688–4697. [Google Scholar] [CrossRef]
- Pierpoint, W. o-Quinones formed in plant extracts. Their reactions with amino acids and peptides. Biochem. J. 1969, 112, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Okura, M.; Nakanishi, Y.; Ojika, M.; Wakamatsu, K.; Yamashita, T. Tyrosinase-catalyzed metabolism of rhododendrol (RD) in B16 melanoma cell: production of RD-pheomelanin and covalent binding with thiol proteins. Pigment Cell Melanoma Res. 2015, 28, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Fujita, K. Oxygen-dependent conjugation of dopa with cysteine catalyzed by iron-EDTA complex. Biochem. Pharmacol. 1984, 33, 2193–2197. [Google Scholar] [CrossRef]
- Imai, Y.; Ito, S.; Fujita, K. Determination of natural thiols by liquid chromatography after derivatization with 3,5-di-tert.-butyl-1,2-benzoquinone. J. Chromatogr. 1987, 420, 404–410. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Lim, T.M. Glutathione conjugates with dopamine-derived quinones to form reactive or non-reactive glutathione-conjugates. Neurochem. Res. 2010, 35, 1805–1818. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar]
- Sian-Hülsmann, J.; Mandel, S.; Youdim, M.B.; Riederer, P. The relevance of iron in the pathogenesis of Parkinson’s disease. J. Neurochem. 2011, 118, 939–957. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Murase, T.; Zucca, F.A.; Zecca, L.; Ito, S. Biosynthetic pathway to neuromelanin and its aging process. Pigment Cell Melanoma Res. 2012, 25, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Pham, A.N.; Hare, D.J.; Waite, T.D. Kinetic modeling of pH-dependent oxidation of dopamine by iron and its relevance to Parkinson’s Disease. Front. Neurosci. 2018, 12, 859. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Pezzella, A.; Land, E.J.; Ramsden, C.A.; Riley, P.A. 5,6-Dihydroxyindoles and indole-5,6-diones. Adv. Heterocycl. Chem. 2005, 89, 1–63. [Google Scholar]
- D’Ischia, M.; Napolitano, A.; Pezzella, A. 5,6-dihydroxyindole chemistry: Unexplored opportunities beyond eumelanin. Eur. J. Org. Chem. 2011, 2011, 5501–5516. [Google Scholar] [CrossRef]
- Corradini, M.G.; Napolitano, A.; Prota, G. A biosynthetic approach to the structure of eumelanins—The isolation of oligomers from 5,6-dihydroxy-1-methylindole. Tetrahedron 1986, 42, 2083–2088. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Manta, B.; Botti, H.; Radi, R.; Trujillo, M.; Denicola, A. Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem. Res. Toxicol. 2011, 24, 434–450. [Google Scholar] [CrossRef]
- Zecca, L.; Casella, L.; Albertini, A.; Bellei, C.; Zucca, F.A.; Engelen, M.; Zadlo, A.; Szewczyk, G.; Zareba, M.; Sarna, T. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J. Neurochem. 2008, 106, 1866–1875. [Google Scholar]
- Winterbourn, C.C. Revisiting the reactions of superoxide with glutathione and other thiols. Arch. Biochem. Biophys. 2016, 595, 68–71. [Google Scholar] [CrossRef]
- Ito, S.; Okura, M.; Wakamatsu, K.; Yamashita, T. The potent pro-oxidant activity of rhododendrol-eumelanin induces cysteine depletion in B16 melanoma cells. Pigment Cell Melanoma Res. 2017, 30, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Kato, T.; Shinpo, K.; Fujita, K. Oxidation of tyrosine residues in proteins by tyrosinase. Formation of protein-bonded 3,4-dihydroxyphenlyalanine and 5-S-cysteinyl-3,4-dihydroxyphenylalanine. Biochem. J. 1984, 222, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Zhou, M.; Panchuk-Voloshina, N. A one-step fluorometric method for the continuous measurement of monoamine oxidase activity. Anal. Biochem. 1997, 253, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hinoshita, M.; Suzuki, E.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of the leukoderma-inducing agent raspberry ketone produces (E)-4-(3-oxo-1-butenyl)-1,2-benzoquinone: Implications for melanocyte toxicity. Chem. Res. Toxicol. 2017, 30, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Prota, G.; Scherillo, G.; Napolano, E.; Nicolaus, R.A. Structure and biogenesis of phaeomelanins. II. Reaction of o-quinones with cysteine. Gazz. Chim. Ital. 1967, 97, 1451–1478. [Google Scholar]
- Ito, S.; Inoue, S.; Yamamoto, Y.; Fujita, K. Synthesis and antitumor activity of cysteinyl-3,4-dihydroxyphenylalanines and related compounds. J. Med. Chem. 1981, 24, 673–677. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakamatsu, K.; Nakao, K.; Tanaka, H.; Kitahori, Y.; Tanaka, Y.; Ojika, M.; Ito, S. The Oxidative Pathway to Dopamine–Protein Conjugates and Their Pro-Oxidant Activities: Implications for the Neurodegeneration of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 2575. https://doi.org/10.3390/ijms20102575
Wakamatsu K, Nakao K, Tanaka H, Kitahori Y, Tanaka Y, Ojika M, Ito S. The Oxidative Pathway to Dopamine–Protein Conjugates and Their Pro-Oxidant Activities: Implications for the Neurodegeneration of Parkinson’s Disease. International Journal of Molecular Sciences. 2019; 20(10):2575. https://doi.org/10.3390/ijms20102575
Chicago/Turabian StyleWakamatsu, Kazumasa, Kenta Nakao, Hitomi Tanaka, Yuki Kitahori, Yui Tanaka, Makoto Ojika, and Shosuke Ito. 2019. "The Oxidative Pathway to Dopamine–Protein Conjugates and Their Pro-Oxidant Activities: Implications for the Neurodegeneration of Parkinson’s Disease" International Journal of Molecular Sciences 20, no. 10: 2575. https://doi.org/10.3390/ijms20102575
APA StyleWakamatsu, K., Nakao, K., Tanaka, H., Kitahori, Y., Tanaka, Y., Ojika, M., & Ito, S. (2019). The Oxidative Pathway to Dopamine–Protein Conjugates and Their Pro-Oxidant Activities: Implications for the Neurodegeneration of Parkinson’s Disease. International Journal of Molecular Sciences, 20(10), 2575. https://doi.org/10.3390/ijms20102575