Pathogenicity and Virulence of Trueperella pyogenes: A Review
Abstract
:1. Introduction
2. Pathogenicity
2.1. T. pyogenes Infections in Animals
2.2. T. pyogenes Infections in Humans
3. Reservoir, Transmission, and Routes of Infection
4. Pathogenesis of T. pyogenes Infection
4.1. T. pyogenes Virulence
4.1.1. Pyolysin
4.1.2. Fimbriae
4.1.3. Extracellular Matrix-Binding Proteins
4.1.4. Neuraminidases
4.1.5. Biofilm
4.1.6. Regulation of Virulence Factor Expression
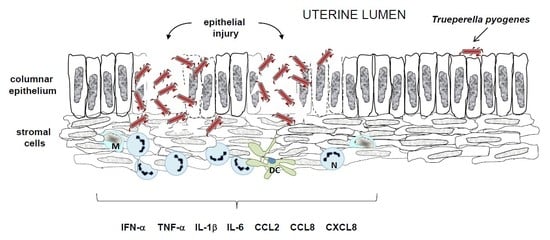
4.2. Induction of Host Defense Mechanisms by T. pyogenes
5. T. pyogenes Genome
6. Immunoprotection and Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yassin, A.F.; Hupfer, H.; Siering, C.; Schumann, P. Comparative chemotaxonomic and phylogenetic studies on the genus Arcanobacterium Collins et al. 1982 emend. Lehnen et al. 2006: Proposal for Trueperella gen. nov. and emended description of the genus Arcanobacterium. Int. J. Syst. Evol. Microbiol. 2011, 61, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.P.; Foster, G.; Collins, M.D. Phylogenetic analysis of the genus Actinomyces based on 16S rRNA gene sequences: Description of Arcanobacterium phocae sp. nov., Arcanobacterium bernardiae comb. nov., and Arcanobacterium pyogenes comb. nov. Int. J. Syst. Bacteriol. 1997, 47, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Jones, D.; Kroppenstedt, R.M.; Schleifer, K.H. Chemical studies as a guide to the classification of Corynebacterium pyogenes and Corynebacterium haemolyticum. J. Gen. Microbiol. 1982, 128, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.A.; Cornell, C.P.; Fraga, A.M. Transfer of Corynebacterium pyogenes (Glage) Eberson to the genus Actinomyces as Actinomyces pyogenes (Glage) comb. nov. Int. J. Syst. Bacteriol. 1982, 32, 419–429. [Google Scholar] [CrossRef]
- Rogosa, M.; Cummins, C.S.; Lelliott, R.A.; Keddie, R.M. Coryneform group of bacteria. In Bergey’s Manual of Determinative Bacteriology, 8th ed.; Buchanan, R.E., Gibbons, N.E., Eds.; The Williams and Wilkins Company: Baltimore, MD, USA, 1974; pp. 599–632. [Google Scholar]
- Zhao, K.; Li, W.; Kang, C.; Du, L.; Huang, T.; Zhang, X.; Wu, M.; Yue, B. Phylogenomics and Evolutionary Dynamics of the Family Actinomycetaceae. Genome Biol. Evol. 2014, 6, 2625–2633. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Lämmler, C. Evaluation of the API Coryne test system for identification of Actinomyces pyogenes. Zentralbl. Veterinarmed. B 1992, 39, 273–276. [Google Scholar] [CrossRef]
- Hijazin, M.; Hassan, A.A.; Alber, J.; Lämmler, C.; Timke, M.; Kostrzewa, M.; Prenger-Bernighoff, E.; Zschöck, M. Evaluation of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) for species identification of bacteria of genera Arcanobacterium and Trueperella. Vet. Microbiol. 2012, 157, 243–245. [Google Scholar] [CrossRef]
- Zhang, W.; Meng, X.; Wang, J. Sensitive and rapid detection of Trueperella pyogenes using loop-mediated isothermal amplification method. J. Microbiol. Methods 2013, 93, 124–126. [Google Scholar] [CrossRef]
- Nagib, S.; Rau, J.; Sammra, O.; Lämmler, C.; Schlez, K.; Zschöck, M.; Prenger-Berninghoff, E.; Klein, G.; Abdulmawjood, A. Identification of Trueperella pyogenes isolated from bovine mastitis by Fourier transform infrared spectroscopy. PLoS ONE 2014, 9, e10465. [Google Scholar] [CrossRef]
- Randall, L.P.; Lemma, F.; Koylass, M.; Rogers, J.; Ayling, R.D.; Worth, D.; Klita, M.; Steventon, A.; Line, K.; Wragg, P.; et al. Evaluation of MALDI-ToF as a method for the identification of bacteria in the veterinary diagnostic laboratory. Res. Vet. Sci. 2015, 101, 42–49. [Google Scholar] [CrossRef]
- Abdulmawjood, A.; Wickhorst, J.; Hashim, O.; Sammra, O.; Hassan, A.A.; Alssahen, M.; Lämmler, C.; Prenger-Berninghoff, E.; Klein, G. Application of a loop-mediated isothermal amplification (LAMP) assay for molecular identification of Trueperella pyogenes isolated from various origins. Mol. Cell Probes 2016, 30, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.Z.; Matajira, C.E.C.; da Costa, B.L.P.; Ferreira, T.S.P.; Silva, G.F.R.; Dutra, M.C.; Gomes, V.T.M.; Silva, A.P.S.; Christ, A.P.G.; Sato, M.I.Z.; et al. Characterization of porcine Trueperella pyogenes by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS), molecular typing and antimicrobial susceptibility profiling in Sao Paulo State. Comp. Immunol. Microbiol. Infect. Dis. 2017, 51, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Rowlinson, M.C.; Bruckner, D.A.; Hinnebusch, C.; Nielsen, K.; Deville, J.G. Clearance of Cellulosimicrobium cellulans Bacteriemia in a Child without Central Venous Catheter Removal. J. Clin. Microbiol. 2006, 44, 2650–2654. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joiningmethod: A newmethod for reconstructingphylogenetictrees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferringverylargephylogenies by using the neighbor-joiningmethod. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidencelimits on phylogenies: Anapproachusing the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: MolecularEvolutionary Genetics Analysis acrosscomputingplatforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Queen, C.; Ward, A.C.; Hunter, D.L. Bacteria isolated from nasal and tonsillar samples of clinically healthy Rocky Mountain bighorn and domestic sheep. J. Wildl. Dis. 1994, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rzewuska, M.; Osińska, B.; Bielecki, W.; Skrzypczak, M.; Stefańska, I.; Binek, M. Microflora of urogenital tract in European bison (Bison bonasus). In Health Threats for the Eurpean Bison Particularly in Free-Roaming Populations in Poland; Kita, J., Anusz, K., Eds.; Warsaw University of Life Sciences: Warsaw, Poland, 2006; pp. 27–28. [Google Scholar]
- Silva, E.; Gaivão, M.; Leitão, S.; Jost, B.H.; Carneiro, C.; Vilela, C.L.; Lopes da Costa, L.; Mateus, L. Genomic characterization of Arcanobacterium pyogenes isolates recovered from the uterus of dairy cows with normal puerperium or clinical metritis. Vet. Microbiol. 2008, 132, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Nagaraja, T.G.; Wallace, N.; Staats, J.; Chengappa, M.M.; Oberst, R.D. Biochemical and ribotypic comparison of Actinomyces pyogenes and A. pyogenes-like organisms from liver abscesses, ruminal wall, and ruminal contents of cattle. Am. J. Vet. Res. 1998, 59, 271–276. [Google Scholar] [PubMed]
- Jost, B.H.; Post, K.W.; Songer, J.G.; Billington, S.J. Isolation of Arcanobacterium pyogenes from the porcine gastric mucosa. Vet. Res. Commun. 2002, 26, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Spittel, S.; Hoedemaker, M. Mastitis diagnosis in dairy cows using PathoProof real-time polymerase chain reaction assay in comparison with conventional bacterial culture in a Northern German field study. Berl. Munchener Tierarztliche Wochenschr. 2012, 125, 494–502. [Google Scholar]
- Williamson, L.H. Caseous lymphadenitis in small ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 359–371. [Google Scholar] [CrossRef]
- Stefańska, I.; Gieryńska, M.; Rzewuska, M.; Binek, M. Survival of Corynebacterium pseudotuberculosis within macrophages and induction of phagocytes death. Pol. J. Vet. Sci. 2010, 13, 143–149. [Google Scholar] [PubMed]
- Muscatello, G. Rhodococcus equi pneumonia in the foal—Part 1: Pathogenesis and epidemiology. Vet. J. 2012, 192, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, L.; Rzewuska, M.; Takai, S.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Feret, M.; Gawryś, M.; Witkowski, M.; Kita, J. Molecular characterization of Rhodococcus equi isolates from horses in Poland: pVapA characteristics and plasmid new variant, 85-kb type V. BMC Vet. Res. 2017, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Rzewuska, M.; Czopowicz, M.; Gawryś, M.; Markowska-Daniel, I.; Bielecki, W. Relationships between antimicrobial resistance, distribution of virulence factor genes and the origin of Trueperella pyogenes isolated from domestic animals and European bison (Bison bonasus). Microb. Pathog. 2016, 96, 35–41. [Google Scholar] [CrossRef]
- Risseti, R.M.; Zastempowska, E.; Twarużek, M.; Lassa, H.; Pantoja, J.C.F.; de Vargas, A.P.C.; Guerra, S.T.; Bolanõs, C.A.D.; de Paula, C.L.; Alves, A.C.; et al. Virulence markers associated with Trueperella pyogenes infections in livestock and companion animals. Lett. Appl. Microbiol. 2017, 65, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Rogovskyy, A.S.; Lawhon, S.; Kuczmanski, K.; Gillis, D.C.; Wu, J.; Hurley, H.; Rogovska, Y.V.; Konganti, K.; Yang, C.Y.; Duncan, K. Phenotypic and genotypic characteristics of Trueperella pyogenes isolated from ruminants. J. Vet. Diagn. Investig. 2018, 30, 348–353. [Google Scholar] [CrossRef]
- Ashrafi Tamai, I.; Mohammadzadeh, A.; Zahraei Salehi, T.; Mahmoodi, P. Genomic characterisation, detection of genes encoding virulence factors and evaluation of antibiotic resistance of Trueperella pyogenes isolated from cattle with clinical metritis. Antonie Van Leeuwenhoek 2018, 111, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Guérin-Faublée, V.; Flandrois, J.P.; Broye, E.; Tupin, F.; Richard, Y. Actinomyces pyogenes: Susceptibility of 103 clinical animal isolates to 22 antimicrobial agents. Vet. Res. 1993, 24, 251–259. [Google Scholar] [PubMed]
- Werckenthin, C.; Alesík, E.; Grobbel, M.; Lübke-Becker, A.; Schwarz, S.; Wieler, L.H.; Wallmann, J. Antimicrobial susceptibility of Pseudomonas aeruginosa from dogs and cats as well as Arcanobacterium pyogenes from cattle and swine as determined in the BfT-GermVet monitoring program 2004–2006. Berl. Munchener Tierarztliche Wochenschr. 2007, 120, 412–422. [Google Scholar]
- Urumova, V.; Lyutzkanov, M.; Tsachev, I.; Marutsov, P.; Zhelev, G. Investigations on the involvement of Arcanobacterium pyogenes in various infections in productive and companion animals and sensitivity of isolates to antibacterials. Revue Méd. Vét. 2009, 160, 582–585. [Google Scholar]
- Hijazin, M.; Ülbegi-Mohyla, H.; Alber, J.; Lämmler, C.; Hassan, A.A.; Abdulmawjood, A.; Prenger-Berninghoff, E.; Weiss, R.; Zschöck, M. Molecular identification and further characterization of Arcanobacterium pyogenes isolated from bovine mastitis and from various other origins. J. Dairy Sci. 2011, 94, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Zastempowska, E.; Lassa, H. Genotypic characterization and evaluation of an antibiotic resistance of Trueperella pyogenes (Arcanobacterium pyogenes) isolated from milk of dairy cows with clinical mastitis. Vet. Microbiol. 2012, 161, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A.; Lübke-Becker, A.; Heuwieser, W. Minimum inhibitory concentrations of frequently used antibiotics against Escherichia coli and Trueperella pyogenes isolated from uteri of postpartum dairy cows. J. Dairy Sci. 2018, 101, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, C.; Liu, Y.; Zhao, J.; Yang, Y.; Shen, J. Identification, susceptibility, and detection of integron-gene cassettes of Arcanobacterium pyogenes in bovine endometritis. J. Dairy Sci. 2009, 92, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.L.; Liu, Y.; Zhang, X.Y.; Palahati, P.; Wang, H.N.; Yue, B.S. Detection and characterization of antibiotic resistance genes in Arcanobacterium pyogenes strains from abscesses of forest musk deer. J. Med. Microbiol. 2011, 60, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.X.; Tian, K.; Han, L.M.; Wang, Q.X.; Liu, Y.C.; Tian, C.L.; Liu, M.C. Resistance to β-lactam antibiotic may influence nanH gene expression in Trueperella pyogenes isolated from bovine endometritis. Microb. Pathog. 2014, 71–72, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, J.; Wang, Q.; Liu, Y.; Tian, C.; Zhao, Y.; Yu, L.; Liu, M. Trueperella pyogenes isolated from dairy cows with endometritis in Inner Mongolia, China: Tetracycline susceptibility and tetracycline-resistance gene distribution. Microb. Pathog. 2017, 105, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Alkasir, R.; Wang, J.; Gao, J.; Ali, T.; Zhang, L.; Szenci, O.; Bajcsy, Á.C.; Han, B. Properties and antimicrobial susceptibility of Trueperella pyogenes isolated from bovine mastitis in China. Acta Vet. Hung. 2016, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Kojima, A.; Ishimaru, M. Antimicrobial susceptibility of Arcanobacterium pyogenes isolated from cattle and pigs. J. Vet. Med. B Infect. Dis. Vet. Public Health 2000, 47, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, D.; Mizomoto, T.; Ueda, C.; Takagi, N.; Shimizu, N.; Matsuura, Y.; Makuuchi, Y.; Watanabe, A.; Shinozuka, Y.; Kawai, K. Factors affecting the incidence and outcome of Trueperella pyogenes mastitis in cows. J. Vet. Med. Sci. 2017, 79, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.G.; Risseti, R.M.; Bolanõs, C.A.D.; Caffaro, K.A.; de Morais, A.C.B.; Lara, G.H.B.; Zamprogna, T.O.; Paes, A.C.; Listoni, F.J.P.; Franco, M.M.J. Trueperella pyogenes multispecies infections in domestic animals: A retrospective study of 144 cases (2002 to 2012). Vet. Q. 2015, 35, 82–87. [Google Scholar] [CrossRef]
- Trinh, H.T.; Billington, S.J.; Field, A.C.; Songer, J.G.; Jost, B.H. Susceptibility of Arcanobacterium pyogenes from different sources to tetracycline, macrolide and lincosamide antimicrobial agents. Vet. Microbiol. 2002, 85, 353–359. [Google Scholar] [CrossRef]
- Jost, B.H.; Billington, S.J. Arcanobacterium pyogenes: Molecular pathogenesis of an animal opportunist. Antonie Van Leeuwenhoek 2005, 88, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.; Caixeta, L.S.; Machado, V.S.; Rauf, A.K.; Gilbert, R.O.; Bicalho, R.C. Antimicrobial resistance and presence of virulence factor genes in Arcanobacterium pyogenes isolated from the uterus of postpartum dairy cows. Vet. Microbiol. 2010, 145, 84–89. [Google Scholar] [CrossRef]
- Bicalho, M.L.; Lima, F.S.; Machado, V.S.; Meira, E.B.; Ganda, E.K.; Foditsch, C.; Bicalho, R.C.; Gilbert, R.O. Associations among Trueperella pyogenes, endometritis diagnosis, and pregnancy outcomes in dairy cows. Theriogenology 2016, 85, 267–274. [Google Scholar] [CrossRef]
- Markowska-Daniel, I.; Urbaniak, K.; Stępniewska, K.; Pejsak, Z. Antibiotic susceptibility of bacteria isolated from respiratory tract of pigs in Poland between 2004 and 2008. Pol. J. Vet. Sci. 2010, 13, 29–36. [Google Scholar]
- Feßler, A.T.; Schwarz, S. Antimicrobial Resistance in Corynebacterium spp., Arcanobacterium spp., and Trueperella pyogenes. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Lavín, S.; Ruiz-Bascarán, M.; Marco, I.; Abarca, M.L.; Crespo, M.J.; Franch, J. Foot infections associated with Arcanobacterium pyogenes in free-living fallow deer (Dama dama). J. Wildl. Dis. 2004, 40, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Belser, E.H.; Cohen, B.S.; Keeler, S.P.; Killmaster, C.H.; Bowers, J.W.; Miller, K.V. Epethelial presence of Trueperella pyogenes predicts site-level presence of cranial abscess disease in white-tailed deer (Odocoileus virginianus). PLoS ONE 2015, 10, e0120028. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, M.L.; Machado, V.S.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Association between virulence factors of Escherichia coli, Fusobacterium necrophorum, and Arcanobacterium pyogenes and uterine diseases of dairy cows. Vet. Microbiol. 2012, 157, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Peter, S.; Wagener, K.; Drillich, M.; Ehling-Schulz, M.; Einspanier, R.; Gabler, C. Bovine Endometrial Epithelial Cells Scale Their Pro-inflammatory Response In vitro to Pathogenic Trueperella pyogenes Isolated from the Bovine Uterus in a Strain-Specific Manner. Front. Cell. Infect. Microbiol. 2017, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, S.T.; Jost, B.H.; Billington, S.J. Transcriptional regulation of pyolysin production in the animal pathogen, Arcanobacterium pyogenes. Vet. Microbiol. 2008, 132, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, M.; Junni, R.; Simojoki, H.; Malinen, E.; Seuna, E.; Klitgaard, K.; Kujala-Wirth, M.; Soveri, T.; Pelkonen, S. Bacterial species associated with interdigital phlegmon outbreaks in Finnish dairy herds. BMC Vet. Res. 2019, 29, 44. [Google Scholar] [CrossRef] [PubMed]
- Tadepalli, S.; Narayanan, S.K.; Stewart, G.C.; Chengappa, M.M.; Nagaraja, T.G. Fusobacterium necrophorum: A ruminal bacterium that invades liver to cause abscesses in cattle. Anaerobe 2009, 15, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, C.M.; Hathcock, T.L. Bovine rumenitis-liver abscess complex: A bacteriological review. Cornell Vet. 1983, 73, 288–297. [Google Scholar] [PubMed]
- Roberts, D.S. The pathogenic synergy of Fusiformis necrophorus and Corynebacterium pyogenes. II. The response of F. necrophorus to a filterable product of C. pyogenes. Br. J. Exp. Pathol. 1967, 48, 674–679. [Google Scholar]
- Azawi, O.I.; Al-Abidy, H.F.; Ali, A.J. Pathological and bacteriological studies of hydrosalpinx in buffaloes. Reprod. Domest. Anim. 2010, 45, 416–420. [Google Scholar] [CrossRef]
- de Boer, M.; Heuer, C.; Hussein, H.; McDougall, S. Minimum inhibitory concentrations of selected antimicrobials against Escherichia coli and Trueperella pyogenes of bovine uterine origin. J. Dairy Sci. 2015, 98, 4427–4438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, W.; Huang, T.; Song, X.; Zhang, X.; Yue, B. Comparative transcriptome analysis of Trueperella pyogenes reveals a novel antimicrobial strategy. Arch. Microbiol. 2017, 199, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, X.; Zhao, K.; Jing, J.; Shen, Y.; Zhang, X.; Yue, B. Quorum-sensing molecules N-acyl homoserine lactones inhibit Trueperella pyogenes infection in mouse model. Vet. Microbiol. 2018, 213, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, E.; Lassa, H.; Markiewicz, H.; Kaptur, M.; Nadolny, M.; Niewitecki, W.; Ziętara, J. Sensitivity to antibiotics of Arcanobacterium pyogenes and Escherichia coli from the uteri of cows with metritis/endometritis. Vet. J. 2011, 187, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Nava, S.; Boscán-Ocando, J.; Nava, J. Normal bacterial flora from vaginas of Criollo Limonero cows. Trop. Anim. Health Prod. 2011, 43, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Amos, M.R.; Healey, G.D.; Goldstone, R.J.; Mahan, S.M.; Düvel, A.; Schuberth, H.J.; Sandra, O.; Zieger, P.; Dieuzy-Labaye, I.; Smith, D.G.; et al. Differential endometrial cell sensitivity to a cholesterol-dependent cytolysin links Trueperella pyogenes to uterine disease in cattle. Biol. Reprod. 2014, 90, 54. [Google Scholar] [CrossRef]
- Griffin, S.; Healey, G.D.; Sheldon, I.M. Isoprenoids increase bovine endometrial stromal cell tolerance to the cholesterol-dependent cytolysin from Trueperella pyogenes. Biol. Reprod. 2018, 99, 749–760. [Google Scholar] [CrossRef]
- Lima, F.S.; Greco, L.F.; Bisinotto, R.S.; Ribeiro, E.S.; Martinez, N.M.; Thatcher, W.W.; Santos, J.E.; Reinhard, M.K.; Galvão, K.N. Effects of intrauterine infusion of Trueperella pyogenes on endometrial mRNA expression of proinflammatory cytokines and luteolytic cascade genes and their association with luteal life span in dairy cows. Theriogenology 2015, 84, 1263–1272. [Google Scholar] [CrossRef]
- Wagener, K.; Grunert, T.; Prunner, I.; Ehling-Schulz, M.; Drillich, M. Dynamics of uterine infections with Escherichia coli, Streptococcus uberis and Trueperella pyogenes in post-partum dairy cows and their association with clinical endometritis. Vet. J. 2014, 202, 527–532. [Google Scholar] [CrossRef]
- Carneiro, L.C.; Cronin, J.G.; Sheldon, I.M. Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod. Biol. 2016, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bonnett, B.N.; Martin, S.W.; Meek, A.H. Associations of clinical findings, bacteriological and histological results of endometrial biopsy with reproductive performance of postpartum dairy cows. Prev. Vet. Med. 1993, 15, 205–220. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Danesh Mesgaran, S.; Gärtner, M.A.; Wagener, K.; Drillich, M.; Ehling-Schulz, M.; Einspanier, R.; Gabler, C. Different inflammatory responses of bovine oviductal epithelial cells in vitro to bacterial species with distinct pathogenicity characteristics and passage number. Theriogenology 2018, 106, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, B.N.; Martin, S.W.; Gannon, V.P.; Miller, R.B.; Etherington, W.G. Endometrial biopsy in Holstein-Friesian dairy cows. III. Bacteriological analysis and correlations with histological findings. Can. J. Vet. Res. 1991, 55, 168–173. [Google Scholar] [PubMed]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Jaureguiberry, M.; Madoz, L.V.; Giuliodori, M.J.; Wagener, K.; Prunner, I.; Grunert, T.; Ehling-Schulz, M.; Drillich, M.; de la Sota, R.L. Identification of Escherichia coli and Trueperella pyogenes isolated from the uterus of dairy cows using routine bacteriological testing and Fourier transform infrared spectroscopy. Acta Vet. Scand. 2016, 58, 81. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Williams, E.J.; Miller, A.N.; Nash, D.M.; Herath, S. Uterine diseases in cattle after parturition. Vet. J. 2008, 176, 115–121. [Google Scholar] [CrossRef]
- Prunner, I.; Wagener, K.; Pothmann, H.; Ehling-Schulz, M.; Drillich, M. Risk factors for uterine diseases on small- and medium-sized dairy farms determined by clinical, bacteriological, and cytological examinations. Theriogenology 2014, 82, 857–865. [Google Scholar] [CrossRef]
- Seno, N.; Azuma, R. A study on heifer mastitis in Japan and its causative microorganisms. Natl. Inst. Anim. Health Q. (Tokyo) 1983, 23, 82–91. [Google Scholar]
- Quinn, A.K.; Vermunt, J.J.; Twiss, D.P. Arcanobacterium pyogenes mastitis in a 18-month-old heifer. N. Z. Vet. J. 2002, 50, 167–168. [Google Scholar] [CrossRef]
- Ericsson Unnerstad, H.; Lindberg, A.; Persson Waller, K.; Ekman, T.; Artursson, K.; Nilsson-Ost, M.; Bengtsson, B. Microbial aetiology of acute clinical mastitis and agent-specific risk factors. Vet. Microbiol. 2009, 137, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertl, J.A.; Schukken, Y.H.; Welcome, F.L.; Tauer, L.W.; Gröhn, Y.T. Effects of pathogen-specific clinical mastitis on probability of conception in Holstein dairy cows. J. Dairy Sci. 2014, 97, 6942–6954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillerton, J.E.; Bramley, A.J.; Watson, C.A. The epidemiology of summer mastitis: A survey of clinical cases. Br. Vet. J. 1987, 143, 520–530. [Google Scholar] [CrossRef]
- Chirico, J.; Jonsson, P.; Kjellberg, S.; Thomas, G. Summer mastitis experimentally induced by Hydrotaea irritans exposed to bacteria. Med. Vet. Entomol. 1997, 11, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.; Aalbaek, B.; Hansen, J.W. Comparative bacteriological studies on summer mastitis in grazing cattle and pyogenes mastitis in stabled cattle in Denmark. Vet. Microbiol. 1992, 32, 81–88. [Google Scholar] [CrossRef]
- Madsen, M.; Høi Sørensen, G.; Aalbaek, B.; Hansen, J.W.; Bjørn, H. Summer mastitis in heifers: Studies on the seasonal occurrence of Actinomyces pyogenes, Peptostreptococcus indolicus and Bacteroidaceae in clinically healthy cattle in Denmark. Vet. Microbiol. 1992, 30, 243–255. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Chengappa, M.M. Liver abscesses in feedlot cattle: A review. J. Anim. Sci. 1998, 76, 287–298. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Lechtenberg, K.F. Liver abscesses in feedlot cattle. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 351–369. [Google Scholar] [CrossRef]
- Amachawadi, R.G.; Nagaraja, T.G. Liver abscesses in cattle: A review of incidence in Holsteins and of bacteriology and vaccine approaches to control in feedlot cattle. J. Anim. Sci. 2016, 94, 1620–1632. [Google Scholar] [CrossRef]
- Oberbauer, A.M.; Berry, S.L.; Belanger, J.M.; McGoldrick, R.M.; Pinos-Rodriquez, J.M.; Famula, T.R. Determining the heritable component of dairy cattle foot lesions. J. Dairy Sci. 2013, 96, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Kontturi, M.; Kujala, M.; Junni, R.; Malinen, E.; Seuna, E.; Pelkonen, S.; Soveri, T.; Simojoki, H. Survey of interdigital phlegmon outbreaks and their risk factors in free stall dairy herds in Finland. Acta Vet. Scand. 2017, 59, 46. [Google Scholar] [CrossRef] [PubMed]
- Bay, V.; Griffiths, B.; Carter, S.; Evans, N.J.; Lenzi, L.; Bicalho, R.C.; Oikonomou, G. 16S rRNA amplicon sequencing reveals a polymicrobial nature of complicated claw horn disruption lesions and interdigital phlegmon in dairy cattle. Sci. Rep. 2018, 8, 15529. [Google Scholar] [CrossRef] [PubMed]
- Power, H.T.; Rebhun, W.C. Bacterial endocarditis in adult dairy cattle. J. Am. Vet. Med. Assoc. 1983, 182, 806–808. [Google Scholar] [PubMed]
- Ertas, H.B.; Kilic, A.; Özbey, G.; Muz, A. Isolation of Arcanobacterium (Actinomyces) pyogenes from abscessed cattle kidney and identification by PCR, Turk. J. Vet. Anim. Sci. 2005, 29, 455–459. [Google Scholar]
- Hinton, M. Corynebacterium pyogenes and bovine abortion. J. Hyg. Camb. 1974, 72, 365–368. [Google Scholar] [CrossRef]
- Turner, G.V. A microbiological study of polyarthritis in slaughter pigs. J. S. Afr. Vet. Assoc. 1982, 53, 99–101. [Google Scholar] [PubMed]
- Høie, S.; Falk, K.; Lium, B.M. An abattoir survey of pneumonia and pleuritis in slaughter weight swine from 9 selected herds. IV. Bacteriological findings in chronic pneumonic lesions. Acta Vet. Scand. 1991, 32, 395–402. [Google Scholar]
- Hariharan, H.; MacDonald, J.; Carnat, B.; Bryenton, J.; Heaney, S. An investigation of bacterial causes of arthritis in slaughter hogs. J. Vet. Diagn. Investig. 1992, 4, 28–30. [Google Scholar] [CrossRef]
- Iwamatsu, S.; Morio, A.; Watanabe, K.; Ikeo, T.; Yamashita, T.; Yoshino, H. Suppurative cerebral lesions caused by infection with Actinomyces pyogenes in breeding swine. J. Jpn. Vet. Med. Assoc. 1986, 39, 238–242. [Google Scholar] [CrossRef]
- Dong, W.L.; Kong, L.C.; Wang, Y.; Gou, C.L.; Xu, B.; Ma, H.X.; Gao, Y.H. Aminoglycoside resistance of Trueperella pyogenes isolated from pigs in China. J. Vet. Med. Sci. 2017, 79, 1836–1839. [Google Scholar] [CrossRef]
- Jarosz, Ł.S.; Gradzki, Z.; Kalinowski, M. Trueperella pyogenes infections in swine: Clinical course and pathology. Pol. J. Vet. Sci. 2014, 17, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Pejsak, Z.; Markowska-Daniel, I.; Samorek, M.; Truszczyński, M. Wykrywanie i ocena właściwości krajowych izolatów Arcanobacterium pyogenes wyosobnionych od świń. Med. Weter. 2006, 62, 781–784. [Google Scholar]
- Tadayon, R.A.; Cheema, A.H.; Muhammed, S.I. Microorganisms associated with abscesses of sheep and goats in the south of Iran. Am. J. Vet. Res. 1980, 41, 798–802. [Google Scholar] [PubMed]
- Al Dughaym, A.M. Isolation of Serratia, Arcanobacterium and Burkholderia species from visceral and cutaneous abscesses in four emaciated ewes. Vet. Rec. 2004, 155, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chen, T.H.; Shyu, C.L.; Su, N.Y.; Chan, J.P. Disseminated abscessation complicated with bone marrow abscess caused by Arcanobacterium pyogenes in a goat. J. Vet. Med. Sci. 2010, 72, 1089–1092. [Google Scholar] [CrossRef]
- Barbour, E.K.; Itani, H.H.; Shaib, H.A.; Saade, M.F.; Sleiman, F.T.; Nour, A.A.; Harakeh, S. Koch’s postulate of Arcanobacterium pyogenes and its immunogenicity in local and imported Saanen goats. Vet. Ital. 2010, 46, 319–327. [Google Scholar]
- Raadsma, H.W.; Egerton, J.R. A review of footrot in sheep: Aetiology, risk factors and control methods. Livest. Sci. 2013, 156, 106–114. [Google Scholar] [CrossRef]
- Wani, A.H.; Verma, S.; Sharma, M.; Wani, A. Infectious lameness among migratory sheep and goats in north-west India, with particular focus on anaerobes. Rev. Sci. Tech. 2015, 34, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Stefańska, I.; Rzewuska, M.; Binek, M. Corynebacterium pseudotuberculosis—Zakażenia u zwierząt. Postęp. Mikrobiol. 2007, 46, 101–112. [Google Scholar]
- Lavin, S.; Marco, I.; Franch, J.; Abarca, L. Report of a case of pyogenic arthritis associated with Actinomyces pyogenes in a chamois (Rupicapra pyrenaica). Zentralbl. Veterinarmed. B 1998, 45, 251–253. [Google Scholar] [CrossRef]
- Seifi, H.A.; Saifzadeh, S.; Farshid, A.A.; Rad, M.; Farrokhi, F. Mandibular pyogranulomatous osteomyelitis in a Sannen goat. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2003, 50, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Kidanemariam, A.; Gouws, J.; van Vuuren, M.; Gummow, B. Ulcerative balanitis and vulvitis of Dorper sheep in South Africa: A study on its aetiology and clinical features. J. S. Afr. Vet. Assoc. 2005, 76, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Billington, S.J.; Post, K.W.; Jost, B.H. Isolation of Arcanobacterium (Actinomyces) pyogenes from cases of feline otitis externa and canine cystitis. J. Vet. Diagn. Investig. 2002, 14, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J.W.; van den Tweel, J.G. Hypertrophic osteopathy in a dog with a chronic lung abscess. J. Am. Vet. Med. Assoc. 1990, 196, 760–762. [Google Scholar]
- Dickie, C.W. Feline pyothorax caused by a Borrelia-like organism and Corynebacterium pyogenes. J. Am. Vet. Med. Assoc. 1979, 174, 516–517. [Google Scholar] [PubMed]
- Wareth, G.; El-Diasty, M.; Melzer, F.; Murugaiyan, J.; Abdulmawjood, A.; Sprague, L.D.; Neubauer, H. Trueperella pyogenes and Brucella abortus coinfection in a dog and a cat on a dairy farm in Egypt with recurrent cases of mastitis and abortion. Vet. Med. Int. 2018, 2018, 2056436. [Google Scholar] [CrossRef] [PubMed]
- Rampacci, E.; Passamonti, F.; Bottinelli, M.; Stefanetti, V.; Cercone, M.; Nannarone, S.; Gialletti, R.; Beccati, F.; Coletti, M.; Pepe, M. Umbilical infections in foals: Microbiological investigation and management. Vet. Rec. 2017, 180, 543. [Google Scholar] [CrossRef]
- Shahbazfar, A.A.; Kolahian, S.; Ashrafi Helan, J.; Mohammadpour, H. Multi abscessation with multinodular abscesses in a New Zealand white rabbit (Oryctolagus cuniculus) following Arcanobacterium pyogenes infection. Revue Méd. Vét. 2013, 164, 23–26. [Google Scholar]
- Barbour, E.K.; Brinton, M.K.; Caputa, A.; Johnson, J.B.; Poss, P.E. Characteristics of Actinomyces pyogenes involved in lameness of male turkeys in north-central United States. Avian Dis. 1991, 35, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Brinton, M.K.; Schellberg, L.C.; Johnson, J.B.; Frank, R.K.; Halvorson, D.A.; Newman, J.A. Description of osteomyelitis lesions associated with Actinomyces pyogenes infection in the proximal tibia of adult male turkeys. Avian Dis. 1993, 37, 259–262. [Google Scholar] [CrossRef]
- Priya, P.M.; Abhinay, G.; Ambily, R.; Siju, J. A Unique Case of Trueperella Pyogenes Causing Hepatic Abscesses in Pigeons. Int. J. Vet. Med. Res. Rep. 2013, 1–3. [Google Scholar] [CrossRef]
- Cohen, B.S.; Belser, E.H.; Keeler, S.P.; Yabsley, M.J.; Miller, K.V. A Headache from Our Past? Intracranial Abscess Disease, Virulence Factors of Trueperella Pyogenes, And A Legacy of Translocating White-Tailed Deer (Odocoileus Virginianus). J. Wildl. Dis. 2018, 54, 671–679. [Google Scholar] [CrossRef]
- Karns, G.R.; Lancia, R.A.; Deperno, C.S.; Conner, M.C.; Stoskopf, M.K. Intracranial abscessation as a natural mortality factor for adult male white-tailed deer (Odocoileus virginianus) in Kent County, Maryland, USA. J. Wildl. Dis. 2009, 45, 196–200. [Google Scholar] [CrossRef]
- Turner, M.M.; Deperno, C.S.; Conner, M.C.; Eyler, T.B.; Lancia, R.A.; Klaver, R.W.; Stoskopf, M.K. Habitat, wildlife, and one health: Arcanobacterium pyogenes in Maryland and Upper Eastern Shore white-tailed deer populations. Infect. Ecol. Epidemiol. 2013, 3, 19175. [Google Scholar] [CrossRef]
- Cohen, B.S.; Belser, E.H.; Keeler, S.P.; Yabsley, M.J.; Miller, K.V. Isolation and genotypic characterization of Trueperella (Arcanobacterium) pyogenes recovered from active cranial abscess infections of male white-tailed deer (Odocoileus virginianus). J. Zoo Wildl. Med. 2015, 46, 62–67. [Google Scholar] [CrossRef]
- Chirino-Trejo, M.; Woodbury, M.R.; Huang, F. Antibiotic sensitivity and biochemical characterization of Fusobacterium spp. and Arcanobacterium pyogenes isolated from farmed white-tailed deer (Odocoileus virginianus) with necrobacillosis. J. Zoo Wildl. Med. 2003, 34, 262–268. [Google Scholar] [CrossRef]
- Tell, L.A.; Brooks, J.W.; Lintner, V.; Matthews, T.; Kariyawasam, S. Antimicrobial susceptibility of Arcanobacterium pyogenes isolated from the lungs of white-tailed deer (Odocoileus virginianus) with pneumonia. J. Vet. Diagn. Investig. 2011, 23, 1009–1013. [Google Scholar] [CrossRef]
- Nettles, V.F.; Quist, C.F.; Lopez, R.R.; Wilmers, T.J.; Frank, P.; Roberts, W.; Chitwood, S.; Davidson, W.R. Morbidity and mortality factors in key deer (Odocoileus virginianus clavium). J. Wildl. Dis. 2002, 38, 685–692. [Google Scholar] [CrossRef]
- Wickhorst, J.P.; Hassan, A.A.; Sheet, O.H.; Eisenberg, T.; Sammra, O.; Alssahen, M.; Lämmler, C.; Prenger-Berninghoff, E.; Zschöck, M.; Timke, M.; et al. Trueperella pyogenes isolated from a brain abscess of an adult roebuck (Capreolus capreolus). Folia Microbiol. (Praha) 2018, 63, 17–22. [Google Scholar] [CrossRef]
- Badger, S.B. Infectious epiphysitis and valvular endocarditis in a red deer (Cervus elaphus). N. Z. Vet. J. 1982, 30, 17–18. [Google Scholar] [CrossRef]
- Muñoz Gutiérrez, J.F.; Sondgeroth, K.S.; Williams, E.S.; Montgomery, D.L.; Creekmore, T.E.; Miller, M.M. Infectious keratoconjunctivitis in free-ranging mule deer in Wyoming: A retrospective study and identification of a novel alphaherpesvirus. J. Vet. Diagn. Investig. 2018, 30, 663–670. [Google Scholar] [CrossRef]
- Zhao, K.; Tian, Y.; Yue, B.; Wang, H.; Zhang, X. Virulence determinants and biofilm production among Trueperella pyogenes recovered from abscesses of captive forest musk deer. Arch. Microbiol. 2013, 195, 203–209. [Google Scholar] [CrossRef]
- Rzewuska, M.; Stefańska, I.; Osińska, B.; Kizerwetter-Świda, M.; Chrobak, D.; Kaba, J.; Bielecki, W. Phenotypic characteristics and virulence genotypes of Trueperella (Arcanobacterium) pyogenes strains isolated from European bison (Bison bonasus). Vet. Microbiol. 2012, 160, 69–76. [Google Scholar] [CrossRef]
- Portas, T.J.; Bryant, B.R. Morbidity and mortality associated with Arcanobacterium pyogenes in a group of captive blackbuck (Antilope cervicapra). J. Zoo Wildl. Med. 2005, 36, 286–289. [Google Scholar] [CrossRef]
- Twomey, D.F.; Boon, J.D.; Sayers, G.; Schock, A. Arcanobacterium pyogenes septicemia in a southern pudu (Pudu puda) following uterine prolapse. J. Zoo Wildl. Med. 2010, 41, 158–160. [Google Scholar] [CrossRef]
- Franzen, D.; Lamberski, N.; Zuba, J.; Richardson, G.L.; Fischer, A.T.; Rantanen, N.W. Diagnosis and Medical and Surgical Management of Chronic Infectious Fibrinous Pleuritis in an Okapi (Okapia Johnstoni). J. Zoo Wildl. Med. 2015, 46, 427–430. [Google Scholar] [CrossRef]
- Zarnke, R.L.; Schlater, L.K. Abscesses in a free-ranging bison in Alaska. J. Wildl. Dis. 1984, 20, 151–152. [Google Scholar]
- Besser, T.E.; Frances Cassirer, E.; Highland, M.A.; Wolff, P.; Justice-Allen, A.; Mansfield, K.; Davis, M.A.; Foreyt, W. Bighorn sheep pneumonia: Sorting out the cause of a polymicrobial disease. Prev. Vet. Med. 2013, 108, 85–93. [Google Scholar] [CrossRef]
- Moustafa, A.M. First observation of camel (Camelus dromedarius) lymphadenitis in Libya. A case report. Rev. Elev. Med. Vet. Pays. Trop. 1994, 47, 313–314. [Google Scholar]
- Wareth, G.; Murugaiyan, J.; Khater, D.F.; Moustafa, S.A. Subclinical pulmonary pathogenic infection in camels slaughtered in Cairo, Egypt. J. Infect. Dev. Ctries. 2014, 8, 909–913. [Google Scholar] [CrossRef]
- Ali, A.; Derar, R.; Al-Sobayil, F.; Al-Hawas, A.; Hassanein, K. A retrospective study on clinical findings of 7300 cases (2007–2014) of barren female dromedaries. Theriogenology 2015, 84, 452–456. [Google Scholar] [CrossRef]
- Tarazi, Y.H.; Al-Ani, F.K. An outbreak of dermatophilosis and caseous lymphadenitis mixed infection in camels (Camelus dromedaries) in Jordan. J. Infect. Dev. Ctries. 2016, 10, 506–511. [Google Scholar] [CrossRef]
- Aschfalk, A.; Josefsen, T.D.; Steingass, H.; Müller, W.; Goethe, R. Crowding and winter emergency feeding as predisposing factors for kerato-conjunctivitis in semi-domesticated reindeer in Norway. Dtsch. Tierarztl. Wochenschr. 2003, 110, 295–298. [Google Scholar]
- Tryland, M.; das Neves, C.G.; Sunde, M.; Mørk, T. Cervid herpesvirus 2, the primary agent in an outbreak of infectious keratoconjunctivitis in semidomesticated reindeer. J. Clin. Microbiol. 2009, 47, 3707–3713. [Google Scholar] [CrossRef]
- Eisenberg, T.; Nagib, S.; Hijazin, M.; Alber, J.; Lämmler, C.; Hassan, A.A.; Timke, M.; Kostrzewa, M.; Prenger-Berninghoff, E.; Schauerte, N.; et al. Trueperella pyogenes as cause of a facial abscess in a grey slender loris (Loris lydekkerianus nordicus)—A case report. Berl. Munchener Tierarztliche Wochenschr. 2012, 125, 407–410. [Google Scholar]
- Nagib, S.; Glaeser, S.P.; Eisenberg, T.; Sammra, O.; Lämmler, C.; Kämpfer, P.; Schauerte, N.; Geiger, C.; Kaim, U.; Prenger-Berninghoff, E.; et al. Fatal infection in three Grey Slender Lorises (Loris lydekkerianus nordicus) caused by clonally related Trueperella pyogenes. BMC Vet. Res. 2017, 13, 273. [Google Scholar] [CrossRef]
- Ülbegi-Mohyla, H.; Hijazin, M.; Alber, J.; Lämmler, C.; Hassan, A.A.; Abdulmawjood, A.; Prenger-Berninghoff, E.; Weiss, R.; Zschöck, M. Identification of Arcanobacteriumpyogenes isolated by post mortem examinations of a bearded dragon and a gecko by phenotypic and genotypic properties. J. Vet. Sci. 2010, 11, 265–267. [Google Scholar] [CrossRef]
- Funke, G.; von Graevenitz, A.; Clarridge, J.E.; Bernard, K.A. Clinical microbiology of coryneform bacteria. Clin. Microbiol. Rev. 1997, 10, 125–159. [Google Scholar] [CrossRef]
- Jootar, P.; Gherunpong, V.; Saitanu, K. Corynebacterium pyogenes endocarditis. Report of a case with necropsy and review of the literature. J. Med. Assoc. Thail. 1978, 61, 596–601. [Google Scholar]
- Kavitha, K.; Latha, R.; Udayashankar, C.; Jayanthi, K.; Oudeacoumar, P. Three cases of Arcanobacterium pyogenes-associated soft tissue infection. J. Med. Microbiol. 2010, 59, 736–739. [Google Scholar] [CrossRef]
- Plamondon, M.; Martinez, G.; Raynal, L.; Touchette, M.; Valiquette, L. A fatal case of Arcanobacterium pyogenes endocarditis in a man with no identified animal contact: Case report and review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 663–666. [Google Scholar] [CrossRef]
- Chesdachai, S.; Larbcharoensub, N.; Chansoon, T.; Chalermsanyakorn, P.; Santanirand, P.; Chotiprasitsakul, D.; Ratanakorn, D.; Boonbaichaiyapruck, S. Arcanobacterium pyogenes endocarditis: A case report and literature review. Southeast. Asian. J. Trop. Med. Public Health 2014, 45, 142–148. [Google Scholar]
- Andy Tang, S.O.; Leong, T.S.; Ruixin, T.; Chua, H.H.; Chew, L.P. Thrombotic thrombocytopenic purpura-like syndrome associated with Arcanobacterium pyogenes endocarditis in a post-transplant patient: A case report. Med. J. Malaysia. 2018, 73, 344–346. [Google Scholar]
- Kotrajaras, R.; Tagami, H. Corynebacterium pyogenes. Its pathogenic mechanism in epidemic leg ulcers in Thailand. Int. J. Dermatol. 1987, 26, 45–50. [Google Scholar] [CrossRef]
- Drancourt, M.; Oulès, O.; Bouche, V.; Peloux, Y. Two cases of Actinomyces pyogenes infection in humans. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Hermida Amejeiras, A.; Romero Jung, P.; Cabarcos Ortiz De Barrón, A.; Treviño Castallo, M. One case of pneumonia with Arcanobacterium pyogenes. An. Med. Int. 2004, 21, 334–336. [Google Scholar]
- Nicholson, P.; Kiely, P.; Street, J.; Mahalingum, K. Septic arthritis due to Actinomyces pyogenes. Injury 1998, 29, 640–642. [Google Scholar] [CrossRef]
- Lynch, M.; O’Leary, J.; Murnaghan, D.; Cryan, B. Actinomyces pyogenes septic arthritis in a diabetic farmer. J. Infect. 1998, 37, 71–73. [Google Scholar] [CrossRef]
- Levy, C.E.; Pedro, R.J.; von Nowakonski, A.; Holanda, L.M.; Brocchi, M.; Ramo, M.C. Arcanobacterium pyogenes sepsis in farmer, Brazil. Emerg. Infect. Dis. 2009, 15, 1131–1132. [Google Scholar] [CrossRef]
- Gahrn-Hansen, B.; Frederiksen, W. Human infections with Actinomyces pyogenes (Corynebacterium pyogenes). Diagn. Microbiol. Infect. Dis. 1992, 15, 349–354. [Google Scholar] [CrossRef]
- Sullivan, D.C.; Chapman, S.W. Bacteria that masquerade as fungi: Actinomycosis/nocardia. Proc. Am. Thorac. Soc. 2010, 7, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Mochon, A.B.; Sussland, D.; Saubolle, M.A. Aerobic Actinomycetes of Clinical Significance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Prescott, J.F. Rhodococcus equi: An animal and human pathogen. Clin. Microbiol. Rev. 1991, 4, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Dorella, F.A.; Pacheco, L.G.; Oliveira, S.C.; Miyoshi, A.; Azevedo, V. Corynebacterium pseudotuberculosis: Microbiology, biochemical properties, pathogenesis and molecular studies of virulence. Vet. Res. 2006, 37, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Rzewuska, M.; Rodo, A.; Bielecki, W. Dermacentor reticulatus ticks as possible vectors of Trueperella pyogenes infection in European bison (Bison bonasus): Preliminary studies. J. Comp. Pathol. 2016, 154, 120. [Google Scholar] [CrossRef]
- Jost, B.H.; Songer, J.G.; Billington, S.J. An Arcanobacterium (Actinomyces) pyogenes mutant deficient in production of the pore-forming cytolysin pyolysin has reduced virulence. Infect. Immun. 1999, 67, 1723–1728. [Google Scholar] [PubMed]
- Billington, S.J.; Songer, J.G.; Jost, B.H. Molecular characterization of the pore-forming toxin, pyolysin, a major virulence determinant of Arcanobacterium pyogenes. Vet. Microbiol. 2001, 82, 261–274. [Google Scholar] [CrossRef]
- Rudnick, S.T.; Jost, B.H.; Songer, J.G.; Billington, S.J. The gene encoding pyolysin, the pore-forming toxin of Arcanobacterium pyogenes, resides within a genomic islet flanked by essential genes. FEMS Microbiol. Lett. 2003, 225, 241–247. [Google Scholar] [CrossRef]
- Alouf, J.E. Cholesterol-binding cytolytic protein toxins. Int. J. Med. Microbiol. 2000, 290, 351–356. [Google Scholar] [CrossRef]
- Heuck, A.P.; Moe, P.C.; Johnson, B.B. The cholesterol-dependent cytolysin family of gram-positive bacterial toxins. Subcell. Biochem. 2010, 51, 551–577. [Google Scholar] [PubMed]
- Ding, H.; Lämmler, C. Purification and further characterization of a haemolysin of Actinomyces pyogenes. Zentralbl. Veterinarmed. B 1996, 43, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R. Further studies on the toxin of Corynebacterium pyogenes. J. Pathol. Bacteriol. 1944, 56, 525–529. [Google Scholar] [CrossRef]
- Kume, T.; Tainaka, M.; Saito, H.; Hiruma, M.; Nishio, S.; Kashiwazaki, M.; Mitani, K.; Nakajima, Y. Research on experimental Corynebacterium pyogenes infections in pigs. Kitasato Arch. Exp. Med. 1983, 56, 119–135. [Google Scholar] [PubMed]
- Hu, Y.; Zhang, W.; Bao, J.; Wu, Y.; Yan, M.; Xiao, Y.; Yang, L.; Zhang, Y.; Wang, J. A chimeric protein composed of the binding domains of Clostridium perfringens phospholipase C and Trueperella pyogenes pyolysin induces partial immunoprotection in a mouse model. Res. Vet. Sci. 2016, 107, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, K.; Serizawa, A.; Hashimoto, N.; Kaidoh, T.; Takeuchi, S. Analysis of the functional domains of Arcanobacterium pyogenes pyolysin using monoclonal antibodies. Vet. Microbiol. 2001, 81, 235–242. [Google Scholar] [CrossRef]
- Jost, B.H.; Field, A.C.; Trinh, H.T.; Songer, J.G.; Billington, S.J. Tylosin resistance in Arcanobacterium pyogenes is encoded by an erm X determinant. Antimicrob. Agents Chemother. 2003, 47, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Liu, M.; Zhang, X.; Wang, H.; Yue, B. In vitro and in vivo expression of virulence genes in Trueperella pyogenes based on a mouse model. Vet. Microbiol. 2013, 163, 344–350. [Google Scholar] [CrossRef]
- Billington, S.J.; Jost, B.H.; Cuevas, W.A. The Arcanobacterium (Actinomyces) pyogenes hemolysin, pyolysin, is a novel member of the thiol-activated cytolysin family. J. Bacteriol. 1997, 179, 6100–6106. [Google Scholar] [CrossRef]
- Ikegami, M.; Hashimoto, N.; Kaidoh, T.; Sekizaki, T.; Takeuchi, S. Genetic and biochemical properties of a hemolysin (pyolysin) produced by a swine isolate of Arcanobacterium (Actinomyces) pyogenes. Microbiol. Immunol. 2000, 44, 1–7. [Google Scholar] [CrossRef]
- Funk, P.G.; Staats, J.J.; Howe, M.; Nagaraja, T.G.; Chengappa, M.M. Identification and partial characterization of an Actinomyces pyogenes hemolysin. Vet. Microbiol. 1996, 50, 129–142. [Google Scholar] [CrossRef]
- Billington, S.J.; Esmay, P.A.; Songer, J.G.; Jost, B.H. Identification and role in virulence of putative iron acquisition genes from Corynebacterium pseudotuberculosis. FEMS Microbiol. Lett. 2002, 208, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, K.; Matsunaga, K.; Higuchi, H.; Kaidoh, T.; Takeuchi, S. Effect of amino acid substitutions in the epitope regions of pyolysin from Arcanobacterium pyogenes. Vet. Microbiol. 2003, 91, 205–213. [Google Scholar] [CrossRef]
- Pokrajac, L.; Harris, J.R.; Sarraf, N.; Palmer, M. Oligomerization and hemolytic properties of the C-terminal domain of pyolysin, a cholesterol-dependent cytolysin. Biochem. Cell Biol. 2013, 91, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Wang, H.; Wang, B.; Zhang, Y.; Hu, Y.; Ma, B.; Wang, J. Replacing the 238th aspartic acid with an arginine impaired the oligomerization activity and inflammation-inducing property of pyolysin. Virulence 2018, 9, 1112–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Hu, Y.; Bao, J.; Xiao, Y.; Zhang, Y.; Yang, L.; Wang, J.; Zhang, W. Isoleucine 61 is important for the hemolytic activity of pyolysin of Trueperella pyogenes. Vet. Microbiol. 2016, 182, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Wang, H.; Ma, B.; Xu, L.; Wang, J.; Zhang, W. Identification of B-cell linear epitopes in domains 1-3 of pyolysin of Trueperella pyogenes using polyclonal antibodies. Vet. Microbiol. 2017, 210, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Pokrajac, L.; Baik, C.; Harris, J.R.; Sarraf, N.S.; Palmer, M. Partial oligomerization of pyolysin induced by a disulfide-tethered mutant. Biochem. Cell Biol. 2012, 90, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.R.; Lewis, R.J.; Baik, C.; Pokrajac, L.; Billington, S.J.; Palmer, M. Cholesterol microcrystals and cochleate cylinders: Attachment of pyolysin oligomers and domain 4. J. Struct. Biol. 2011, 173, 38–45. [Google Scholar] [CrossRef]
- Preta, G.; Jankunec, M.; Heinrich, F.; Griffin, S.; Sheldon, I.M.; Valincius, G. Tethered bilayer membranes as a complementary tool for functional and structural studies: The pyolysin case. Biochim. Biophys. Acta 2016, 1858, 2070–2080. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, B.; Liang, H.; Ma, B.; Wang, J.; Zhang, W. Determination of the expression of three fimbrial subunit proteins in cultured Trueperella pyogenes. Acta Vet. Scand. 2018, 60, 53. [Google Scholar] [CrossRef]
- Bisinotto, R.S.; Filho, J.C.O.; Narbus, C.; Machado, V.S.; Murray, E.; Bicalho, R.C. Identification of fimbrial subunits in the genome of Trueperella pyogenes and association between serum antibodies against fimbrial proteins and uterine conditions in dairy cows. J. Dairy Sci. 2016, 99, 3765–3776. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, G.; Valtulina, V.; Rindi, S.; Jost, B.H.; Speziale, P. Functional and structural properties of CbpA, a collagen-binding protein from Arcanobacterium pyogenes. Microbiology 2007, 153, 3380–3389. [Google Scholar] [CrossRef] [PubMed]
- Lämmler, C.; Ding, H. Characterization of Fibrinogen-binding Properties of Actinomyces pyogenes. J. Vet. Med. B 1994, 41, 588–596. [Google Scholar] [CrossRef]
- Esmay, P.A.; Billington, S.J.; Link, M.A.; Songer, J.G.; Jost, B.H. The Arcanobacterium pyogenes collagen-binding protein, CbpA, promotes adhesion to host cells. Infect. Immun. 2003, 71, 4368–4374. [Google Scholar] [CrossRef]
- Taylor, G. Sialidases: Structures, biological significance and therapeutic potential. Curr. Opin. Struct. Biol. 1996, 6, 830–837. [Google Scholar] [CrossRef]
- Pettigrew, M.M.; Fennie, K.P.; York, M.P.; Daniels, J.; Ghaffar, F. Variation in the Presence of Neuraminidase Genes among Streptococcus pneumoniae Isolates with Identical Sequence Types. Infect. Immun. 2006, 74, 3360–3365. [Google Scholar] [CrossRef]
- Moriyama, T.; Barksdale, L. Neuraminidase of Corynebacterium diphtheriae. J. Bacteriol. 1967, 9, 1565–1581. [Google Scholar]
- Soong, G.; Muir, A.; Gomez, M.I.; Waks, J.; Reddy, B.; Planet, P.; Singh, P.K.; Kanetko, Y.; Wolfgang, M.C.; Hsiao, Y.S.; et al. Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J. Clin. Investig. 2006, 116, 2297–2305. [Google Scholar] [CrossRef] [Green Version]
- Moncla, B.J.; Braham, P.; Hillier, S.L. Sialidase (neuraminidase) activity among gram-negative anaerobic and capnophilic bacteria. J. Clin. Microbiol. 1990, 28, 422–425. [Google Scholar] [Green Version]
- Crennell, S.J.; Garman, E.F.; Philippon, C.; Vasella, A.; Laver, W.G.; Vim, E.R.; Taylor, G.L. The structures ofSalmonella typhimurium LT2 neuraminidase and its complexes with three inhibitors at high resolution. J. Mol. Biol. 1996, 259, 264–280. [Google Scholar] [CrossRef]
- Ada, G.L.; French, E.L.; Lind, P.E. Purification and properties of neuraminidase from Vibrio cholera. J. Gen. Microbiol. 1961, 24, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Godoy, V.G.; Dallas, M.M.; Russo, T.A.; Malamy, M.H. A role for Bacteroides fragilis neuraminidase in bacterial growth in two model systems. Infect. Immun. 1993, 61, 4415–4426. [Google Scholar] [PubMed]
- Schaufuss, P.; Lämmler, C. Characterization of Extracellular Neuraminidase produced by Actinomyces pyogenes. Zentralblatt. Bakteriol. 1989, 271, 28–35. [Google Scholar] [CrossRef]
- Jost, B.H.; Songer, J.G.; Billington, S.J. Cloning, expression, and characterization of a neuraminidase gene from Arcanobacterium pyogenes. Infect. Immun. 2001, 69, 4430–4437. [Google Scholar] [CrossRef] [PubMed]
- Jost, B.H.; Songer, J.G.; Billington, S.J. Identification of a second Arcanobacterium pyogenes neuraminidase and involvement of neuraminidase activity in host cell adhesion. Infect. Immun. 2002, 70, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, D.; Turutoglu, H.; Pehlivanoglu, F.; Guler, L. Virulence Genes, Biofilm Production and Antibiotic Susceptibility in Trueperella pyogenes isolated from Cattle. Isr. J. Vet. Med. 2016, 71, 36–42. [Google Scholar]
- Zhang, S.; Qiu, J.; Yang, R.; Shen, K.; Xu, G.; Fu, L. Complete genome sequence of Trueperella pyogenes, isolated from infected farmland goats. Genome Announc. 2016, 4, e01421-16. [Google Scholar] [CrossRef]
- Kasimanickam, V.R.; Owen, K.; Kasimanickam, R.K. Detection of genes encoding multidrug resistance and biofilm virulence factor in uterine pathogenic bacteria in postpartum dairy cows. Theriogenology 2016, 85, 173–179. [Google Scholar] [CrossRef]
- da Silva Duarte, V.; Dias, R.S.; Kropinski, A.M.; da Silva Xavier, A.; Ferro, C.G.; Vidigal, P.M.P.; da Silva, C.C.; de Paula, S.O. A T4virus prevents biofilm formation by Trueperella pyogenes. Vet. Microbiol. 2018, 218, 45–51. [Google Scholar] [CrossRef]
- Zschiedrich, C.P.; Keidel, V.; Szurmant, H. Molecular Mechanisms of Two-Component Signal Transduction. J. Mol. Biol. 2016, 428, 3752–3775. [Google Scholar] [CrossRef] [Green Version]
- Herrou, J.; Crosson, S.; Fiebig, A. Structure and function of HWE/HisKA2-family sensor histidine kinases. Curr. Opin. Microbiol. 2017, 36, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hupało-Sikorska, A.; Gregorczyk, K.P.; Rzewuska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Gieryńska, M. Involvement of macrophages during Trueperella pyogenes infection. In Proceedings of XVIII Conference DIAGMOL 2017—Molecular Biology in Diagnostics of Infectious Diseases and Biotechnology; Warsaw University of Life Sciences: Warsaw, Poland, 2017; pp. 45–46. [Google Scholar]
- Machado, V.S.; Bicalho, R.C. Complete genome sequence of Trueperella pyogenes, an important opportunistic pathogen of livestock. Genome Announc. 2014, 2, e00400-14. [Google Scholar] [CrossRef] [PubMed]
- da Silva Duarte, V.; Dias, R.S.; Kropinski, A.M.; Vidigal, P.M.P.; Sousa, F.O.; da Silva Xavier, A.; da Silva, C.C.; de Paula, S.O. Complete genome sequence of vB_EcoM_UFV13, a new bacteriophage able to disrupt Trueperella pyogenes biofilm. Genome Announc. 2016, 4, e01292-16. [Google Scholar]
- Bernier, A.M.; Bernard, K. Draft genome sequence of Trueperella bernardiae LCDC 89-0504T, isolated from a human blood culture. Genome Announc. 2016, 4, e01634-15. [Google Scholar] [PubMed]
- Billington, S.J.; Jost, B.H.; Songer, J.G. The Arcanobacterium (Actinomyces) pyogenes plasmid pAP1 is a member of the pIJ101/pJV1 family of rolling circle replication plasmids. J. Bacteriol. 1998, 180, 3233–3236. [Google Scholar]
- Tauch, A.; Götker, S.; Pühler, A.; Kalinowski, J.; Thierbach, G. The 27.8-kb R-plasmid pTET3 from Corynebacterium glutamicum encodes the aminoglycoside adenyltransferase gene cassette aadA9 and the regulated tetracycline efflux system Tet 33 flanked by active copies of the widespread insertion sequence IS6100. Plasmid 2002, 48, 117–129. [Google Scholar] [CrossRef]
- Lovell, R.; Foggie, A.; Pearson, J.K. Field trials with Corynebacterium pyogenes alum-precipitated toxoid. J. Comp. Pathol. 1950, 60, 225–229. [Google Scholar] [CrossRef]
- Hunter, P.; van der Lugt, J.J.; Gouws, J.J.; Onderstepoort, J. Failure of an Actinomyces pyogenes vaccine to protect sheep against an intravenous challenge. Vet. Res. 1990, 57, 239–241. [Google Scholar]
- Kostro, K.; Lisiecka, U.; Żmuda, A.; Niemczuk, K.; Stojecki, K.; Taszkun, I. Selected parameters of immune response in pregnant sows vaccinated against Trueperella pyogenes. Bull. Vet. Inst. Pulawy 2014, 58, 17–21. [Google Scholar] [CrossRef]
- Machado, V.S.; Bicalho, M.L.; Meira Junior, E.B.; Rossi, R.; Ribeiro, B.L.; Lima, S.; Santos, T.; Kussler, A.; Foditsch, C.; Ganda, E.K.; et al. Subcutaneous immunization with inactivated bacterial components and purified protein of Escherichia coli, Fusobacterium necrophorum and Trueperella pyogenes prevents puerperal metritis in Holstein dairy cows. PLoS ONE 2014, 9, e91734. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Wang, B.; Ma, B.; Wang, J. A combined Clostridium perfringens/Trueperella pyogenes inactivated vaccine induces complete immunoprotection in a mouse model. Biologicals 2017, 47, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhao, K.; Zhang, Z.; Tang, C.; Zhang, X.; Yue, B. DNA vaccination based on pyolysin co-immunized with IL-1β enhances host antibacterial immunity against Trueperella pyogenes infection. Vaccine 2016, 34, 3469–3477. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, X.; Jing, J.; Zhao, K.; Shen, Y.; Zhang, X.; Yue, B. Chitosan-DNA nanoparticles enhanced the immunogenicity of multivalent DNA vaccination on mice against Trueperella pyogenes infection. J. Nanobiotechnol. 2018, 16, 8. [Google Scholar] [CrossRef] [PubMed]






| Strain Designation | Strain Origin | Size (Mb) | GC% | Number of | GenBank Accession nr | |||
|---|---|---|---|---|---|---|---|---|
| Genes | CDS | RNA | Pseudogenes | |||||
| TP4 | swine | 2.43 | 59.4 | 2202 | 2102 | 59 | 41 | CP033905.1 |
| TP3 | swine | 2.38 | 59.4 | 2156 | 2058 | 58 | 40 | CP033904.1 |
| TP2 | cattle | 2.25 | 59.7 | 2023 | 1938 | 58 | 27 | CP033903.1 |
| TP1 | cattle | 2.33 | 59.8 | 2125 | 1993 | 58 | 74 | CP033902.1 |
| TP-2849 | swine | 2.38 | 59.4 | 2158 | 2063 | 58 | 37 | CP029004.1 |
| TP4479 | swine | 2.38 | 59.4 | 2153 | 2058 | 58 | 37 | CP029001.1 |
| Arash114 | water buffalo | 2.34 | 59.5 | 2145 | 2054 | 56 | 35 | CP028833.1 |
| 2012CQ-ZSH | goat | 2.30 | 59.7 | 2050 | 1806 | 53 | 191 | CP012649.1 |
| TP8 | forest musk deer | 2.27 | 59.6 | 2091 | 2001 | 50 | 40 | CP007003.1 |
| TP6375 | cattle | 2.34 | 59.5 | 2082 | 1984 | 53 | 45 | CP007519.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzewuska, M.; Kwiecień, E.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Stefańska, I.; Gieryńska, M. Pathogenicity and Virulence of Trueperella pyogenes: A Review. Int. J. Mol. Sci. 2019, 20, 2737. https://doi.org/10.3390/ijms20112737
Rzewuska M, Kwiecień E, Chrobak-Chmiel D, Kizerwetter-Świda M, Stefańska I, Gieryńska M. Pathogenicity and Virulence of Trueperella pyogenes: A Review. International Journal of Molecular Sciences. 2019; 20(11):2737. https://doi.org/10.3390/ijms20112737
Chicago/Turabian StyleRzewuska, Magdalena, Ewelina Kwiecień, Dorota Chrobak-Chmiel, Magdalena Kizerwetter-Świda, Ilona Stefańska, and Małgorzata Gieryńska. 2019. "Pathogenicity and Virulence of Trueperella pyogenes: A Review" International Journal of Molecular Sciences 20, no. 11: 2737. https://doi.org/10.3390/ijms20112737
APA StyleRzewuska, M., Kwiecień, E., Chrobak-Chmiel, D., Kizerwetter-Świda, M., Stefańska, I., & Gieryńska, M. (2019). Pathogenicity and Virulence of Trueperella pyogenes: A Review. International Journal of Molecular Sciences, 20(11), 2737. https://doi.org/10.3390/ijms20112737