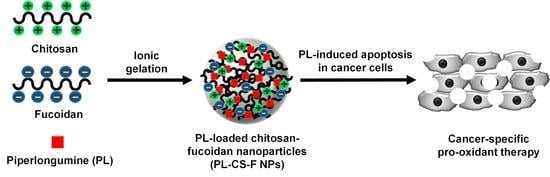
Selective Anticancer Therapy Using Pro-Oxidant Drug-Loaded Chitosan–Fucoidan Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of PL-CS–F NPs
2.2. Drug Release of PL-CS–F NPs
2.3. Cancer-Specific Cytotoxicity of PL-CS–F NPs
2.4. Elevation of Intracellular ROS by PL-CS–F NPs
2.5. Apoptosis of PL-CS–F NPs
3. Materials and Methods
3.1. Materials
3.2. Preparation of CS–F NPs and PL-CS–F NPs
3.3. Characterization of PL-CS–F NPs
3.4. Quantification of PL Encapsulation Efficiency
3.5. Drug Release Study of PL-CS–F NPs
3.6. Cell Culture
3.7. Assessment of In Vitro Cytotoxicity
3.8. Quantification of Intracellular ROS Levels
3.9. Apoptosis Analysis by Annexin V-FITC/PI Staining
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Y.; Zhang, A.Q.; Cheng, S.X.; Rong, L.; Zhang, X.Z. Drug self-delivery systems for cancer therapy. Biomaterials 2017, 112, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer. Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006, 58, 1532–1555. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef]
- Venkatesan, J.; Alam, M.S.; Hong, E.J.; Kim, S.-K.; Shim, M.S. Preparation of piperlongumine-loaded chitosan nanoparticles for safe and efficient cancer therapy. RSC Adv. 2016, 6, 79307–79316. [Google Scholar] [CrossRef]
- Chávez de Paz, L.E.; Resin, A.; Howard, K.A.; Sutherland, D.S.; Wejse, P.L. Antimicrobial effect of chitosan nanoparticles on streptococcus mutans biofilms. Appl. Environ. Microbiol. 2011, 77, 3892–3895. [Google Scholar] [CrossRef]
- Duttagupta, D.S.; Jadhav, V.M.; Kadam, V.J. Chitosan: A propitious biopolymer for drug delivery. Curr. Drug Deliv. 2015, 12, 369–381. [Google Scholar] [CrossRef]
- Kumar, M.N.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell. 2006, 10, 175–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Jiang, H.; Corbet, C.; de Mey, S.; Law, K.; Gevaert, T.; Feron, O.; De Ridder, M. Piperlongumine increases sensitivity of colorectal cancer cells to radiation: Involvement of ROS production via dual inhibition of glutathione and thioredoxin systems. Cancer Lett. 2019, 450, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.J.; Lee, D.; Kang, H.C.; Kim, Y.-C.; Shim, M.S. Cancer-specific pro-oxidant therapy using low-toxic polypeptide micelles encapsulating piperlongumine. J. Ind. Eng. Chem. 2018, 63, 57–64. [Google Scholar] [CrossRef]
- Makhov, P.; Golovine, K.; Teper, E.; Kutikov, A.; Mehrazin, R.; Corcoran, A.; Tulin, A.; Uzzo, R.G.; Kolenko, V.M. Piperlongumine promotes autophagy via inhibition of Akt/mTOR signalling and mediates cancer cell death. Br. J. Cancer 2014, 110, 899–907. [Google Scholar] [CrossRef] [Green Version]
- Hang, W.; Yin, Z.-X.; Liu, G.; Zeng, Q.; Shen, X.-F.; Sun, Q.-H.; Li, D.-D.; Jian, Y.-P.; Zhang, Y.-H.; Wang, Y.-S.; et al. Piperlongumine and p53-reactivator APR-246 selectively induce cell death in HNSCC by targeting GSTP1. Oncogene 2018, 37, 3384–3398. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z. Increased oxidative stress as a selective anticancer therapy. Oxid. Med. Cell Longev. 2015, 2015, 294303. [Google Scholar] [CrossRef]
- Wang, J.; Yao, K.; Wang, C.; Tang, C.; Jiang, X. Synthesis and drug delivery of novel amphiphilic block copolymers containing hydrophobic dehydroabietic moiety. J. Mater. Chem. B 2013, 17, 2324–2332. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, S.; Kang, H.C.; Shim, M.S. ROS-responsive thioether-based nanocarriers for efficient pro-oxidant cancer therapy. J. Ind. Eng. Chem. 2019, 75, 238–245. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Liu, T.J. Mobilization of mesenchymal stem cells by stromal cell-derived factor-1 released from chitosan/tripolyphosphate/fucoidan nanoparticles. Acta Biomater. 2012, 8, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Application of pH-responsive fucoidan/chitosan nanoparticles to improve oral quercetin delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, J.-L.; Kim, E.H.; Park, J.Y.; Kim, J.W.; Kwon, M.; Lee, B.-H. Piperlongumine selectively kills cancer cells and increases cisplatin antitumor activity in head and neck cancer. Oncotarget 2014, 5, 9227–9238. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Samples | Size (nm) | PDI | Zeta Potential (mV) | Encapsulation Efficiency of PL (%) |
|---|---|---|---|---|
| CS–F NPs | 234.73 ± 12.82 | 0.162 ± 0.004 | 7.86 ± 0.72 | N/A |
| PL-CS–F NPs | 215.70 ± 13.38 | 0.163 ± 0.030 | 19.26 ± 2.02 | 16.87 + 1.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.G.; Venkatesan, J.; Shim, M.S. Selective Anticancer Therapy Using Pro-Oxidant Drug-Loaded Chitosan–Fucoidan Nanoparticles. Int. J. Mol. Sci. 2019, 20, 3220. https://doi.org/10.3390/ijms20133220
Choi DG, Venkatesan J, Shim MS. Selective Anticancer Therapy Using Pro-Oxidant Drug-Loaded Chitosan–Fucoidan Nanoparticles. International Journal of Molecular Sciences. 2019; 20(13):3220. https://doi.org/10.3390/ijms20133220
Chicago/Turabian StyleChoi, Dae Gun, Jayachandran Venkatesan, and Min Suk Shim. 2019. "Selective Anticancer Therapy Using Pro-Oxidant Drug-Loaded Chitosan–Fucoidan Nanoparticles" International Journal of Molecular Sciences 20, no. 13: 3220. https://doi.org/10.3390/ijms20133220
APA StyleChoi, D. G., Venkatesan, J., & Shim, M. S. (2019). Selective Anticancer Therapy Using Pro-Oxidant Drug-Loaded Chitosan–Fucoidan Nanoparticles. International Journal of Molecular Sciences, 20(13), 3220. https://doi.org/10.3390/ijms20133220