In Vivo Comparative Study on Acute and Sub-acute Biological Effects Induced by Ultrafine Particles of Different Anthropogenic Sources in BALB/c Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. DEP Induced Higher Inflammatory Response than BB in BALf of Treated Mice
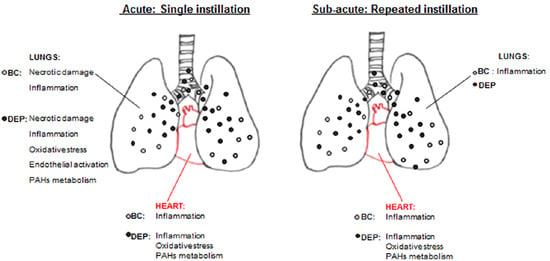
2.2. Both UFPs Induced Inflammatory Response in Lung and Heart Parenchyma of Treated Mice
2.3. DEP Induced Higher Oxidative Stress Response than BB in Lung and Heart Parenchyma of Treated Mice
2.4. The Interdependence between Oxidative Stress and Inflammation Following DEP-Exposure
3. Materials and Methods
3.1. UFPs Characterization
3.2. Animals and Treatments
3.2.1. Animal Housing
3.2.2. Intratracheal Instillation
3.3. Bronchoalveolar Lavage Fluid Analysis
3.3.1. Cell Counts
3.3.2. Biochemical Analyses on cell-free BALf Supernatants
3.4. Lung and Heart Homogenization
3.5. Histopathological Analysis
3.6. Electrophoresis and Immunoblotting
3.7. Fluorescence Molecular Tomography
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Air Pollution and Child Health: Prescribing Clean Air; WHO/CED/PHE/18.01; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/ceh/publications/Advance-copy-Oct24_18150_Air-Pollution-and-Child-Health-merged-compressed.pdf?ua=1 in press (accessed on 10 April 2019).
- Cassee, F.R.; Mills, N.L.; Newby, D.E. Cardiovascular Effects of Inhaled Ultrafine and Nano-Sized Particles; John Wiley & Sons: Hoboken, NJ, USA, 2011; p. 585. ISBN 978-0-470-43353-9. [Google Scholar]
- Bai, N.; Khazaei, M.; Van Eeden, S.F.; Laher, I. The pharmacology of particulate matter air pollution-induced cardiovascular dysfunction. Pharmacol Ther. 2007, 113, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; Pope, C.A., 3rd; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Shannahan, J.H.; Kodavanti, U.P.; Brown, J.M. Manufactured and airborne nanoparticle cardiopulmonary interactions: A review of mechanisms and the possible contribution of mast cells. Inhal. Toxicol. 2012, 24, 320–339. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.Y.; Wang, J.S.; Chao, M.W. Causation by Diesel Exhaust Particles of Endothelial Dysfunctions in Cytotoxicity, Pro-inflammation, Permeability, and Apoptosis Induced by ROS Generation. Cardiovasc. Toxicol. 2017, 17, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Stone, V.; Seaton, A.; MacNee, W. Ambient particle inhalation and the cardiovascular system: Potential mechanisms. Environ. Health Perspect. 2001, 109, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Manigrasso, M.; Vernale, C.; Avino, P. Traffic aerosol lobar doses deposited in the human respiratory system. Environ. Sci. Pollut. Res. Int. 2017, 24, 13866–13873. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Tamrakar, S.; Aglawe, A.; Lad, H.; Srivastava, R.K.; Mishra, D.K.; Tiwari, R.; Chaudhury, K.; Goryacheva, I.Y.; Mishra, P.K. Ultrafine particulate matter impairs mitochondrial redox homeostasis and activates phosphatidylinositol 3-kinase mediated DNA damage responses in lymphocytes. Environ. Pollut. 2018, 234, 406–419. [Google Scholar] [CrossRef]
- Heinzerling, A.; Hsu, J.; Yip, F. Respiratory Health Effects of Ultrafine Particles in Children: A Literature Review. Water Air Soil Pollut. 2016, 227, 32. [Google Scholar] [CrossRef]
- Dye, J.A.; Lehmann, J.R.; McGee, J.K.; Winsett, D.W.; Ledbetter, A.D.; Everitt, J.I.; Ghio, A.J.; Costa, D.L. Acute pulmonary toxicity of particulate matter filter extracts in rats: Coherence with epidemiologic studies in Utah Valley residents. Environ. Health Perspect. 2001, 109, 395–403. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Nel, A.E. Impairment of mitochondrial function by particulate matter (PM) and their toxic components: Implications for PM-induced cardiovascular and lung disease. Front. Biosci. 2007, 12, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, W.; Zhang, R.; Liu, P.; Wang, Q.; Shang, Y.; Wu, M.; Donaldson, K.; Wang, Q. Comparison of cellular toxicity caused by ambient ultrafine particles and engineered metal oxide nanoparticles. Part. Fibre Toxicol. 2015, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Arpa Inemar. Inemar Inventory Emission of Air Pollutants for Lombardy Region [WWW Document]. 2012. Available online: http://inemar.arpalombardia.it/inemar/webdata/main.seam (accessed on 10 April 2019).
- Maricq, M.M. Chemical characterization of particulate emissions from diesel engines: A review. J. Aerosol Sci. 2007, 38, 1079–1118. [Google Scholar] [CrossRef]
- Zheng, M.; Cass, G.R.; Ke, L.; Wang, F.; Schauer, J.J.; Edgerton, E.S.; Russell, A.G. Source apportionment of daily fine particulate matter at Jefferson Street, Atlanta, GA, during summer and winter. J. Air Waste Manag. Assoc. 2007, 57, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Bisig, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Diesel Exhaust: Current knowledge of adverse effects and underlying cellular mechanisms. Arch. Toxicol. 2016, 90, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Liati, A.; Eggenschwiler, P.D. Characterization of particulate matter deposited in diesel particulate filters: Visual and analytical approach in macro-, micro- and nano-scales. Combust. Flame 2010, 157, 1658–1670. [Google Scholar] [CrossRef]
- Jones, P.; Higenbottam, T. Quantifying of severity of exacerbations in chronic obstructive pulmonary disease: Adaptations to the definition to allow quantification. Proc. Am. Thorac. Soc. 2007, 4, 597–601. [Google Scholar] [CrossRef]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke health effects: A review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Vecchi, R.; Marabini, L.; Fermo, P.; Becagli, S.; Bernardoni, V.; Caruso, D.; Corbella, L.; Dell’Acqua, M.; Galli, C.L.; et al. The chemical composition of ultrafine particles and associated biological effects at an alpine town impacted by wood burning. Sci. Total. Environ. 2017, 587, 223–231. [Google Scholar] [CrossRef]
- Mantecca, P.; Sancini, G.; Moschini, E.; Farina, F.; Gualtieri, M.; Rohr, A.; Miserocchi, G.; Palestini, P.; Camatini, M. Lung toxicity induced by intratracheal instillation of size-fractionated tire particles. Toxicol. Lett. 2009, 189, 206–214. [Google Scholar] [CrossRef]
- Mazzoli-Rocha, F.; Magalhães, C.B.; Malm, O.; Saldiva, P.H.; Zin, W.A.; Faffe, D.S. Comparative respiratory toxicity of particles produced by traffic and sugar cane burning. Environ. Res. 2008, 108, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Zia, S.; Marzouqi, F.; Al-Dhaheri, A.; Subramaniyan, D.; Dhanasekaran, S.; Yasin, J.; Ali, B.H.; Kazzam, E.E. Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Br. J. Pharmacol. 2011, 164, 1871–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unosson, J.; Blomberg, A.; Sandström, T.; Muala, A.; Boman, C.; Nyström, R.; Westerholm, R.; Mills, N.L.; Newby, D.E.; Langrish, J.P.; et al. Exposure to wood smoke increases arterial stiffness and decreases heart rate variability in humans. Part. Fibre Toxicol. 2013, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Seagrave, J.; McDonald, J.D.; Reed, M.D.; Seilkop, S.K.; Mauderly, J.L. Responses to subchronic inhalation of low concentrations of diesel exhaust and hardwood smoke measured in rat bronchoalveolar lavage fluid. Inhal. Toxicol. 2005, 17, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Kocbach, A.; Namork, E.; Schwarze, P.E. Pro-inflammatory potential of wood smoke and traffic-derived particles in a monocytic cell line. Toxicology 2008, 247, 123–132. [Google Scholar] [CrossRef]
- Totlandsdal, A.I.; Øvrevik, J.; Cochran, R.E.; Herseth, J.-I.; Bølling, A.K.; Låg, M.; Schwarze, P.; Lilleaas, E.; Holme, J.A.; Kubátová, A. The occurrence of polycyclic aromatic hydrocarbons and their derivatives and the proinflammatory potential of fractionated extracts of diesel exhaust and wood smoke particles. J. Environ. Sci. Health Part A Tox. Hazard. Subst. Environ. Eng. 2014, 49, 383–396. [Google Scholar] [CrossRef]
- Farina, F.; Sancini, G.; Battaglia, C.; Tinaglia, V.; Mantecca, P.; Camatini, M.; Palestini, P. Milano summer particulate matter (PM10) triggers lung inflammation and extra pulmonary adverse events in mice. PLoS ONE 2013, 8, e56636. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Corsetto, P.A.; Farina, F.; Montorfano, G.; Pani, G.; Battaglia, C.; Sancini, G.; Palestini, P. Repeated intratracheal instillation of PM10 induces lipid reshaping in lung parenchyma and in extra-pulmonary tissues. PLoS ONE 2014, 9, e106855. [Google Scholar] [CrossRef]
- Sancini, G.; Farina, F.; Battaglia, C.; Cifola, I.; Mangano, E.; Mantecca, P.; Camatini, M.; Palestini, P. Health risk assessment for air pollutants: Alterations in lung and cardiac gene expression in mice exposed to Milano winter fine particulate matter (PM2.5). PLoS ONE 2014, 9, e109685. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, B.; Ke, W.; Feng, B.; Lin, H.; Xiao, J.; Zeng, W.; Li, X.; Tao, J.; Yang, Z.; et al. Associations of Short-Term and Long-Term Exposure to Ambient Air Pollutants with Hypertension: A Systematic Review and Meta-Analysis. Hypertension 2016, 68, 62–70. [Google Scholar] [CrossRef]
- Byrne, A.J.; Mathie, S.A.; Gregory, L.G.; Lloyd, C.M. Pulmonary macrophages: Key players in the innate defence of the airways. Thorax 2015, 70, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Ishii, H.; Hogg, J.C.; Shih, C.H.; Yatera, K.; Vincent, R.; Van Eeden, S.F. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am. J. Respir. Crit. Care Med. 2004, 170, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, H. Alveolar epithelial type II cell: Defender of the alveolus revisited. Respir. Res. 2001, 2, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Rydell-Törmänen, K.; Uller, L.; Erjefält, J.S. Neutrophil cannibalism—A backup when the macrophage clearance system is insufficient. Respir. Res. 2006, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, Composition, and Lung Diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, S.; Zee, J.M.; Patel, K.D. Regulation of matrix metalloproteinase-9 (MMP-9) in TNF-stimulated neutrophils: Novel pathways for tertiary granule release. J. Leukoc. Biol. 2005, 79, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Löffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- Gupta, R.C. Biomarkers in Toxicology; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-12-404630-6. [Google Scholar]
- Henderson, R.F. Use of bronchoalveolar lavage to detect respiratory tract toxicity of inhaled material. Exp. Toxicol. Pathol. 2005, 57, 155–159. [Google Scholar] [CrossRef]
- Fagan, K.A.; McMurtry, I.F.; Rodman, D.M. Role of endothelin-1 in lung disease. Respir. Res. 2001, 2, 90–101. [Google Scholar] [CrossRef]
- Fielding, C.A.; McLoughlin, R.M.; McLeod, L.; Colmont, C.S.; Najdovska, M.; Grail, D.; Ernst, M.; Jones, S.A.; Topley, N.; Jenkins, B.J. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J. Immunol. 2008, 181, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, T.; Izumi, H.; Yoshiura, Y.; Myojo, T.; Oyabu, T.; Lee, B.W.; Okada, T.; Marui, T.; Wang, K.Y.; Kubo, M.; et al. Usefulness of myeloperoxidase as a biomarker for the ranking of pulmonary toxicity of nanomaterials. Part. Fibre Toxicol. 2018, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.K.; Yoon, H.K.; Jee, B.K.; Ko, H.J.; Lee, K.H.; Kim, H.J.; Lim, Y. COX-2 expression and inflammatory effects by diesel exhaust particles in vitro and in vivo. Toxicol. Lett. 2008, 176, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Tranfield, E.M.; Kavanagh, T.J.; Kaufman, J.D.; Rosenfeld, M.E.; Van Eeden, S.F. Exposure to diesel exhaust upregulates COX-2 expression in ApoE knockout mice. Inhal. Toxicol. 2012, 24, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Bromberg, P.A.; Samet, J.M. COX-2 expression induced by diesel particles involves chromatin modification and degradation of HDAC1. Am. J. Respir. Cell Mol. Biol. 2007, 37, 232–239. [Google Scholar] [CrossRef]
- Marcet, B.; Libert, F.; Boeynaems, J.M.; Communi, D. Extracellular nucleotides induce COX-2 up-regulation and prostaglandin E2 production in human A549 alveolar type II epithelial cells. Eur. J. Pharmacol. 2007, 566, 167–171. [Google Scholar] [CrossRef]
- Kawabe, J.; Ushikubi, F.; Hasebe, N. Prostacyclin in vascular diseases. Recent insights and future perspectives. Circ. J. 2010, 74, 836–843. [Google Scholar] [CrossRef]
- Barbieri, S.S.; Weksler, B.B. Tobacco smoke cooperates with interleukin-1β to alter β-catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenase-2 expression in vitro and in vivo. FASEB J. 2007, 21, 1831–1843. [Google Scholar] [CrossRef]
- Schindhelm, R.K.; Van Der Zwan, L.P.; Teerlink, T.; Scheffer, P.G. Myeloperoxidase: A Useful Biomarker for Cardiovascular Disease Risk Stratification? Clin. Chem. 2009, 55, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Morrow, D.A.; Sabatine, M.S.; Brennan, M.L.; De Lemos, J.A.; Murphy, S.A.; Ruff, C.T.; Rifai, N.; Cannon, C.P.; Hazen, S.L. Concurrent evaluation of novel cardiac biomarkers in acute coronary syndrome: Myeloperoxidase and soluble CD40 ligand and the risk of recurrent ischaemic events in TACTICS-TIMI 18. Eur. Heart J. 2008, 29, 1096–1102. [Google Scholar] [CrossRef]
- Latronico, T.; Branà, M.T.; Merra, E.; Fasano, A.; Di Bari, G.; Casalino, E.; Liuzzi, G.M. Impact of manganese neurotoxicity on MMP-9 production and superoxide dismutase activity in rat primary astrocytes. Effect of resveratrol and therapeutical implications for the treatment of CNS diseases. Toxicol. Sci. 2013, 135, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Longhin, E.; Gualtieri, M.; Capasso, L.; Bengalli, R.; Mollerup, S.; Holme, J.A.; Øvrevik, J.; Casadei, S.; Di Benedetto, C.; Parenti, P.; et al. Physico-chemical properties and biological effects of diesel and biomass particles. Environ. Pollut. 2016, 215, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Huertero, F.; Apàtiga-Vega, E.; Miguel-Pèrez, G.; Villeda Cuevas, D.; Trillo-Trinoco, J. Molecular Markers Associated with the Biological Response to Aromatic Hydrocarbons from Urban Air in Humans. In Air Pollution—New Developments; Moldoveanu, A.M., Ed.; InrechOpen: London, UK, 2011; ISBN 978-953-307-527-3. [Google Scholar] [Green Version]
- Nebert, D.W.; Dalton, P.T. The role of cytochrome p-450 enzymes in endogenous signaling pathways and environmental carcinogenesis. Nat. Rev. Cancer 2006, 6, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Gerlofs-Nijland, M.E.; Rummelhard, M.; Boere, A.J.; Leseman, D.L.; Duffin, R.; Schins, R.P.; Borm, P.J.; Sillanpää, M.; Salonen, R.O.; Cassee, F.R. Particle induced toxicity in relation to transition metal and polycyclic aromatic hydrocarbon contents. Environ. Sci. Technol. 2009, 43, 4729–4736. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, C.J.; Padalko, E. iNOS (NOS2) at a glance. J. Cell Sci. 2004, 117, 2865–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, B.M.; Pae, H.O.; Chung, H.T. Nitric oxide priming protects nitric oxide-mediated apoptosis via heme oxygenase-1 induction. Free Radic. Biol. Med. 2003, 34, 1136–1145. [Google Scholar] [CrossRef]
- Farina, F.; Sancini, G.; Mantecca, P.; Gallinotti, D.; Camatini, M.; Palestini, P. The acute toxic effects of particulate matter in mouse lung are related to size and season of collection. Toxicol. Lett. 2011, 202, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Watterson, T.L.; Hamilton, B.; Martin, R.; Coulombe, R.A. Urban Particulate Matter Causes ER Stress and the Unfolded Protein Response in Human Lung Cells. Toxicol. Sci. 2009, 112, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binková, B.; Srám, R.J.; Rössner, P., Jr. Heat shock proteins hsp32 and hsp70 as biomarkers of an early response? In vitro induction of heat shock proteins after exposure of cell culture to carcinogenic compounds and their real mixtures. Mutat. Res. 2003, 542, 105–116. [Google Scholar]
- Graff, D.W.; Cascio, W.E.; Brackhan, J.A.; Devlin, R.B. Metal particulate matter components affect gene expression and beat frequency of neonatal rat ventricular myocytes. Environ. Health Perspect. 2004, 112, 792–798. [Google Scholar] [CrossRef]
- Gerde, P.; Muggenburg, B.A.; Lundborg, M.; Dahl, A.R. The rapid alveolar absorption of diesel soot-adsorbed benzo[a]pyrene: Bioavailability, metabolism and dosimetry of an inhaled particle-borne carcinogen. Carcinogenesis 2001, 22, 741–749. [Google Scholar] [CrossRef]
- Kure, E.H.; Andreassen, A.; Ovrebø, S.; Grzybowska, E.; Fiala, Z.; Strózyk, M.; Chorazy, M.; Haugen, A. Benzo(a)pyrene-albumin adducts in humans exposed to polycyclic aromatic hydrocarbons in an industrial area of Poland. Occup. Environ. Med. 1997, 54, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Grollino, M.G.; Consales, C.; Costabile, F.; Manigrasso, M.; Avino, P.; Aufderheide, M.; Cordelli, E.; Di Liberto, L.; Petralia, E.; et al. Is it the time to study air pollution effects under environmental conditions? A case study to support the shift of in vitro toxicology from the bench to the field. Chemosphere 2018, 207, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Godschalk, R.; Alexandrov, K.; Cascorbi, I.; Kriek, E.; Ostertag, J.; Van Schooten, F.J.; Bartsch, H. Myeloperoxidase–463A variant reduces benzo[a]pyrene diol epoxide DNA adducts in skin of coal tar treated patients. Carcinogenesis 2001, 22, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Fialkow, L.; Wang, Y.; Downey, G.P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 2007, 42, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Mantecca, P.; Farina, F.; Moschini, E.; Gallinotti, D.; Gualtieri, M.; Rohr, A.; Sancini, G.; Palestini, P.; Camatini, M. Comparative acute lung inflammation induced by atmospheric PM and size-fractionated tire particles. Toxicol. Lett. 2010, 198, 244–254. [Google Scholar] [CrossRef]
- Kaewamatawong, T.; Shimada, A.; Morita, T.; Banlunara, W.; Bintvihok, A. Acute and Subacute Pulmonary Effects of Diesel Exhaust Particles in Mice: Pathological Changes and Translocation Pathways to the Circulation. Thai J. Vet. Med. 2009, 39, 311–318. [Google Scholar]
- Win-Shwe, T.T.; Fujitani, Y.; Sone, H.; Furuyama, A.; Nitta, H.; Hirano, S. Effects of acute single intranasal instillation of secondary organic aerosol on neurological and immunological biomarkers in the brain and lung of BALB/c mice. J. Toxicol. Sci. 2013, 38, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Happo, M.S.; Salonen, R.O.; Hälinen, A.I.; Jalava, P.I.; Pennanen, A.S.; Dormans, J.A.; Gerlofs-Nijland, M.E.; Cassee, F.R.; Kosma, V.M.; Sillanpää, M.; et al. Inflammation and tissue damage in mouse lung by single and repeated dosing of urban air coarse and fine particles collected from six European cities. Inhal. Toxicol. 2010, 22, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Stoeger, T.; Takenaka, S.; Frankenberger, B.; Ritter, B.; Karg, E.; Maier, K.; Schulz, H.; Schmid, O. Deducing in vivo toxicity of combustion-derived nanoparticles from a cell-free oxidative potency assay and metabolic activation of organic compounds. Environ. Health Perspect. 2009, 117, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Stoeger, T.; Reinhard, C.; Takenaka, S.; Schroeppel, A.; Karg, E.; Ritter, B.; Heyder, J.; Schulz, H. Instillation of Six Different Ultrafine Carbon Particles Indicates a Surface Area Threshold Dose for Acute Lung Inflammation in Mice. Environ. Health Perspect. 2006, 114, 328–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmour, P.S.; Schladweiler, M.C.; Richards, J.H.; Ledbetter, A.D.; Kodavanti, U.P. Hypertensive rats are susceptible to TLR4-mediated signaling following exposure to combustion source particulate matter. Inhal. Toxicol. 2004, 16, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Afgan, E.; Baker, D.; Batut, B.; Van Den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golan, R.; Ladva, C.; Greenwald, R.; Krall, J.R.; Raysoni, A.U.; Kewada, P.; Winquist, A.; Flanders, W.D.; Liang, D.; Sarnat, J.A. Acute pulmonary and inflammatory response in young adults following a scripted car commute. Air Qual. Atmos. Health. 2018, 11, 123–136. [Google Scholar] [CrossRef]
- Liang, D.; Ladva, C.N.; Golan, R.; Yu, T.; Walker, D.I.; Sarnat, S.E.; Greenwald, R.; Uppal, K.; Tran, V.; Jones, D.P.; et al. Perturbations of the arginine metabolome following exposures to traffic-related air pollution in a panel of commuters with and without asthma. Environ. Int. 2019, 127, 503–513. [Google Scholar] [CrossRef]
- Sinharay, R.; Gong, J.; Barratt, B.; Ohman-Strickland, P.; Ernst, S.; Kelly, F.J.; Zhang, J.J.; Collins, P.; Cullinan, P.; Chung, K.F. Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: A randomised, crossover study. Lancet 2018, 391, 339–349. [Google Scholar] [CrossRef]









| Single Instillation | Repeated Instillation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sham | BB | DEP | sham | BB | DEP | |||||||
| mean | st.er. | mean | st.er. | mean | st.er. | mean | st.er. | mean | st.er. | mean | st.er. | |
| Total Cells (E+06/mL BALf) | 2 | 0.4 | 1.3 | 0.4 | 3 *§ | 0.7 | 1.46 | 0.72 | 0.94 | 0.16 | 0.79 | 0.28 |
| AMs% | 85.7 | 9.8 | 59.5 | 12.2 | 29 *§ | 10 | 65.03 | 11.5 | 47.14 | 4.5 | 43.27 | 6.5 |
| PMNs % | 14 | 9.9 | 40 | 12.4 | 71 *§ | 10 | 34.56 | 0.39 | 0.43 | 0.25 | 0.35 | 0.16 |
| Ls % | 0.3 | 0.3 | 0.5 | 0.4 | 0.1 | 0.1 | 0.39 | 0.24 | 0.23 | 0.04 | 0.22 | 0.02 |
| Total Protein (mg/mL) | 0.2 | 0.1 | 0.2 | 0.1 | 0.4*§ | 0.1 | 0.21 | 0.02 | 108.91 | 7.7 | 88.32 | 19.7 |
| LDH (mU/mL) | 75.2 | 7.1 | 119.3 * | 8.8 | 124.3 * | 9.0 | 76.1 | 14.7 | 0.11 | 0.04 | 0.09 | 0.01 |
| ALP (IU/L) | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.12 | 0.01 | 2.03 | 0.11 | 1.83 | 0.2 |
| TAC (Trolox (nmol/mL)/Tot Pt (mg/mL)) | 1.7 | 0.1 | 1.9 | 0.2 | 1.5 § | 0.1 | 2.44 | 0.29 | 6.67 | 1.02 | 5.01 | 0.55 |
| ET-1 (pg/mL) | 3.8 | 0.6 | 3.6 | 0.3 | 8.5 *§ | 1.1 | 6.91 | 1.05 | ||||
| Hsp70 (ng/mL) | 0.6 | 0.1 | 0.7 | 0.1 | 0.7 | 0.1 | 0.46 | 0.05 | 0.43 | 0.02 | 0.43 | 0.02 |
| TNFα (pg/mL) | 55.5 | 31.6 | 90.8 | 39.2 | 238.2 | 97 | <LOD | <LOD | <LOD | |||
| IL-6 (pg/mL) | 17.1 | 13.3 | 41.1. | 20.3 | 70.9 | 11.4 | <LOD | <LOD | <LOD | |||
| MMP-9 (pg/mL) | 918.55 | 709.5 | 3932.9 | 2053.1 | 9100.4 | 3772.6 | 606.4 | 230.8 | 433.8 | 67.1 | 413.4 | 102.7 |
| Elements | Unit | DEP | BB |
|---|---|---|---|
| Al | ng/μg | 135 ± 4 | ND |
| K | ng/μg | 50 ± 0.02 | 195 ± 12.5 |
| Ca | ng/μg | 198 ± 8 | 70 ± 4 |
| Fe | ng/μg | 4 ± 0.001 | ND |
| Zn | ng/μg | 70 ± 2 | 4 ± 0.001 |
| Cr | ng/μg | 0.04 ± 0.001 | ND |
| Mn | ng/μg | 0.03 ± 0.001 | 0.42 ± 0.03 |
| V | ng/μg | 0.05 ± 0.007 | ND |
| Ni | ng/μg | 0.02 ± 0.001 | ND |
| Pb | ng/μg | 0.02 ± 0.001 | ND |
| Total PAHs | ng/mg | 600 ± 150 | 50 ± 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, F.; Lonati, E.; Milani, C.; Massimino, L.; Ballarini, E.; Donzelli, E.; Crippa, L.; Marmiroli, P.; Botto, L.; Corsetto, P.A.; et al. In Vivo Comparative Study on Acute and Sub-acute Biological Effects Induced by Ultrafine Particles of Different Anthropogenic Sources in BALB/c Mice. Int. J. Mol. Sci. 2019, 20, 2805. https://doi.org/10.3390/ijms20112805
Farina F, Lonati E, Milani C, Massimino L, Ballarini E, Donzelli E, Crippa L, Marmiroli P, Botto L, Corsetto PA, et al. In Vivo Comparative Study on Acute and Sub-acute Biological Effects Induced by Ultrafine Particles of Different Anthropogenic Sources in BALB/c Mice. International Journal of Molecular Sciences. 2019; 20(11):2805. https://doi.org/10.3390/ijms20112805
Chicago/Turabian StyleFarina, Francesca, Elena Lonati, Chiara Milani, Luca Massimino, Elisa Ballarini, Elisabetta Donzelli, Luca Crippa, Paola Marmiroli, Laura Botto, Paola Antonia Corsetto, and et al. 2019. "In Vivo Comparative Study on Acute and Sub-acute Biological Effects Induced by Ultrafine Particles of Different Anthropogenic Sources in BALB/c Mice" International Journal of Molecular Sciences 20, no. 11: 2805. https://doi.org/10.3390/ijms20112805
APA StyleFarina, F., Lonati, E., Milani, C., Massimino, L., Ballarini, E., Donzelli, E., Crippa, L., Marmiroli, P., Botto, L., Corsetto, P. A., Sancini, G., Bulbarelli, A., & Palestini, P. (2019). In Vivo Comparative Study on Acute and Sub-acute Biological Effects Induced by Ultrafine Particles of Different Anthropogenic Sources in BALB/c Mice. International Journal of Molecular Sciences, 20(11), 2805. https://doi.org/10.3390/ijms20112805