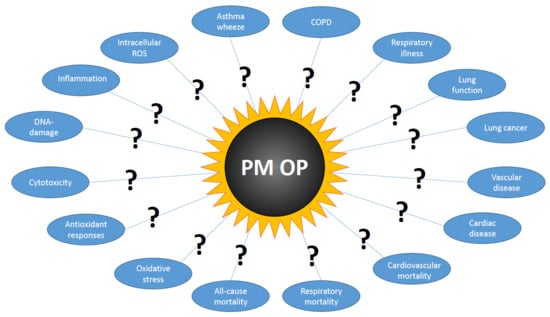
Oxidative Potential Versus Biological Effects: A Review on the Relevance of Cell-Free/Abiotic Assays as Predictors of Toxicity from Airborne Particulate Matter
Abstract
:1. Introduction
2. Literature Search and Selection of Studies
- (1)
- Multiple particles types or samples;
- (2)
- Assessment of OP in cell-free systems;
- (3)
- Assessment of biological effects in cells, animals or humans.
3. Results
Consistency of Associations Between OP and Biological Effects
4. Discussion
4.1. Considerations Regarding the Methods Used to Measure OP
4.2. Redox-Independent Mechanisms of PM Toxicity
4.3. Correlation is not Causation
4.4. The Relative Toxicity of Particles Varies across Different Endpoints and Target Cells or Tissues
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Borm, P.J. Particle toxicology: From coal mining to nanotechnology. Inhal. Toxicol. 2002, 14, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Grahame, T.J.; Klemm, R.; Schlesinger, R.B. Public health and components of particulate matter: The changing assessment of black carbon. J. Air Waste Manag. Assoc. 2014, 64, 620–660. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Fussell, J.C. Air pollution and airway disease. Clin. Exp. Allergy 2011, 41, 1059–1071. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74C, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.N.; Balde, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [Green Version]
- Landrigan, P.J. Air pollution and health. Lancet. Public Health 2017, 2, e4–e5. [Google Scholar] [CrossRef]
- Salvi, S.; Holgate, S.T. Mechanisms of particulate matter toxicity. Clin. Exp. Allergy 1999, 29, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Stone, V.; Seaton, A.; MacNee, W. Ambient particle inhalation and the cardiovascular system: Potential mechanisms. Environ. Health Perspect. 2001, 109 (Suppl. 4), 523–527. [Google Scholar]
- Øvrevik, J.; Refsnes, M.; Lag, M.; Holme, J.A.; Schwarze, P.E. Activation of Proinflammatory Responses in Cells of the Airway Mucosa by Particulate Matter: Oxidant- and Non-Oxidant-Mediated Triggering Mechanisms. Biomolecules 2015, 5, 1399–1440. [Google Scholar] [CrossRef] [Green Version]
- Brits, E.; Schoeters, G.; Verschaeve, L. Genotoxicity of PM10 and extracted organics collected in an industrial, urban and rural area in Flanders, Belgium. Environ. Res. 2004, 96, 109–118. [Google Scholar] [CrossRef]
- Gualtieri, M.; Rigamonti, L.; Galeotti, V.; Camatini, M. Toxicity of tire debris extracts on human lung cell line A549. Toxicol. In Vitro 2005, 19, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Øvrevik, J.; Mollerup, S.; Longhin, E.; Dahlman, H.J.; Camatini, M.; Holme, J.A. Airborne urban particles (Milan winter- PM2.5) cause mitotic arrest and cell death: Effects on DNA, mitochondria, AhR binding and spindle organization. Mutat. Res. 2011, in press. [Google Scholar] [CrossRef] [PubMed]
- Longhin, E.; Holme, J.A.; Gutzkow, K.B.; Arlt, V.M.; Kucab, J.E.; Camatini, M.; Gualtieri, M. Cell cycle alterations induced by urban PM2.5 in bronchial epithelial cells: Characterization of the process and possible mechanisms involved. Part. Fibre Toxicol. 2013, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Stone, V.; Borm, P.J.A.; Jimenez, L.A.; Gilmour, P.S.; Schins, R.P.F.; Knaapen, A.M.; Rahman, I.; Faux, S.P.; Brown, D.M.; et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic. Biol. Med. 2003, 34, 1369–1382. [Google Scholar] [CrossRef]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moller, P.; Danielsen, P.H.; Karottki, D.G.; Jantzen, K.; Roursgaard, M.; Klingberg, H.; Jensen, D.M.; Christophersen, D.V.; Hemmingsen, J.G.; Cao, Y.; et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat. Res. Rev. Mutat. Res. 2014, 762, 133–166. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Castranova, V.; Halliwell, B.; Vallyathan, V. Reactive oxygen species and silica-induced carcinogenesis. J. Toxicol. Environ. Health B Crit. Rev. 1998, 1, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Gulumian, M.; Hei, T.K.; Kamp, D.; Rahman, Q.; Mossman, B.T. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic. Biol. Med. 2003, 34, 1117–1129. [Google Scholar] [CrossRef]
- Ayres, J.G.; Borm, P.; Cassee, F.R.; Castranova, V.; Donaldson, K.; Ghio, A.; Harrison, R.M.; Hider, R.; Kelly, F.; Kooter, I.M.; et al. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential--a workshop report and consensus statement. Inhal. Toxicol. 2008, 20, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.; Kelly, F.; Kunzli, N.; Schins, R.P.; Donaldson, K. Oxidant generation by particulate matter: From biologically effective dose to a promising, novel metric. Occup. Environ. Med. 2007, 64, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Kunzli, N.; Mudway, I.S.; Gotschi, T.; Shi, T.; Kelly, F.J.; Cook, S.; Burney, P.; Forsberg, B.; Gauderman, J.W.; Hazenkamp, M.E.; et al. Comparison of oxidative properties, light absorbance, total and elemental mass concentration of ambient PM2.5 collected at 20 European sites. Environ. Health Perspect. 2006, 114, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, S.E.; Chang, H.H.; Weber, R.J. Ambient PM2.5 and Health: Does PM2.5 Oxidative Potential Play a Role? Am. J. Respir. Crit. Care Med. 2016, 194, 530–531. [Google Scholar] [CrossRef]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.Y.; Sarnat, S.E.; Klein, M.; Mulholland, J.A.; et al. Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Forman, H.J.; Finch, C.E. A critical review of assays for hazardous components of air pollution. Free Radic. Biol. Med. 2018, 117, 202–217. [Google Scholar] [CrossRef]
- Frampton, M.W.; Ghio, A.J.; Samet, J.M.; Carson, J.L.; Carter, J.D.; Devlin, R.B. Effects of aqueous extracts of PM(10) filters from the Utah valley on human airway epithelial cells. Am. J. Physiol. 1999, 277, L960–L967. [Google Scholar]
- Ghio, A.J.; Stonehuerner, J.; Dailey, L.A.; Carter, J.D. Metals associated with both the water-soluble and insoluble fractions of an ambient air pollution particle catalyze an oxidative stress. Inhal. Toxicol. 1999, 11, 37–49. [Google Scholar] [CrossRef]
- Van Maanen, J.M.S.; Borm, P.J.A.; Knaapen, A.; van Herwijnen, M.; Schilderman, P.A.E.L.; Smith, K.R.; Aust, A.E.; Tomatis, M.; Fubini, B. In vitro effects of coal fly ashes: Hydroxyl radical generation, iron release, and DNA damage and toxicity in rat lung epithelial cells. Inhal. Toxicol. 1999, 11, 1123–1141. [Google Scholar]
- Dellinger, B.; Pryor, W.A.; Cueto, R.; Squadrito, G.L.; Hegde, V.; Deutsch, W.A. Role of free radicals in the toxicity of airborne fine particulate matter. Chem. Res. Toxicol. 2001, 14, 1371–1377. [Google Scholar] [CrossRef]
- Molinelli, A.R.; Madden, M.C.; McGee, J.K.; Stonehuerner, J.G.; Ghio, A.J. Effect of metal removal on the toxicity of airborne particulate matter from the Utah Valley. Inhal. Toxicol. 2002, 14, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Knaapen, A.M.; Begerow, J.; Birmili, W.; Borm, P.J.; Schins, R.P. Temporal variation of hydroxyl radical generation and 8-hydroxy-2’-deoxyguanosine formation by coarse and fine particulate matter. Occup. Environ. Med. 2003, 60, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Salonen, R.O.; Halinen, A.I.; Pennanen, A.S.; Hirvonen, M.R.; Sillanpaa, M.; Hillamo, R.; Shi, T.; Borm, P.; Sandell, E.; Koskentalo, T.; et al. Chemical and in vitro toxicologic characterization of wintertime and springtime urban-air particles with an aerodynamic diameter below 10 microm in Helsinki. Scand. J. Work Environ. Health 2004, 30 (Suppl. 2), 80–90. [Google Scholar]
- Schins, R.P.; Lightbody, J.H.; Borm, P.J.; Shi, T.; Donaldson, K.; Stone, V. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol. Appl. Pharm. 2004, 195, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Beck-Speier, I.; Dayal, N.; Karg, E.; Maier, K.L.; Schumann, G.; Schulz, H.; Semmler, M.; Takenaka, S.; Stettmaier, K.; Bors, W.; et al. Oxidative stress and lipid mediators induced in alveolar macrophages by ultrafine particles. Free. Radic. Biol. Med. 2005, 38, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- De Vizcaya-Ruiz, A.; Gutierrez-CastiBarbier, O.; Usachenko, J.L.; Uribe-Ramirez, M.; Cebrian, M.E.; Mugica-Alvarez, V.; Sepulveda, J.; Rosas, I.; Salinas, E.; Garcia-Cuellar, C.; et al. Characterization and in vitro biological effects of concentrated particulate matter from Mexico City. Atmos. Environ. 2006, 40, S583–S592. [Google Scholar] [CrossRef]
- Shi, T.; Duffin, R.; Borm, P.J.; Li, H.; Weishaupt, C.; Schins, R.P. Hydroxyl-radical-dependent DNA damage by ambient particulate matter from contrasting sampling locations. Environ. Res. 2006, 101, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.S.; Castranova, V.; Chen, B.T.; Schwegler-Berry, D.; Hoover, M.; Piacitelli, C.; Gaughan, D.M. Particle size-dependent radical generation from wildland fire smoke. Toxicology 2007, 236, 103–113. [Google Scholar] [CrossRef]
- Repine, J.E.; Reiss, O.K.; Elkins, N.; Chughtai, A.R.; Smith, D.M. Effects of fine carbonaceous particles containing high and low unpaired electron spin densities on lungs of female mice. Transl. Res. 2008, 152, 185–193. [Google Scholar] [CrossRef]
- Verma, V.; Polidori, A.; Schauer, J.J.; Shafer, M.M.; Cassee, F.R.; Sioutas, C. Physicochemical and toxicological profiles of particulate matter in Los Angeles during the October 2007 southern California wildfires. Environ. Sci. Technol. 2009, 43, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, U.S.; McWhinney, R.D.; Rastogi, N.; Abbatt, J.P.; Evans, G.J.; Scott, J.A. Cytotoxic and proinflammatory effects of ambient and source-related particulate matter (PM) in relation to the production of reactive oxygen species (ROS) and cytokine adsorption by particles. Inhal. Toxicol. 2010, 22 (Suppl. 2), 37–47. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.; Birmili, W.; Albrecht, C.; Hellack, B.; Jermann, E.; Wick, G.; Harrison, R.M.; Schins, R.P. Oxidant generation and toxicity of size-fractionated ambient particles in human lung epithelial cells. Environ. Sci. Technol. 2010, 44, 3539–3545. [Google Scholar] [CrossRef] [PubMed]
- Steenhof, M.; Gosens, I.; Strak, M.; Godri, K.J.; Hoek, G.; Cassee, F.R.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Lebret, E.; et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential--the RAPTES project. Part. Fibre Toxicol. 2011, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.H.; Moller, P.; Jensen, K.A.; Sharma, A.K.; Wallin, H.; Bossi, R.; Autrup, H.; Molhave, L.; Ravanat, J.L.; Briede, J.J.; et al. Oxidative stress, DNA damage, and inflammation induced by ambient air and wood smoke particulate matter in human A549 and THP-1 cell lines. Chem. Res. Toxicol. 2011, 24, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.H.; Loft, S.; Jacobsen, N.R.; Jensen, K.A.; Autrup, H.; Ravanat, J.L.; Wallin, H.; Moller, P. Oxidative stress, inflammation, and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicol. Sci. 2010, 118, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Quintana, R.; Serrano, J.; Gomez, V.; de Foy, B.; Miranda, J.; Garcia-Cuellar, C.; Vega, E.; Vazquez-Lopez, I.; Molina, L.T.; Manzano-Leon, N.; et al. The oxidative potential and biological effects induced by PM10 obtained in Mexico City and at a receptor site during the MILAGRO Campaign. Environ. Pollut. 2011, 159, 3446–3454. [Google Scholar] [CrossRef] [PubMed]
- Gerlofs-Nijland, M.E.; Totlandsdal, A.I.; Tzamkiozis, T.; Leseman, D.L.; Samaras, Z.; Lag, M.; Schwarze, P.; Ntziachristos, L.; Cassee, F.R. Cell toxicity and oxidative potential of engine exhaust particles: Impact of using particulate filter or biodiesel fuel blend. Environ. Sci. Technol. 2013, 47, 5931–5938. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Gillen, D.L.; Schauer, J.J.; Shafer, M.M. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J. Expos. Sci. Environ. Epidemiol. 2013, 23, 466–473. [Google Scholar] [CrossRef]
- Lu, Y.; Su, S.; Jin, W.; Wang, B.; Li, N.; Shen, H.; Li, W.; Huang, Y.; Chen, H.; Zhang, Y.; et al. Characteristics and cellular effects of ambient particulate matter from Beijing. Environ. Pollut. 2014, 191, 63–69. [Google Scholar] [CrossRef]
- Badding, M.A.; Fix, N.R.; Antonini, J.M.; Leonard, S.S. A comparison of cytotoxicity and oxidative stress from welding fumes generated with a new nickel-, copper-based consumable versus mild and stainless steel-based welding in RAW 264.7 mouse macrophages. PLoS ONE 2014, 9, e101310. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Baumgartner, J.; Zhang, Y.; Liu, Y.; Sun, Y.; Zhang, M. Oxidative potential and inflammatory impacts of source apportioned ambient air pollution in Beijing. Environ. Sci. Technol. 2014, 48, 12920–12929. [Google Scholar] [CrossRef] [PubMed]
- Velali, E.; Papachristou, E.; Pantazaki, A.; Choli-Papadopoulou, T.; Planou, S.; Kouras, A.; Manoli, E.; Besis, A.; Voutsa, D.; Samara, C. Redox activity and in vitro bioactivity of the water-soluble fraction of urban particulate matter in relation to particle size and chemical composition. Environ. Pollut. 2015, 208, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.R.; Cox, D.P.; Drury, B.E.; Gould, T.R.; Kavanagh, T.J.; Paulsen, M.H.; Sheppard, L.; Simpson, C.D.; Stewart, J.A.; Larson, T.V.; et al. Chemical characterization and in vitro toxicity of diesel exhaust particulate matter generated under varying conditions. Air Qual. Atmos. Health 2015, 8, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Van Den Heuvel, R.; Staelens, J.; Koppen, G.; Schoeters, G. Toxicity of Urban PM10 and Relation with Tracers of Biomass Burning. Int. J. Environ. Res. Public Health 2018, 15, 320. [Google Scholar] [CrossRef] [PubMed]
- Van Den Heuvel, R.; Den Hond, E.; Govarts, E.; Colles, A.; Koppen, G.; Staelens, J.; Mampaey, M.; Janssen, N.; Schoeters, G. Identification of PM10 characteristics involved in cellular responses in human bronchial epithelial cells (Beas-2B). Environ. Res. 2016, 149, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Karavalakis, G.; Gysel, N.; Schmitz, D.A.; Cho, A.K.; Sioutas, C.; Schauer, J.J.; Cocker, D.R.; Durbin, T.D. Impact of biodiesel on regulated and unregulated emissions, and redox and proinflammatory properties of PM emitted from heavy-duty vehicles. Sci. Total. Environ. 2017, 584–585, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Crobeddu, B.; Aragao-Santiago, L.; Bui, L.C.; Boland, S.; Baeza Squiban, A. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ. Pollut. 2017, 230, 125–133. [Google Scholar] [CrossRef] [PubMed]
- He, R.W.; Shirmohammadi, F.; Gerlofs-Nijland, M.E.; Sioutas, C.; Cassee, F.R. Pro-inflammatory responses to PM0.25 from airport and urban traffic emissions. Sci. Total Environ. 2018, 640–641, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, I.C.; Sturrock, A.; Ghiassi, H.; Woller, D.J.; Deering-Rice, C.E.; Lighty, J.S.; Paine, R.; Reilly, C.; Kelly, K.E. Effects of fuel components and combustion particle physicochemical properties on toxicological responses of lung cells. J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng. 2018, 53, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Devlin, R.B. Inflammatory lung injury after bronchial instillation of air pollution particles. Am. J. Respir. Crit. Care Med. 2001, 164, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Schaumann, F.; Borm, P.J.; Herbrich, A.; Knoch, J.; Pitz, M.; Schins, R.P.; Luettig, B.; Hohlfeld, J.M.; Heinrich, J.; Krug, N. Metal-rich ambient particles (particulate matter 2.5) cause airway inflammation in healthy subjects. Am. J. Respir. Crit. Care Med. 2004, 170, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, J.G.; de Kok, T.M.; Briede, J.J.; Wesseling, G.; Kleinjans, J.C.; van Schayck, C.P. Relationship between radical generation by urban ambient particulate matter and pulmonary function of school children. J. Toxicol. Environ. Health A 2006, 69, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Tonne, C.; Yanosky, J.D.; Beevers, S.; Wilkinson, P.; Kelly, F.J. PM mass concentration and PM oxidative potential in relation to carotid intima-media thickness. Epidemiology 2012, 23, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Canova, C.; Minelli, C.; Dunster, C.; Kelly, F.; Shah, P.L.; Caneja, C.; Tumilty, M.K.; Burney, P. PM10 oxidative properties and asthma and COPD. Epidemiology 2014, 25, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.T.; Weber, R.J.; Abrams, J.; Verma, V.; Fang, T.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; et al. Reactive Oxygen Species Generation Linked to Sources of Atmospheric Particulate Matter and Cardiorespiratory Effects. Environ. Sci. Technol. 2015, 49, 13605–13612. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.; Strak, M.; Yang, A.; Hellack, B.; Kelly, F.J.; Kuhlbusch, T.A.; Harrison, R.M.; Brunekreef, B.; Cassee, F.R.; Steenhof, M.; et al. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occup. Environ. Med. 2015, 72, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Steenhof, M.; Mudway, I.S.; Gosens, I.; Hoek, G.; Godri, K.J.; Kelly, F.J.; Harrison, R.M.; Pieters, R.H.; Cassee, F.R.; Lebret, E.; et al. Acute nasal pro-inflammatory response to air pollution depends on characteristics other than particle mass concentration or oxidative potential: The RAPTES project. Occup. Environ. Med. 2013, 70, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Steenhof, M.; Janssen, N.A.; Strak, M.; Hoek, G.; Gosens, I.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Pieters, R.H.; Cassee, F.R.; et al. Air pollution exposure affects circulating white blood cell counts in healthy subjects: The role of particle composition, oxidative potential and gaseous pollutants—the RAPTES project. Inhal. Toxicol. 2014, 26, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Strak, M.; Janssen, N.A.; Godri, K.J.; Gosens, I.; Mudway, I.S.; Cassee, F.R.; Lebret, E.; Kelly, F.J.; Harrison, R.M.; Brunekreef, B.; et al. Respiratory health effects of airborne particulate matter: The role of particle size, composition, and oxidative potential-the RAPTES project. Environ. Health Perspect. 2012, 120, 1183–1189. [Google Scholar] [CrossRef]
- Strak, M.; Hoek, G.; Steenhof, M.; Kilinc, E.; Godri, K.J.; Gosens, I.; Mudway, I.S.; van Oerle, R.; Spronk, H.M.; Cassee, F.R.; et al. Components of ambient air pollution affect thrombin generation in healthy humans: The RAPTES project. Occup. Environ. Med. 2013, 70, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Strak, M.; Hoek, G.; Godri, K.J.; Gosens, I.; Mudway, I.S.; van Oerle, R.; Spronk, H.M.; Cassee, F.R.; Lebret, E.; Kelly, F.J.; et al. Composition of PM affects acute vascular inflammatory and coagulative markers—The RAPTES project. PLoS ONE 2013, 8, e58944. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Samoli, E.; Analitis, A.; Fuller, G.W.; Green, D.C.; Anderson, H.R.; Purdie, E.; Dunster, C.; Aitlhadj, L.; Kelly, F.J.; et al. Short-term associations between particle oxidative potential and daily mortality and hospital admissions in London. Int. J. Hyg. Environ. Health 2016, 219, 566–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, A.; Janssen, N.A.; Brunekreef, B.; Cassee, F.R.; Hoek, G.; Gehring, U. Children’s respiratory health and oxidative potential of PM2.5: The PIAMA birth cohort study. Occup. Environ. Med. 2016, 73, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.Y.; Weber, R.J.; Klein, M.; Sarnat, S.E.; Chang, H.H.; Strickland, M.J.; Verma, V.; Fang, T.; Bates, J.T.; Mulholland, J.A.; et al. Associations between Ambient Fine Particulate Oxidative Potential and Cardiorespiratory Emergency Department Visits. Environ. Health Perspect. 2017, 125, 107008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Tolbert, P.E.; et al. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 2016, 16, 3865–3879. [Google Scholar] [CrossRef]
- Zhang, X.; Staimer, N.; Tjoa, T.; Gillen, D.L.; Schauer, J.J.; Shafer, M.M.; Hasheminassab, S.; Pakbin, P.; Longhurst, J.; Sioutas, C.; et al. Associations between microvascular function and short-term exposure to traffic-related air pollution and particulate matter oxidative potential. Environ. Health 2016, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Maikawa, C.L.; Weichenthal, S.; Wheeler, A.J.; Dobbin, N.A.; Smargiassi, A.; Evans, G.; Liu, L.; Goldberg, M.S.; Pollitt, K.J. Particulate Oxidative Burden as a Predictor of Exhaled Nitric Oxide in Children with Asthma. Environ. Health Perspect. 2016, 124, 1616–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weichenthal, S.A.; Lavigne, E.; Evans, G.J.; Godri Pollitt, K.J.; Burnett, R.T. Fine Particulate Matter and Emergency Room Visits for Respiratory Illness. Effect Modification by Oxidative Potential. Am. J. Respir. Crit. Care Med. 2016, 194, 577–586. [Google Scholar] [CrossRef]
- Weichenthal, S.; Lavigne, E.; Evans, G.; Pollitt, K.; Burnett, R.T. Ambient PM2.5 and risk of emergency room visits for myocardial infarction: Impact of regional PM2.5 oxidative potential: A case-crossover study. Environ. Health 2016, 15, 46. [Google Scholar] [CrossRef]
- Weichenthal, S.; Crouse, D.L.; Pinault, L.; Godri-Pollitt, K.; Lavigne, E.; Evans, G.; van Donkelaar, A.; Martin, R.V.; Burnett, R.T. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian Census Health and Environment Cohort (CanCHEC). Environ. Res. 2016, 146, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavigne, E.; Burnett, R.T.; Stieb, D.M.; Evans, G.J.; Godri Pollitt, K.J.; Chen, H.; van Rijswijk, D.; Weichenthal, S. Fine Particulate Air Pollution and Adverse Birth Outcomes: Effect Modification by Regional Nonvolatile Oxidative Potential. Environ. Health Perspect. 2018, 126, 077012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strak, M.; Janssen, N.; Beelen, R.; Schmitz, O.; Vaartjes, I.; Karssenberg, D.; van den Brink, C.; Bots, M.L.; Dijst, M.; Brunekreef, B.; et al. Long-term exposure to particulate matter, NO2 and the oxidative potential of particulates and diabetes prevalence in a large national health survey. Environ. Int. 2017, 108, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Urch, B.; Szyszkowicz, M.; Evans, G.; Speck, M.; Van Huang, A.; Leingartner, K.; Shutt, R.H.; Pelletier, G.; Gold, D.R.; et al. Metals and oxidative potential in urban particulate matter influence systemic inflammatory and neural biomarkers: A controlled exposure study. Environ. Int. 2018, 121, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012, 12, 11317–11350. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Dalvi, L.T.; Oliveira, R.G.; Ginani, J.S.; Hermes-Lima, M. Reevaluation of the 2-deoxyribose assay for determination of free radical formation. Biochim. Biophys. Acta 2009, 1790, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Hedayat, F.; Stevanovic, S.; Miljevic, B.; Bottle, S.; Ristovski, Z.D. Review-evaluating the molecular assays for measuring the oxidative potential of particulate matter. Chem. Ind. Chem. Engin. Quart. 2014, 21, 201–210. [Google Scholar] [CrossRef]
- Jones, D.P. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods Enzym. 2002, 348, 93–112. [Google Scholar]
- Myhre, O.; Andersen, J.M.; Aarnes, H.; Fonnum, F. Evaluation of the probes 2’,7’-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharm. 2003, 65, 1575–1582. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Ng, A.W.; Bidani, A.; Heming, T.A. Innate host defense of the lung: Effects of lung-lining fluid pH. Lung 2004, 182, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, N.; van Eldik, R. A mechanistic study of the reduction of quinones by ascorbic acid. J. Chem. Soc. Perkin Trans. 2 1997, 8, 1465–1468. [Google Scholar] [CrossRef]
- Guin, P.S.; Das, S.; Mandal, P.C. Electrochemical Reduction of Quinones in Different Media: A Review. Int. J. Electrochem. 2011, 2011, 22. [Google Scholar] [CrossRef]
- Calas, A.; Uzu, G.; Martins, J.M.F.; Voisin, D.; Spadini, L.; Lacroix, T.; Jaffrezo, J.L. The importance of simulated lung fluid (SLF) extractions for a more relevant evaluation of the oxidative potential of particulate matter. Sci. Rep. 2017, 7, 11617. [Google Scholar] [CrossRef]
- Hussain, S.; Boland, S.; Baeza-Squiban, A.; Hamel, R.; Thomassen, L.C.; Martens, J.A.; Billon-Galland, M.A.; Fleury-Feith, J.; Moisan, F.; Pairon, J.C.; et al. Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: Role of particle surface area and internalized amount. Toxicology 2009, 260, 142–149. [Google Scholar] [CrossRef]
- Donaldson, K.; Borm, P.J.; Castranova, V.; Gulumian, M. The limits of testing particle-mediated oxidative stress in vitro in predicting diverse pathologies; relevance for testing of nanoparticles. Part. Fibre Toxicol. 2009, 6, 13. [Google Scholar] [CrossRef]
- Kocbach, A.; Totlandsdal, A.I.; Lag, M.; Refsnes, M.; Schwarze, P.E. Differential binding of cytokines to environmentally relevant particles: A possible source for misinterpretation of in vitro results? Toxicol. Lett. 2008, 176, 131–137. [Google Scholar] [CrossRef]
- Kroll, A.; Pillukat, M.H.; Hahn, D.; Schnekenburger, J. Interference of engineered nanoparticles with in vitro toxicity assays. Arch. Toxicol. 2012, 86, 1123–1136. [Google Scholar] [CrossRef]
- Kong, B.; Seog, J.H.; Graham, L.M.; Lee, S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomed. (Lond. Engl.) 2011, 6, 929–941. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, J.D.; Armstrong, D.A. Free radical inactivation of lactate dehydrogenase. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1976, 30, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Fariss, M.W.; Gilmour, M.I.; Reilly, C.A.; Liedtke, W.; Ghio, A.J. Emerging mechanistic targets in lung injury induced by combustion-generated particles. Toxicol. Sci. 2013, 132, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.N.; Fanick, E.R.; Brooks, E.G. TRP channels and traffic-related environmental pollution-induced pulmonary disease. Semin. Immunopathol. 2016, 38, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, S.; Fenton, M.J.; Soukup, J.M. Involvement of microbial components and Toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am. J. Respir. Cell Mol. Biol. 2002, 27, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Øvrevik, J.; Holme, J.A.; Perrone, M.G.; Bolzacchini, E.; Schwarze, P.E.; Camatini, M. Differences in cytotoxicity versus pro-inflammatory potency of different PM fractions in human epithelial lung cells. Toxicol. In Vitro 2010, 24, 29–39. [Google Scholar] [CrossRef]
- Fardel, O. Cytokines as molecular targets for aryl hydrocarbon receptor ligands: Implications for toxicity and xenobiotic detoxification. Expert. Opin. Drug Metab. Toxicol. 2013, 9, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; Di, M.P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Rabson, A.B.; Gallo, M.A. Ah receptor and NF-kappaB interactions: Mechanisms and physiological implications. Chem. Biol. Interact. 2002, 141, 97–115. [Google Scholar] [CrossRef]
- Chen, P.H.; Chang, H.; Chang, J.T.; Lin, P. Aryl hydrocarbon receptor in association with RelA modulates IL-6 expression in non-smoking lung cancer. Oncogene 2012, 31, 2555–2565. [Google Scholar] [CrossRef]
- N’Diaye, M.; Le, F.E.; Lagadic-Gossmann, D.; Corre, S.; Gilot, D.; Lecureur, V.; Monteiro, P.; Rauch, C.; Galibert, M.D.; Fardel, O. Aryl hydrocarbon receptor- and calcium-dependent induction of the chemokine CCL1 by the environmental contaminant benzo[a]pyrene. J. Biol. Chem. 2006, 281, 19906–19915. [Google Scholar] [CrossRef]
- Podechard, N.; Lecureur, V.; Le Ferrec, E.; Guenon, I.; Sparfel, L.; Gilot, D.; Gordon, J.R.; Lagente, V.; Fardel, O. Interleukin-8 induction by the environmental contaminant benzo(a)pyrene is aryl hydrocarbon receptor-dependent and leads to lung inflammation. Toxicol. Lett. 2008, 177, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Sciullo, E.; Matsumura, F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem. Biophys. Res. Commun. 2007, 363, 722–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, C.F.; Matsumura, F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem. Pharm. 2009, 77, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.W.; Duckett, C.S. The aryl hydrocarbon nuclear translocator alters CD30-mediated NF-kappaB-dependent transcription. Science 2009, 323, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Holme, J.A.; Brinchmann, B.C.; Refsnes, M.; Lag, M.; Ovrevik, J. Potential role of polycyclic aromatic hydrocarbons as mediators of cardiovascular effects from combustion particles. Environ. Health 2019, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Holme, J.A.; Brinchmann, B.C.; Le Ferrec, E.; Lagadic-Gossmann, D.; Ovrevik, J. Combustion Particle-Induced Changes in Calcium Homeostasis: A Contributing Factor to Vascular Disease? Cardiovasc. Toxicol. 2019, 19, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Wang, J.; Zhu, K.; Tang, Y.; Huang, S.; Shui, X.; Ding, Y.; Chen, C.; Lei, W. Aryl Hydrocarbon Receptor: A New Player of Pathogenesis and Therapy in Cardiovascular Diseases. Biomed. Res. Int. 2018, 2018, 6058784. [Google Scholar] [CrossRef]
- Levonen, A.L.; Hill, B.G.; Kansanen, E.; Zhang, J.; Darley-Usmar, V.M. Redox regulation of antioxidants, autophagy, and the response to stress: Implications for electrophile therapeutics. Free Radic. Biol. Med. 2014, 71, 196–207. [Google Scholar] [CrossRef] [Green Version]
- Kodavanti, U.P.; Schladweiler, M.C.J.; Ledbetter, A.D.; Hauser, R.; Christiani, D.C.; Samet, J.M.; McGee, J.; Richards, J.H.; Costa, D.L. Pulmonary and systemic effects of zinc-containing emission particles in three rat strains: Multiple exposure scenarios. Toxicol. Sci. 2002, 70, 73–85. [Google Scholar] [CrossRef]
- Tal, T.L.; Graves, L.M.; Silbajoris, R.; Bromberg, P.A.; Wu, W.; Samet, J.M. Inhibition of protein tyrosine phosphatase activity mediates epidermal growth factor receptor signaling in human airway epithelial cells exposed to Zn(2+). Toxicol. Appl. Pharm. 2006, 214, 16–23. [Google Scholar] [CrossRef]
- Samet, J.M.; Graves, L.M.; Quay, J.; Dailey, L.A.; Devlin, R.B.; Ghio, A.J.; Wu, W.; Bromberg, P.A.; Reed, W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Ajp Lung Cell. Mol. Physiol. 1998, 275, L551–L558. [Google Scholar] [CrossRef] [PubMed]
- Øvrevik, J.; Lag, M.; Holme, J.A.; Schwarze, P.E.; Refsnes, M. Cytokine and chemokine expression patterns in lung epithelial cells exposed to components characteristic of particulate air pollution. Toxicology 2009, 259, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Baxter, L.K.; Crooks, J.L.; Sacks, J.D. Influence of exposure differences on city-to-city heterogeneity in PM2.5-mortality associations in US cities. Environ. Health 2017, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Amatullah, H.; North, M.L.; Akhtar, U.S.; Rastogi, N.; Urch, B.; Silverman, F.S.; Chow, C.W.; Evans, G.J.; Scott, J.A. Comparative cardiopulmonary effects of size-fractionated airborne particulate matter. Inhal. Toxicol. 2012, 24, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Dailey, L.A.; Soukup, J.M.; Grambow, S.C.; Devlin, R.B.; Huang, Y.C. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ. Health Perspect. 2005, 113, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Happo, M.S.; Salonen, R.O.; Halinen, A.I.; Jalava, P.I.; Pennanen, A.S.; Kosma, V.M.; Sillanpaa, M.; Hillamo, R.; Brunekreef, B.; Katsouyanni, K.; et al. Dose and time dependency of inflammatory responses in the mouse lung to urban air coarse, fine, and ultrafine particles from six European cities. Inhal. Toxicol. 2007, 19, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Duffin, R.; Bradley, M.; Megson, I.L.; MacNee, W.; Lee, J.K.; Jeong, J.; Donaldson, K. Predictive value of in vitro assays depends on the mechanism of toxicity of metal oxide nanoparticles. Part. Fibre Toxicol. 2013, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Tong, H.; McGee, J.K.; Baldauf, R.W.; Krantz, Q.T.; Gilmour, M.I. Comparative toxicity of size-fractionated airborne particulate matter collected at different distances from an urban highway. Environ. Health Perspect. 2009, 117, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, M.I.; McGee, J.; Duvall, R.M.; Dailey, L.; Daniels, M.; Boykin, E.; Cho, S.H.; Doerfler, D.; Gordon, T.; Devlin, R.B. Comparative toxicity of size-fractionated airborne particulate matter obtained from different cities in the United States. Inhal. Toxicol. 2007, 19 (Suppl. 1), 7–16. [Google Scholar] [CrossRef]
- Refsnes, M.; Hetland, R.B.; Øvrevik, J.; Sundfor, I.; Schwarze, P.E.; Låg, M. Different particle determinants induce apoptosis and cytokine release in primary alveolar macrophage cultures. Part. Fibre Toxicol. 2006, 3, 10. [Google Scholar] [CrossRef]
- Iskandar, A.; Andersen, Z.J.; Bonnelykke, K.; Ellermann, T.; Andersen, K.K.; Bisgaard, H. Coarse and fine particles but not ultrafine particles in urban air trigger hospital admission for asthma in children. Thorax 2012, 67, 252–257. [Google Scholar] [CrossRef]
- Øvrevik, J.; Myran, T.; Refsnes, M.; Lag, M.; Becher, R.; Hetland, R.B.; Schwarze, P.E. Mineral particles of varying composition induce differential chemokine release from epithelial lung cells: Importance of physico-chemical Characteristics. Ann. Occup. Hyg. 2005, 49, 219–231. [Google Scholar] [PubMed]
- Samet, J.M.; Graff, D.; Berntsen, J.; Ghio, A.J.; Huang, Y.C.; Devlin, R.B. A comparison of studies on the effects of controlled exposure to fine, coarse and ultrafine ambient particulate matter from a single location. Inhal. Toxicol. 2007, 19 (Suppl. 1), 29–32. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Duffin, R.; Poland, C.; Daly, P.; Murphy, F.; Drost, E.; MacNee, W.; Stone, V.; Donaldson, K. Efficacy of simple short-term in vitro assays for predicting the potential of metal oxide nanoparticles to cause pulmonary inflammation. Environ. Health Perspect. 2009, 117, 241–247. [Google Scholar] [CrossRef] [PubMed]





| Assay | Species Detected |
|---|---|
| AA-depletion | Used to measure oxidative potential of transition metals (●OH from H2O2). Interacts with several other reactive species. |
| GSH-depletion | Most ROS as well as peroxides, alkenals, protein disulfides and sulfenic acids |
| Congo Red | Hydroxylic, peroxide and hydroperoxide radicals |
| DCF (DCFH-DA) | ●NO2, ●OH, ONOO−, peroxyl, aloxyl and carbon-centered radicals, peroxides. Can be used to measure H2O2 in presence of a peroxidase catalyst (HRP). Prone to photooxidation. |
| 2-deoxyribose | ●OH and ●OH-like species (used as a simple and inexpensive substitute for ESR). |
| DHE | Can be specific for O2●− (require separation of products by HPLC). Interacts with several other reactive species. |
| DTT | Diverse range of free radicals and reactive species. Reduced by transition metals and quinones, in PM. |
| ESR (or EPR) | ESR with DMPO as spin-trap measures production of ●OH, and is often used in combination with H2O2. ESR without spin-trapping can be used to measure surface radicals on particles (measures unpaired electrons). |
| Luminol | HOCl, H2O2 and ONOO− |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Øvrevik, J. Oxidative Potential Versus Biological Effects: A Review on the Relevance of Cell-Free/Abiotic Assays as Predictors of Toxicity from Airborne Particulate Matter. Int. J. Mol. Sci. 2019, 20, 4772. https://doi.org/10.3390/ijms20194772
Øvrevik J. Oxidative Potential Versus Biological Effects: A Review on the Relevance of Cell-Free/Abiotic Assays as Predictors of Toxicity from Airborne Particulate Matter. International Journal of Molecular Sciences. 2019; 20(19):4772. https://doi.org/10.3390/ijms20194772
Chicago/Turabian StyleØvrevik, Johan. 2019. "Oxidative Potential Versus Biological Effects: A Review on the Relevance of Cell-Free/Abiotic Assays as Predictors of Toxicity from Airborne Particulate Matter" International Journal of Molecular Sciences 20, no. 19: 4772. https://doi.org/10.3390/ijms20194772
APA StyleØvrevik, J. (2019). Oxidative Potential Versus Biological Effects: A Review on the Relevance of Cell-Free/Abiotic Assays as Predictors of Toxicity from Airborne Particulate Matter. International Journal of Molecular Sciences, 20(19), 4772. https://doi.org/10.3390/ijms20194772