Assessment of the Antiangiogenic and Anti-Inflammatory Properties of a Maslinic Acid Derivative and its Potentiation using Zinc Chloride
Abstract
:1. Introduction
2. Results
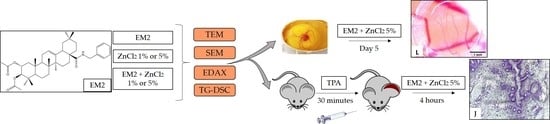
2.1. Transmission Electron Microscopy (TEM) Analysis of EM2
2.2. Scanning Electron Microscope (SEM) Analysis of EM2
2.3. Energy Dispersive X-ray Analysis (EDAX) of EM2
2.4. Thermogravimetry-Differential Scanning Calorimetry (TG-DSC) Analysis of EM2
2.5. EM2 Effects on Normal CAM
2.6. Evaluation of EM2 and ZnCl2 Formulations Topic Application
2.7. Assessment of the Histopathologic Analysis of Mice Ears
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Transmission Electron Microscopy (TEM) Analysis
4.3. Scanning Electron Microscope (SEM) and Energy Dispersive x-ray (EDAX) Analysis
4.4. Thermogravimetry (TG) and Differential Scanning Calorimetry (DSC) Analysis
4.5. Chorioallantoic Membrane Assay (CAM)
Normal Angiogenesis on Chorioallantoic Membrane Assay
4.6. Preparation of EM2 and ZnCl2 Formulations
4.7. In Vivo TPA-Induced Ear Inflammation Protocol
4.8. Histopathological Assessment of Mice Ears
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAM | Chorioallantoic membrane |
| DMSO | Dimethyl sulfoxide |
| EDAX | Energy dispersive X-ray analysis |
| EDD | Embryonic day of development |
| EM2 | Benzyl (2α, 3β) 2,3-diacetoxy-olean−12-en-28-amide |
| H-E | Hematoxilin-eosine |
| HPMC | Hydroxypropylmethylcellulose |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscopy |
| TG-DSC | Thermogravimetry-differential scanning calorimetry |
| TNF-α | Tumor necrosis factor alpha |
| TPA | 12-o-tetradecanoylphorbol−13-acetate |
| VEGF | Vascular endothelial growth factor |
| ZnCl2 | Zinc chloride |
References
- Pan, S.Y.; Zhou, S.F.; Gao, S.H.; Yu, Z.L.; Zhang, S.F.; Tang, M.K.; Sun, J.N.; Ma, D.L.; Han, Y.F.; Fong, W.F.; et al. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evid. Based. Complement. Alternat. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Soica, C.; Antal, D.; Alexa, E.; Pavel, I.Z.; Ghiulai, R.; Ardelean, F.; Babuta, R.M.; Popescu, A.; Dehelean, C.A. Natural Compounds in the Chemoprevention of Malignant Melanoma. Anticancer. Agents Med. Chem. 2018, 18, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.M.; Ferreira, L.M.; Branco, P.S. Molecules of natural origin, semi-synthesis and synthesis with anti-inflammatory and anticancer utilities. Curr. Pharm. Des. 2012, 18, 3979–4046. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Berkó, S.; Varju, G.; Balázs, B.; Kemény, L.; Németh, I.B.; Cioca, A.; Petruș, A.; Dehelean, C.; Cosmin, C.I.; et al. The Effect of Electroporation of a Lyotroic Liquid Crystal Genistein-Based Formulation in the Recovery of Murine Melanoma Lesions. Int. J. Mol. Sci. 2015, 16, 15425–15441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.; Shaw, D.; Simmonds, M.S.J.; Leon, C.J.; Xu, Q.; Lu, A.; Sutherland, I.; Ignatova, S.; Zhu, Y.P.; Verpoorte, R.; et al. Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional Chinese medicine and Chinese materia medica. J. Ethnopharmacol. 2012, 140, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, J.; Chen, Y. Betulinic acid and the pharmacological effects of tumor suppression (Review). Mol. Med. Rep. 2016, 14, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Al-Harrasi, A.; Csuk, R.; Shamraiz, U.; Green, I.R.; Ahmed, I.; Khan, I.A.; Ali, Z. Therapeutic potential of boswellic acids: A patent review (1990-2015). Expert Opin. Ther. Pat. 2017, 27, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Punia, S.; Sharma, A.K. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Pat. Inflamm. Allergy Drug Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ozbey, U.; Sabitaliyevich, U.Y.; Attar, R.; Ozcelik, B.; Zhang, Y.; Guo, M.; Liu, M.; Alhewairini, S.S.; Farooqi, A.A. Maslinic acid as an effective anticancer agent. Cell. Mol. Biol. (Noisy-le-grand). 2018, 64, 87–91. [Google Scholar] [CrossRef]
- Lozano-Mena, G.; Sánchez-González, M.; Juan, M.E.; Planas, J.M. Maslinic acid, a natural phytoalexin-type triterpene from olives--a promising nutraceutical? Molecules 2014, 19, 11538–11559. [Google Scholar] [CrossRef]
- Sánchez-González, M.; Lozano-Mena, G.; Juan, M.E.; García-Granados, A.; Planas, J.M. Assessment of the safety of maslinic acid, a bioactive compound from Olea europaea L. Mol. Nutr. Food Res. 2013, 57, 339–346. [Google Scholar] [CrossRef]
- Siewert, B.; Pianowski, E.; Obernauer, A.; Csuk, R. Towards cytotoxic and selective derivatives of maslinic acid. Bioorg. Med. Chem. 2014, 22, 594–615. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wen, X.; Sun, H. Oleanolic acid derivatives for pharmaceutical use: A patent review. Expert Opin. Ther. Pat. 2016, 26, 643–655. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Perez-Jimenez, A.J.; Reyes-Zurita, F.; Garcia-Salguero, L.; Mokhtari, K.; Herrera-Merchan, A.P.; Medina, P.; Peragon, J.A.; Lupianez, J. Anti-cancer and Anti-angiogenic Properties of Various Natural Pentacyclic Tri-terpenoids and Some of their Chemical Derivatives. Curr. Org. Chem. 2015, 19, 919–947. [Google Scholar] [CrossRef]
- Strüh, C.M.; Jäger, S.; Kersten, A.; Schempp, C.M.; Scheffler, A.; Martin, S.F. Triterpenoids amplify anti-tumoral effects of mistletoe extracts on murine B16.f10 melanoma in vivo. PLoS ONE 2013, 8, e62168. [Google Scholar] [CrossRef] [PubMed]
- Thakor, P.; Song, W.; Subramanian, R.B.; Thakkar, V.R.; Vesey, D.A.; Gobe, G.C. Maslinic Acid Inhibits Proliferation of Renal Cell Carcinoma Cell Lines and Suppresses Angiogenesis of Endothelial Cells. J. kidney cancer VHL 2017, 4, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehelean, C.A.; Feflea, S.; Gheorgheosu, D.; Ganta, S.; Cimpean, A.M.; Muntean, D.; Amiji, M.M. Anti-angiogenic and anti-cancer evaluation of betulin nanoemulsion in chicken chorioallantoic membrane and skin carcinoma in Balb/c mice. J. Biomed. Nanotechnol. 2013, 9, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Dupertuis, Y.M.; Delie, F.; Cohen, M.; Pichard, C. In ovo method for evaluating the effect of nutritional therapies on tumor development, growth and vascularization. Clin. Nutr. Exp. 2015, 2, 9–17. [Google Scholar] [CrossRef]
- Danciu, C.; Avram, S.; Pavel, I.Z.; Ghiulai, R.; Dehelean, C.A.; Ersilia, A.; Minda, D.; Petrescu, C.; Moaca, E.A.; Soica, C. Main Isoflavones Found in Dietary Sources as Natural Anti-inflammatory Agents. Curr. Drug Targets 2018, 19, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Prasad, S.; Reuter, S.; Kannappan, R.; Yadev, V.R.; Park, B.; Kim, J.H.; Gupta, S.C.; Phromnoi, K.; Sundaram, C.; et al. Identification of novel anti-inflammatory agents from Ayurvedic medicine for prevention of chronic diseases: “reverse pharmacology” and “bedside to bench” approach. Curr. Drug Targets 2011, 12, 1595–1653. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Romero-Estrada, A.; Maldonado-Magaña, A.; González-Christen, J.; Bahena, S.M.; Garduño-Ramírez, M.L.; Rodríguez-López, V.; Alvarez, L. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera copallifera. BMC Complement. Altern. Med. 2016, 16, 422. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wei, X.H.; Chen, F.Y.; Li, C.J.; Yang, J.Z.; Ma, J.; Bao, X.Q.; Zhang, D.; Zhang, D.M. Anti-inflammatory pentacyclic triterpenes from the stems of Euonymus carnosus. Fitoterapia 2017, 118, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Safayhi, H.; Sailer, E.R. Anti-inflammatory actions of pentacyclic triterpenes. Planta Med. 1997, 63, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Emri, E.; Miko, E.; Bai, P.; Boros, G.; Nagy, G.; Rózsa, D.; Juhász, T.; Hegedűs, C.; Horkay, I.; Remenyik, É.; et al. Effects of non-toxic zinc exposure on human epidermal keratinocytes. Metallomics 2015, 7, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minda, D.; Coricovac, D.; Pinzaru, I.; Dehelean, C.; Borcan, F.; Muntean, D. Lupeol a potent anti-inflammatory agent in acute inflammation mouse ear model. Fiziol. Physiol. 2015, 25, 25–28. [Google Scholar]
- Pavel, I.Z. Experimental Research Regarding the In vitro and In vivo Effects of a Maslinic Acid Derivative. Ph.D. Thesis, Victor Babes University of Medicine and Pharmacy of Timisoara, Timişoara, Romania, 2017. [Google Scholar]
- Rashidi, H.; Sottile, V. The chick embryo: Hatching a model for contemporary biomedical research. Bioessays 2009, 31, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Zeisser-Labouebe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef]
- Harris, R.J. Multiplication of Rous No. 1 sarcoma agent in the chorioallantoic membrane of the embryonated egg. Br. J. Cancer 1954, 8, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Cotran, R. Relation of vascular proliferation to tumor growth. Int. Rev. Exp. Pathol. 1976, 16, 207–248. [Google Scholar] [PubMed]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane in the Study of Angiogenesis and Metastasis; Springer: Dordrecht, the Netherlands, 2010. [Google Scholar]
- Avram, S.; Ghiulai, R.; Pavel, I.Z.; Mioc, M.; Babuta, R.; Voicu, M.; Coricovac, D.; Danciu, C.; Dehelean, C.; Soica, C. Phytocompounds Targeting Cancer Angiogenesis Using the Chorioallantoic Membrane Assay. In Natural Products and Cancer Drug Discovery; Badria, F.A., Ed.; InTech: London, UK, 2017; pp. 45–66. [Google Scholar]
- Park, S.Y.; Nho, C.W.; Kwon, D.Y.; Kang, Y.H.; Lee, K.W.; Park, J.H.Y. Maslinic acid inhibits the metastatic capacity of DU145 human prostate cancer cells: Possible mediation via hypoxia-inducible factor−1α signalling. Br. J. Nutr. 2013, 109, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Huang, C.Y.; Mong, M.C.; Chan, C.Y.; Yin, M.C. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J Agric Food Chem. 2011, 59, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, C.; López-Biedma, A.; Gaforio, J.J. Maslinic Acid enhances signals for the recruitment of macrophages and their differentiation to m1 state. Evid. Based. Complement. Alternat. Med. 2015, 2015, 654721. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.; Steinberg, S.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef]
- Güran, Ş.; Çoban, Z.D.; Fermanli, O.; Aydingöz, E.; Ipek, E. Folic acid and zinc inhibit angiogenesis in chicken chorioallontoic membrane model via angiogenic factor genes. Gulhane Med. J. 2018, 60, 67–70. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef]
- Huang, L.; Guan, T.; Qian, Y.; Huang, M.; Tang, X.; Li, Y.; Sun, H. Anti-inflammatory effects of maslinic acid, a natural triterpene, in cultured cortical astrocytes via suppression of nuclear factor-kappa B. Eur. J. Pharmacol. 2011, 672, 169–174. [Google Scholar] [CrossRef]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Taguchi, Y.; Akazawa, H.; Ukiya, M.; Kimura, Y.; Suzuki, T.; Nishino, H. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol. Pharm. Bull. 2005, 28, 1995–1999. [Google Scholar] [CrossRef]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Higashihara, H.; Ukiya, M.; Watanabe, K.; Kimura, Y.; Hasegawa, J.I.; Nishino, H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci. Biotechnol. Biochem. 2004, 68, 85–90. [Google Scholar] [CrossRef]
- Pavel, I.Z.; Danciu, C.; Oprean, C.; Dehelean, C.A.; Muntean, D.; Csuk, R.; Muntean, D.M. In vitro Evaluation of the Antimicrobial Ability and Cytotoxicity on Two Melanoma Cell Lines of a Benzylamide Derivative of Maslinic Acid. Anal. Cell. Pathol. 2016, 2016, 2787623. [Google Scholar] [CrossRef]
- Pavel, I.Z.; Pârvu, A.E.; Dehelean, C.A.; Vlase, L.; Csuk, R.; Muntean, D. Assessment of the Antioxidant Effect of a Maslinic Acid Derivative in an Experimental Model of Acute Inflammation. Farmacia 2017, 65, 390–395. [Google Scholar]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar] [CrossRef] [PubMed]
- Sharquie, K.E.; Noaimi, A.A.; Al-Salih, M.M. Topical therapy of acne vulgaris using 2% tea lotion in comparison with 5% zinc sulphate solution. Saudi Med. J. 2008, 29, 1757–1761. [Google Scholar] [PubMed]
- Sharquie, K.E.; Najim, R.A.; Al-Salman, H.N. Oral zinc sulfate in the treatment of rosacea: A double-blind, placebo-controlled study. Int. J. Dermatol. 2006, 45, 857–861. [Google Scholar] [CrossRef]
- Ikeda, N.E.A.; Novak, E.M.; Maria, D.A.; Velosa, A.S.; Pereira, R.M.S. Synthesis, characterization and biological evaluation of Rutin–zinc(II) flavonoid -metal complex. Chem. Biol. Interact. 2015, 239, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A.; Roncali, L.; Dammacco, F. The chick embryo chorioallantoic membrane as a model for in vivo research on anti-angiogenesis. Curr. Pharm. Biotechnol. 2000, 1, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Caunii, A.; Oprean, C.; Cristea, M.; Ivan, A.; Danciu, C.; Tatu, C.; Paunescu, V.; Marti, D.; Tzanakakis, G.; Spandidos, D.A.; et al. Effects of ursolic and oleanolic on SK-MEL-2 melanoma cells: In vitro and in vivo assays. Int. J. Oncol. 2017, 51, 1651–1660. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef] [PubMed]









| Element | Wt % | At % | K-Ratio | Z | A | F |
|---|---|---|---|---|---|---|
| C K | 86.95 | 90.59 | 0.6886 | 1.0043 | 0.7885 | 1.0001 |
| N K | 1.17 | 1.05 | 0.0009 | 0.9956 | 0.0778 | 1.0001 |
| O K | 10.58 | 8.28 | 0.0130 | 0.9876 | 0.1241 | 1.0000 |
| Au M | 1.30 | 0.08 | 0.0147 | 0.6616 | 1.7150 | 1.0000 |
| Total | 100.00 | 100.00 |
| Element | Wt % | At % | K-Ratio | Z | A | F |
|---|---|---|---|---|---|---|
| C K | 88.94 | 92.87 | 0.5971 | 1.0046 | 0.6682 | 1.0001 |
| O K | 8.29 | 6.50 | 0.0092 | 0.9905 | 0.1123 | 1.0000 |
| Zn L | 2.15 | 0.41 | 0.0109 | 0.8490 | 0.5946 | 1.0000 |
| Cl K | 0.61 | 0.22 | 0.0060 | 0.9108 | 1.0682 | 1.0000 |
| Total | 100.00 | 100.00 |
| Formulations | EM2 (mg) | ZnCl2 (mg) | HPMC (mg) | Propylene Glycol (mg) | Preservative Solution (mL) | |
|---|---|---|---|---|---|---|
| 1. | EM2 1% | 1 | − | 2 | 10 | 87 |
| 2. | ZnCl2 1% | − | 1 | 2 | 10 | 87 |
| 3. | ZnCl2 5% | − | 5 | 2 | 10 | 83 |
| 4. | EM2 1% + ZnCl2 1% | 1 | 1 | 2 | 10 | 86 |
| 5. | EM2 1% + ZnCl2 5% | 1 | 5 | 2 | 10 | 82 |
| 6. | Blank hydrogel | − | − | 2 | 10 | 88 |
| Group No. | Group Name | Group Description |
|---|---|---|
| 1 | Control | With no intervention |
| 2 | Acetone | Acetone solution application (20 µl/ear) |
| 3 | TPA | TPA solution application (20 µl/ear) |
| 4 | TPA + indomethacin | A 4% indomethacin cream was topically applied 30 min after applying the TPA solution |
| 5 | Blank hydrogel | Blank hydrogel was topically applied 30 min after TPA solution application |
| 6 | EM2 1% | EM2 1% hydrogel was topically applied 30 min after TPA solution application |
| 7 | ZnCl2 1% | ZnCl2 1% hydrogel was topically applied 30 min after TPA solution application |
| 8 | ZnCl2 5% | ZnCl2 5% hydrogel was topically applied 30 min after TPA solution application |
| 9 | EM2 1% + ZnCl2 1% | EM2 + ZnCl2 1% hydrogel was topically applied 30 min after TPA solution application |
| 10 | EM2 1% + ZnCl2 5% | EM2 + ZnCl2 5% hydrogel was topically applied 30 min after TPA solution application |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavel, I.Z.; Csuk, R.; Danciu, C.; Avram, S.; Baderca, F.; Cioca, A.; Moacă, E.-A.; Mihali, C.-V.; Pinzaru, I.; Muntean, D.M.; et al. Assessment of the Antiangiogenic and Anti-Inflammatory Properties of a Maslinic Acid Derivative and its Potentiation using Zinc Chloride. Int. J. Mol. Sci. 2019, 20, 2828. https://doi.org/10.3390/ijms20112828
Pavel IZ, Csuk R, Danciu C, Avram S, Baderca F, Cioca A, Moacă E-A, Mihali C-V, Pinzaru I, Muntean DM, et al. Assessment of the Antiangiogenic and Anti-Inflammatory Properties of a Maslinic Acid Derivative and its Potentiation using Zinc Chloride. International Journal of Molecular Sciences. 2019; 20(11):2828. https://doi.org/10.3390/ijms20112828
Chicago/Turabian StylePavel, Ioana Zinuca, Rene Csuk, Corina Danciu, Stefana Avram, Flavia Baderca, Andreea Cioca, Elena-Alina Moacă, Ciprian-Valentin Mihali, Iulia Pinzaru, Danina Mirela Muntean, and et al. 2019. "Assessment of the Antiangiogenic and Anti-Inflammatory Properties of a Maslinic Acid Derivative and its Potentiation using Zinc Chloride" International Journal of Molecular Sciences 20, no. 11: 2828. https://doi.org/10.3390/ijms20112828
APA StylePavel, I. Z., Csuk, R., Danciu, C., Avram, S., Baderca, F., Cioca, A., Moacă, E.-A., Mihali, C.-V., Pinzaru, I., Muntean, D. M., & Dehelean, C. A. (2019). Assessment of the Antiangiogenic and Anti-Inflammatory Properties of a Maslinic Acid Derivative and its Potentiation using Zinc Chloride. International Journal of Molecular Sciences, 20(11), 2828. https://doi.org/10.3390/ijms20112828