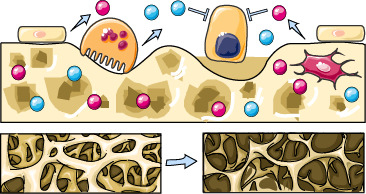
Bone Matrix Levels of Dickkopf and Sclerostin are Positively Correlated with Bone Mass and Strength in Postmenopausal Osteoporosis
Abstract
:1. Introduction
2. Results
2.1. Bone Matrix DKK1 and SOST and Association with Bone Mass and Strength
2.2. Bone Matrix DKK1 and SOST and Correlation with Bone Turn–Over and Fractures
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Serum Biochemistry
4.3. Densitometry
4.4. pQCT and Biomechanical Testing
4.5. Preparation and Extraction of Bone Specimens
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eastell, R.; Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017, 5, 908–923. [Google Scholar] [CrossRef]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Rodda, S.J.; McMahon, A.P. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 2006, 133, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Bellido, T.; Manolagas, S.C.; Kousteni, S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta–catenin–dependent and –independent signaling cascades involving Src/ERK and phosphatidylinositol 3–kinase/AKT. J. Biol. Chem. 2005, 280, 41342–41351. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.A., 2nd; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.A.; et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Ahn, V.E.; Chu, M.L.; Choi, H.J.; Tran, D.; Abo, A.; Weis, W.I. Structural basis of Wnt signaling inhibition by Dickkopf binding to LRP5/6. Dev. Cell 2011, 21, 862–873. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef]
- Mirza, F.S.; Padhi, I.D.; Raisz, L.G.; Lorenzo, J.A. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J. Clin. Endocrinol. Metab. 2010, 95, 1991–1997. [Google Scholar] [CrossRef]
- Tian, J.; Xu, X.J.; Shen, L.; Yang, Y.P.; Zhu, R.; Shuai, B.; Zhu, X.W.; Li, C.G.; Ma, C.; Lv, L. Association of serum Dkk–1 levels with beta–catenin in patients with postmenopausal osteoporosis. J. Huazhong Univ. Sci. Technolog Med. Sci. 2015, 35, 212–218. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Avramidis, A.; Toulis, K.A.; Papatheodorou, A.; Terpos, E. The effect of teriparatide on serum Dickkopf–1 levels in postmenopausal women with established osteoporosis. Clin. Endocrinol. (Oxf) 2010, 72, 752–757. [Google Scholar] [CrossRef]
- Lim, Y.; Kim, C.H.; Lee, S.Y.; Kim, H.; Ahn, S.H.; Lee, S.H.; Koh, J.M.; Rhee, Y.; Baek, K.H.; Min, Y.K.; et al. Decreased Plasma Levels of Sclerostin But Not Dickkopf–1 are Associated with an Increased Prevalence of Osteoporotic Fracture and Lower Bone Mineral Density in Postmenopausal Korean Women. Calcif. Tissue Int. 2016, 99, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Dovjak, P.; Dorfer, S.; Foger-Samwald, U.; Kudlacek, S.; Marculescu, R.; Pietschmann, P. Serum levels of sclerostin and dickkopf–1: Effects of age, gender and fracture status. Gerontology 2014, 60, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Reppe, S.; Noer, A.; Grimholt, R.M.; Halldórsson, B.V.; Medina-Gomez, C.; Gautvik, V.T.; Olstad, O.K.; Berg, J.P.; Datta, H.; Estrada, K. Methylation of bone SOST, its mRNA, and serum sclerostin levels correlate strongly with fracture risk in postmenopausal women. J. Bone Miner. Res. 2015, 30, 249–256. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Roato, I.; D’Amico, L.; Veneziano, L.; Suman, E.; Sassi, F.; Bisignano, G.; Ferracini, R.; Gargiulo, G.; Castoldi, F. Bone and bone marrow pro–osteoclastogenic cytokines are up–regulated in osteoporosis fragility fractures. Osteoporos Int. 2011, 22, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Jemtland, R.; Holden, M.; Reppe, S.; Olstad, O.K.; Reinholt, F.P.; Gautvik, V.T.; Refvem, H.; Frigessi, A.; Houston, B.; Gautvik, K.M. Molecular disease map of bone characterizing the postmenopausal osteoporosis phenotype. J. Bone Miner. Res. 2011, 26, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Reppe, S.; Refvem, H.; Gautvik, V.T.; Olstad, O.K.; Høvring, P.I.; Reinholt, F.P.; Holden, M.; Frigessi, A.; Jemtland, R.; Gautvik, K.M. Eight genes are highly associated with BMD variation in postmenopausal Caucasian women. Bone 2010, 46, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Olarescu, N.C.; Jorgensen, A.P. Increased serum and bone matrix levels of the secreted Wnt antagonist DKK–1 in patients with growth hormone deficiency in response to growth hormone treatment. J. Clin. Endocrinol. Metab. 2015, 100, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Yang, C.; Li, Y.; Dai, Z. An overview of osteocalcin progress. J. Bone Miner. Metab. 2016, 34, 367–379. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; Bradley, A.; Karsenty, G. Increased bone formation in osteocalcin–deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Lambert, L.J.; Challa, A.K.; Niu, A.; Zhou, L.; Tucholski, J.; Johnson, M.S.; Nagy, T.R.; Eberhardt, A.W.; Estep, P.N.; Kesterson, R.A. Increased trabecular bone and improved biomechanics in an osteocalcin–null rat model created by CRISPR/Cas9 technology. Dis. Model Mech. 2016, 9, 1169–1179. [Google Scholar] [CrossRef]
- DeFranco, D.J.; Glowacki, J.; Cox, K.A.; Lian, J.B. Normal bone particles are preferentially resorbed in the presence of osteocalcin–deficient bone particles in vivo. Calcif. Tissue Int. 1991, 49, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Amano, S. Osteocalcin fragment in bone matrix enhances osteoclast maturation at a late stage of osteoclast differentiation. J. Bone Miner. Metab. 2004, 22, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Brixen, K.; Mosekilde, L.; Mosekilde, L.; Flyvbjerg, A.; Bollerslev, J. Age–related changes in cortical bone content of insulin–like growth factor binding protein (IGFBP)–3, IGFBP–5, osteoprotegerin, and calcium in postmenopausal osteoporosis: A cross–sectional study. J. Clin. Endocrinol. Metab. 2003, 88, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bao, Q.; Chen, S.; Liu, H.; Feng, J.; Qin, H.; Li, A.; Liu, D.; Shen, Y.; Zhao, Y.; et al. Different bone remodeling levels of trabecular and cortical bone in response to changes in Wnt/beta–catenin signaling in mice. J. Orthop. Res. 2017, 35, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.; Alkhouli, M.; Gerard-O’Riley, R.L.; Wright, W.; Acton, D.; Gray, A.K.; Patel, B.; Reilly, A.M.; Lim, K.E.; Robling, A.G.; et al. Osteoblast–Specific Overexpression of Human WNT16 Increases Both Cortical and Trabecular Bone Mass and Structure in Mice. Endocrinology 2016, 157, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Choi, H.J.; Sun, H.J.; Yang, J.Y.; An, J.H.; Cho, S.W.; Kim, S.W.; Kim, S.Y.; Kim, J.E.; Shin, C.S. Transgenic mice overexpressing secreted frizzled–related proteins (sFRP)4 under the control of serum amyloid P promoter exhibit low bone mass but did not result in disturbed phosphate homeostasis. Bone 2010, 47, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, R.; Kitazawa, R.; Mori, K. sFRP4–dependent Wnt signal modulation is critical for bone remodeling during postnatal development and age–related bone loss. Sci. Rep. 2016, 6, 25198. [Google Scholar] [CrossRef]
- Cook, G.J.; Lodge, M.A.; Blake, G.M.; Marsden, P.K.; Fogelman, I. Differences in skeletal kinetics between vertebral and humeral bone measured by 18F–fluoride positron emission tomography in postmenopausal women. J. Bone Miner. Res. 2000, 15, 763–769. [Google Scholar] [CrossRef]
- Duan, Y.; Tabensky, A.; DeLuca, V.; Seeman, E. The benefit of hormone replacement therapy on bone mass is greater at the vertebral body than posterior processes or proximal femur. Bone 1997, 21, 447–451. [Google Scholar] [CrossRef]
- Li, J.; Sarosi, I.; Cattley, R.C.; Pretorius, J.; Asuncion, F.; Grisanti, M.; Morony, S.; Adamu, S.; Geng, Z.; Qiu, W. Dkk1–mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 2006, 39, 754–766. [Google Scholar] [CrossRef]
- Morvan, F.; Boulukos, K.; Clement-Lacroix, P.; Roman Roman, S.; Suc-Royer, I.; Vayssière, B.; Ammann, P.; Martin, P.; Pinho, S.; Pognonec, P. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J. Bone Miner. Res. 2006, 21, 934–945. [Google Scholar] [CrossRef]
- Tian, E.; Zhan, F.; Walker, R.; Rasmussen, E.; Ma, Y.; Barlogie, B.; Shaughnessy, J.D., Jr. The role of the Wnt–signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003, 349, 2483–2494. [Google Scholar] [CrossRef]
- Brunetti, G.; Papadia, F.; Tummolo, A.; Fischetto, R.; Nicastro, F.; Piacente, L.; Ventura, A.; Mori, G.; Oranger, A.; Gigante, I.; et al. Impaired bone remodeling in children with osteogenesis imperfecta treated and untreated with bisphosphonates: The role of DKK1, RANKL, and TNF–alpha. Osteoporos Int. 2016, 27, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Ventura, A.; Delvecchio, M.; Fusillo, A.; Piacente, L.; Aceto, G.; Colaianni, G.; Colucci, S.; Cavallo, L.; Grano, M. High Sclerostin and Dickkopf–1 (DKK–1) Serum Levels in Children and Adolescents With Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2017, 102, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Van Bezooijen, R.L.; Roelen, B.A.; Visser, A.; van der Wee-Pals, L.; de Wilt, E.; Karperien, M.; Hamersma, H.; Papapoulos, S.E.; ten Dijke, P.; Löwik, C.W. Sclerostin is an osteocyte–expressed negative regulator of bone formation, but not a classical BMP antagonist. J. Exp. Med. 2004, 199, 805–814. [Google Scholar] [CrossRef]
- Piters, E.; Culha, C.; Moester, M.; Van Bezooijen, R.; Adriaensen, D.; Mueller, T.; Weidauer, S.; Jennes, K.; de Freitas, F.; Löwik, C. First missense mutation in the SOST gene causing sclerosteosis by loss of sclerostin function. Hum. Mutat. 2010, 31, E1526–E1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bezooijen, R.L.; ten Dijke, P.; Papapoulos, S.E.; Lowik, C.W. SOST/sclerostin, an osteocyte–derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005, 16, 319–327. [Google Scholar] [CrossRef]
- Niida, A.; Hiroko, T.; Kasai, M.; Furukawa, Y.; Nakamura, Y.; Suzuki, Y.; Sugano, S.; Akiyama, T. DKK1, a negative regulator of Wnt signaling, is a target of the beta–catenin/TCF pathway. Oncogene 2004, 23, 8520–8526. [Google Scholar] [CrossRef]
- Crane, J.L.; Cao, X. Function of matrix IGF–1 in coupling bone resorption and formation. J. Mol. Med. (Berl.) 2014, 92, 107–115. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Schaffler, M.B.; Cheung, W.Y.; Majeska, R.; Kennedy, O. Osteocytes: Master orchestrators of bone. Calcif. Tissue Int. 2014, 94, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Wijenayaka, A.R.; Kogawa, M.; Lim, H.P.; Bonewald, L.F.; Findlay, D.M.; Atkins, G.J. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL–dependent pathway. PLoS ONE 2011, 6, e25900. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Janz, S. Attenuation of WNT signaling by DKK–1 and –2 regulates BMP2–induced osteoblast differentiation and expression of OPG, RANKL and M–CSF. Mol. Cancer 2007, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, O.; Pena, C.; Garcia, J.M.; Larriba, M.J.; Ordóñez-Morán, P.; Navarro, D.; Barbáchano, A.; López de Silanes, I.; Ballestar, E.; Fraga, M.F. The Wnt antagonist DICKKOPF–1 gene is induced by 1alpha,25–dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 2007, 28, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Otterdal, K.; Lekva, T.; Halvorsen, B.; Gabrielsen, A.; Sandberg, W.J.; Paulsson-Berne, G.; Pedersen, T.M.; Folkersen, L.; Gullestad, L. Dickkopf–1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Akerblom, A.; Ghukasyan, T.; Michelsen, A.E.; Becker, R.C.; Bertilsson, M.; Himmelmann, A.; James, S.K.; Siegbahn, A.; Storey, R.F. Admission Levels of DKK1 (Dickkopf–1) Are Associated With Future Cardiovascular Death in Patients With Acute Coronary Syndromes. Arterioscler. Thromb.Vasc. Biol. 2019, 39, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, S.; Santilli, F.; Liani, R.; Vazzana, N.; Ueland, T.; Di Fulvio, P.; Formoso, G.; Consoli, A.; Aukrust, P.; Davì, G. Circulating dickkopf–1 in diabetes mellitus: Association with platelet activation and effects of improved metabolic control and low–dose aspirin. J. Am. Heart Assoc. 2014, 3, e001000. [Google Scholar] [CrossRef] [PubMed]
- Celer, O.; Akalin, A.; Oztunali, C. Effect of teriparatide treatment on endothelial function, glucose metabolism and inflammation markers in patients with postmenopausal osteoporosis. Clin. Endocrinol. (Oxf.) 2016, 85, 556–560. [Google Scholar] [CrossRef]
- Recommended methods for the determination of four enzymes in blood. Scand. J. Clin. Lab. Investig. 1974, 33, 291–306. [CrossRef]
- Brixen, K.; Nielsen, H.K.; Eriksen, E.F.; Charles, P.; Mosekilde, L. Efficacy of wheat germ lectin–precipitated alkaline phosphatase in serum as an estimator of bone mineralization rate: Comparison to serum total alkaline phosphatase and serum bone Gla–protein. Calcif. Tissue Int. 1989, 44, 93–98. [Google Scholar] [CrossRef]
- Ebbesen, E.N.; Thomsen, J.S.; Mosekilde, L. Nondestructive determination of iliac crest cancellous bone strength by pQCT. Bone 1997, 21, 535–540. [Google Scholar] [CrossRef]
- Ueland, T.; Bollerslev, J.; Hansen, T.B.; Ebbesen, E.N.; Mosekilde, L.; Brixen, K.; Flyvbjerg, A.; Djøseland, O. Increased cortical bone content of insulin–like growth factors in acromegalic patients. J. Clin. Endocrinol. Metab. 1999, 84, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Bollerslev, J.; Flyvbjerg, A.; Hansen, T.B.; Vahl, N.; Mosekilde, L. Effects of 12 months of GH treatment on cortical and trabecular bone content of IGFs and OPG in adults with acquired GH deficiency: A double–blind, randomized, placebo–controlled study. J. Clin. Endocrinol. Metab. 2002, 87, 2760–2763. [Google Scholar] [CrossRef] [PubMed]
- Boonen, S.; Aerssens, J.; Dequeker, J.; Nicholson, P.; Cheng, X.; Lowet, G.; Verbeke, G.; Bouillon, R. Age–associated decline in human femoral neck cortical and trabecular content of insulin–like growth factor I: Potential implications for age–related (type II) osteoporotic fracture occurrence. Calcif. Tissue Int. 1997, 61, 173–178. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Postmenopausal Osteoporosis | Normal Postmenopausal Range |
|---|---|---|
| Age in yr (mean, range) | 63 (47–74) | |
| Years since menopause | 16 ± 10 | |
| sPTH (ng/L) | 37 ± 14 | 30 ± 11 |
| sICTP (μg/L) | 3.2 ± 1.1 | 3.2 ± 1.0 |
| sOCN (μg/mL) | 16.6 ± 10.8 | 13.7 ± 7.1 |
| sAP (U/L) | 20 ± 5 | 11 ± 5 |
| Prevalent vertebral compression fractures | ||
| 0/1/ > 1 | 49/4/3 | |
| BMD (g/cm2) | ||
| Lumbar spine, n = 52 | 0.71 ± 0.10 | 0.89 ± 0.11 † |
| Femoral neck, n = 50 | 0.61 ± 0.07 | 0.73 ± 0.10 † |
| pQCT (mg/cm−3), n = 38 | 198 ± 76 | |
| Fmax (N), n = 37 | 67 ± 54 |
| Characteristic | Cortical | Trabecular | ||
|---|---|---|---|---|
| DKK1 | SOST | DKK1 | SOST | |
| Age | −0.13 | −0.04 | −0.19 | −0.09 |
| Years since menopause | −0.23 | 0.00 | −0.30 | −0.19 |
| sPTH | −0.16 | −0.20 | −0.14 | −0.04 |
| sICTP | −0.10 | 0.07 | 0.00 | 0.09 |
| sAP | 0.22 | 0.03 | 0.20 | 0.08 |
| sOCN | 0.02 | 0.06 | 0.12 | 0.09 |
| OCN † | 0.28 * | 0.11 | 0.40 ** | 0.45 ** |
| Calcium † | 0.20 | 0.13 | −0.08 | −0.09 |
| BMD lumbar spine, n = 52 | 0.32 * | 0.10 | 0.33 * | 0.20 |
| BMD femoral neck, n = 50 | 0.41 ** | 0.21 | 0.17 | 0.10 |
| pQCT, n = 38 | 0.34 * | 0.51 ** | 0.11 | 0.28 |
| Fmax, n = 37 | 0.50 ** | 0.46 ** | 0.14 | 0.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueland, T.; Stilgren, L.; Bollerslev, J. Bone Matrix Levels of Dickkopf and Sclerostin are Positively Correlated with Bone Mass and Strength in Postmenopausal Osteoporosis. Int. J. Mol. Sci. 2019, 20, 2896. https://doi.org/10.3390/ijms20122896
Ueland T, Stilgren L, Bollerslev J. Bone Matrix Levels of Dickkopf and Sclerostin are Positively Correlated with Bone Mass and Strength in Postmenopausal Osteoporosis. International Journal of Molecular Sciences. 2019; 20(12):2896. https://doi.org/10.3390/ijms20122896
Chicago/Turabian StyleUeland, Thor, Lis Stilgren, and Jens Bollerslev. 2019. "Bone Matrix Levels of Dickkopf and Sclerostin are Positively Correlated with Bone Mass and Strength in Postmenopausal Osteoporosis" International Journal of Molecular Sciences 20, no. 12: 2896. https://doi.org/10.3390/ijms20122896
APA StyleUeland, T., Stilgren, L., & Bollerslev, J. (2019). Bone Matrix Levels of Dickkopf and Sclerostin are Positively Correlated with Bone Mass and Strength in Postmenopausal Osteoporosis. International Journal of Molecular Sciences, 20(12), 2896. https://doi.org/10.3390/ijms20122896