Affinity Immobilization of a Bacterial Prolidase onto Metal-Ion-Chelated Magnetic Nanoparticles for the Hydrolysis of Organophosphorus Compounds
Abstract
:1. Introduction
2. Results
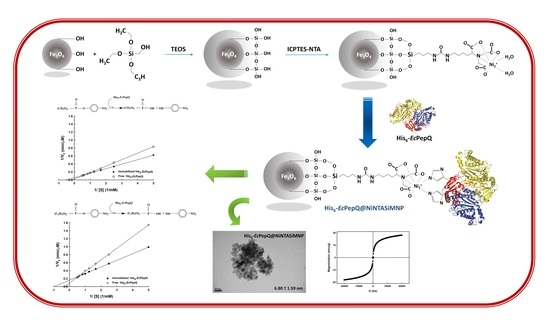
2.1. Preparation of Ni2+-Functionalized Silica-Coated MNPs (NiNTASiMNPs)
2.2. Enzyme Immobilization
2.3. Molecular Properties of Enzyme-Immobilized NiNTASiMNPs
2.4. Effects of Temperature and pH on the Activity of Free and Immobilized Enzymes
2.5. Storage Stability and Reusability of the Immobilized Enzyme
2.6. Hydrolysis of Organophosphorus Compounds by Free and Immobilized Enzymes
3. Discussion
4. Materials and Methods
4.1. Materials and Instrumentation
4.2. Preparation of Surface-Functionalized Magnetic Nanoparticles
4.3. Enzyme Immobilization
4.4. Activity Assay
4.5. Effects of Temperature and pH on the Activity of Free and Immobilized Enzymes
4.6. Storage Stability of Free and Immobilized Enzymes
4.7. Reusability of the Immobilized Enzyme
4.8. Hydrolysis of Two Selected Organophosphorus Compounds by the Recombinant Enzyme
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Regil, R.; Sandoval, G. Biocatalysis for biobased chemicals. Biomolecules 2013, 3, 812–847. [Google Scholar] [PubMed]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and cost analysis for catalyst production in biocatalytic processes. Org. Process. Res. Dev. 2011, 15, 266–274. [Google Scholar]
- Khan, A.A.; Alzohairy, M.A. Recent advances and applications of immobilized enzyme technologies: A review. Res. J. Biol. Sci. 2010, 5, 565–575. [Google Scholar]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar]
- Sirisha, V.L.; Jain, A.; Jain, A. Enzyme immobilization: An overview on methods, support materials and applications of immobilized enzymes. Adv. Food Nutr. Res. 2016, 79, 179–211. [Google Scholar] [PubMed]
- Sari, M.; Akgöl, S.; Karatas, M.; Denizli, A. Reversible immobilization of catalase by metal chelate affinity interaction on magnetic beads. Ind. Eng. Chem. Res. 2006, 45, 3036–3043. [Google Scholar]
- Wang, F.; Guo, C.; Liu, H.Z.; Liu, C.Z. Immobilization of Pycnoporus sanguineus laccase by metal affinity adsorption on magnetic chelator particles. J. Chem. Technol. Biotechnol. 2008, 83, 97–104. [Google Scholar]
- Chen, T.; Yang, W.; Guo, Y.; Yuan, R.; Xu, L.; Yan, Y. Enhancing catalytic performance of β-glucosidase via immobilization on metal ions chelated magnetic nanoparticles. Enzyme Microb. Technol. 2014, 63, 50–57. [Google Scholar]
- Lin, J.; Liu, Y.; Chen, S.; Le, X.; Zhou, X.; Zhao, Z.; Ou, Y.; Yang, J. Reversible immobilization of laccase onto metal-ion-chelated magnetic microspheres for bisphenol A removal. Int. J. Biol. Macromol. 2016, 84, 189–199. [Google Scholar]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process. Res. Dev. 2011, 15, 213–223. [Google Scholar]
- Jia, J.; Zhang, W.; Yang, Z.; Yang, X.; Wang, N.; Yu, X. Novel magnetic cross-linked cellulose aggregates with a potential application in lignocellulosic biomass bioconversion. Molecules 2017, 22, 269. [Google Scholar]
- Cruz-Izquierdo, Á.; Picó, E.A.; López, C.; Serra, J.L.; Llama, M.J. Magnetic cross-linked enzyme aggregates (mCLEAs) of Candida antarctica lipase: An efficient and stable biocatalyst for biodiesel synthesis. PLoS ONE 2014, 9, e115202. [Google Scholar]
- Chen, Y.Y.; Tsai, M.G.; Chi, M.C.; Wang, T.F.; Lin, L.L. Covalent immobilization of Bacillus licheniformis γ-glutamyltranspeptidase on aldehyde-functionalized magnetic nanoparticles. Int. J. Mol. Sci. 2013, 14, 4613–4628. [Google Scholar] [PubMed]
- Juang, T.Y.; Kan, S.J.; Chen, Y.Y.; Tsai, Y.L.; Lin, M.G.; Lin, L.L. Preparation of surface-functionized hyperbranched poly(amido acids) magnetic nanocarriers for the covalent immobilization of a bacterial γ-glutamyltranspeptidase. Molecules 2014, 19, 4997–5012. [Google Scholar] [PubMed]
- Gülay, S.; Şanli-Mohamed, G. Immobilization of thermoalkalophilic recombiant esterase enzyme by entrapment in silicate coated Ca-alginate neads and its hydolytic properties. Int. J. Biol. Macromol. 2012, 50, 545–551. [Google Scholar] [PubMed]
- Raghu, H.S.; Rajeshwara, N.A. Immobilization of α-amylase (1,4-α-D-glucanglucanohydrolase) by calcium alginate encapsulation. Int. Food Res. J. 2015, 22, 869–871. [Google Scholar]
- Šafarik, I.; Šafariková, M. Use of magnetic techiques for the isolation of cells. J. Chromatogr. B 1999, 722, 33–53. [Google Scholar]
- Rusetski, A.N.; Ruuge, E.K. Magnetic fluid as a possible drug carrier for thrombosis treatment. J. Magn. Magn. Mater. 1990, 85, 299–302. [Google Scholar]
- Štafarik, I.; Šafariková, M. Magnetic techniques for the isolation and purification of proteins and peptides. Biomagn. Res. Technol. 2004, 2, 7. [Google Scholar]
- Shen, L.; Zhang, X.; Jin, W. Signal amplification based on DNA hybridization–dehybridization reaction on the surface of magnet submicrobeads for ultrasensitive DNA detection. Analyst 2012, 137, 4849–4854. [Google Scholar]
- Situ, S.F.; Samia, A.C. Highly efficient antibacterial iron oxide@carbon nanochains from wüstite precursor nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 20154–20163. [Google Scholar] [PubMed]
- Tian, T.; Shi, X.; Liang, C.; Luo, Y.; Dong, Z.; Gong, H.; Xu, L.; Zhong, Z.; Peng, R.; Liu, Z. Graphene-based nanocomposite as an effective, multifunctional, and recyclable antibacterial agent. ACS Appl. Mater. Interfaces 2014, 6, 8542–8548. [Google Scholar] [PubMed]
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of iron magnetic nanoparticles in protein immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [PubMed]
- Chen, Y.H.; Chi, M.C.; Wang, T.F.; Chen, J.C.; Lin, L.L. Preparation of magnetic nanoparticles and thier use for immobilization of C-terminally lysine-tagged Bacillus sp. TS-23 α-amylase. Appl. Biochem. Biotechnol. 2012, 166, 1711–1722. [Google Scholar] [PubMed]
- Sommaruga, S.; Galbiati, E.; Peñaranda-Avila, J.; Brambilla, C.; Tortora, P.; Colombo, M.; Prosperi, D. Immobilization of of carboxypeptidase from Sulfolobus solfataricus on magnetic nanoparticles improves enzyme stability and functionality in organic media. BMC Biotechnol. 2014, 14, 82. [Google Scholar]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Taroog, M.V.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar]
- Johnson, P.A.; Park, H.J.; Driscoll, A.J. Enzyme nanoparticle fabrication: Magnetic nanoparticle synthesis and enzyme immobilization. Methods Mol. Biol. 2011, 679, 183–191. [Google Scholar]
- Schenk, G.; Mateen, I.; Ng, T.K.; Pedroso, M.M.; Mitić, N.; Jafelicci, M., Jr.; Marques, R.F.C.; Gahan, L.R.; Ollis, D.L. Organophosphate-degrading metallohydrolases: Structure and function of potent catalysts for applications in bioremediation. Coord. Chem. Rev. 2016, 317, 122–131. [Google Scholar] [Green Version]
- Weaver, J.; Watts, T.; Li, P.; Rye, H.S. Structural basis of substrate selectivity of E. coli prolidase. PLoS ONE 2014, 9, e111531. [Google Scholar]
- DeFrank, J.J.; Cheng, T.C. Purification and properties of an organophosphorus acid anhydrase from a halophilic bacterial isolate. J. Bacteriol. 1991, 173, 1938–1943. [Google Scholar]
- Cheng, T.C.; Rastogi, V.K.; DeFrank, J.J.; Sawiris, G.P. G-type nerve agent decontamination by Alteromonas prolidase. Annu. N. Y. Acad. Sci. 1998, 864, 253–258. [Google Scholar]
- diTargiani, R.C.; Chandrasekaran, L.; Belinskaya, T.; Saxena, A. In search of a catalytic bioscavenger for the prophylaxis of nerve agent toxicity. Chem. Biol. Interact. 2010, 187, 349–354. [Google Scholar] [PubMed]
- Theriot, C.M.; Semcer, R.L.; Shah, S.S.; Grunden, A.M. Improving the catalytic activity of hyperthermophilic Pyrococcus horikoshii prolidase for detoxification of organophosphorus nerve agents over a broad range of temperatures. Archaea 2011, 2011, 565127. [Google Scholar] [PubMed]
- Chandrasekaran, L.; Belinskaya, T.; Saxena, A. In Vitro characterization of organophosphorus compound hydrolysis by native and recombinant human prolidase. Toxicol. Vitro 2013, 27, 499–506. [Google Scholar]
- Yuh, H.; Lee, S.; Kim, S.; Yu, J.; Lee, N.; Lee, J.; Kim, N.D.; Yu, C.; Rho, J. Improved hydrolysis of organophosphorus compounds by engineered human prolidases. Protein Pept. Lett. 2017, 24, 617–625. [Google Scholar]
- Theriot, C.M.; Du, X.; Tove, S.R.; Grunden, A.M. Improving the catalytic activity of hyperthermophilic Pyrococcus prolidases for detoxification of organophosphorus nerve agents over a broad range of temperatures. Appl. Microbiol. Biotechnol. 2010, 87, 1715–1726. [Google Scholar] [PubMed]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar]
- Wang, T.F.; Chi, M.C.; Lai, G.L.; Lin, M.G.; Chen, Y.Y.; Lo, H.F.; Lin, L.L. High-level expression and molecular characterization of a recombinant prolidase from Escherichia coli NovaBlue. PeerJ 2018, 6, e5863. [Google Scholar]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.L.; Zboril, R.; Varma, R.S. Core-shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar]
- Woo, E.J.; Ponvel, K.M.; Ahn, I.S.; Lee, C.H. Synthesis of magnetic/silica nanoparticles with a core of magnetic clusters and their application for the immobilization of His-tagged enzymes. J. Mater. Chem. 2010, 20, 1511–1515. [Google Scholar]
- Mazzucchelli, S.; Verderio, P.; Sommaruga, S.; Colombo, M.; Salvade, A.; Corsi, F.; Galeffi, P.; Tortora, P.; Properi, D. Multiple presentation of Scfv800E6 on silica nanospheres enhances targeting efficiency toward HER-2 receptor. Bioconjugate Chem. 2011, 22, 2296–2303. [Google Scholar]
- Cui, J.; Sun, B.; Lin, T.; Feng, Y.; Jia, S. Enzyme shielding by mesoporous organosilica shell on Fe3O4@silica yolk-shell nanospheres. Int. J. Biol. Macromol. 2018, 117, 673–682. [Google Scholar] [PubMed]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, X.; Zhao, D. Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [PubMed]
- Khalil, M.I. Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron(III) salts as precursors. Arab. J. Chem. 2015, 8, 279–284. [Google Scholar] [Green Version]
- Medvedeva, I.; Bakhteeva, Y.; Zhakov, S.; Revvo, A.; Byzov, I.; Uimin, M.; Yermakov, A.; Mysik, A. Sedimentation and aggregation of magnetic nanoparticles in water by a gradient magnetic field. J. Nanopart. Res. 2013, 15, 2054. [Google Scholar]
- Tang, S.C.N.; Lo, I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [PubMed]
- Vikesland, P.J.; Rebodos, R.L.; Bottero, J.Y.; Rose, J.; Maison, A. Aggregation and sedimentation of magnetite nanoparticle clusters. Environ. Sci. Nano 2016, 3, 567. [Google Scholar]
- Radoń, A.; Łoński, S.; Warski, T.; Babilas, R.; Tański, T.; Dudziak, M.; Łukowiec, D. Catalytic activity of non-spherical shaped magnetic nanoparticles in degradation of Sudan I, Rhodamine B and Methylene Blue dyes. Appl. Surf. Sci. 2019, 487, 1018–1025. [Google Scholar]
- Vasylkiv, O.; Bezdorozhev, O.; Sakka, Y. Synthesis of iron oxide nanoparticles with different morphologies by precipitation method with and without chitosan addition. J. Ceram. Soc. Jp. 2016, 124, 489–494. [Google Scholar] [Green Version]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar]
- Namduri, H.; Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar]
- Song, J.; Su, P.; Yang, Y.; Yang, Y. Efficient immobilization of enzymes onto magnetic nanoparticles by DNA strand displacement: A stable and high-performance biocatalyst. New J. Chem. 2017, 41, 6089–6097. [Google Scholar]
- Yang, Y.; Zhu, G.; Wang, G.; Li, Y.; Tang, R. Robust glucose oxidase with a Fe3O4@C-silica nanohybrid structure. J. Mater. Chem. B 2016, 4, 4726–4731. [Google Scholar]
- Xiao, A.; Xu, C.; Lin, Y.; Ni, H.; Zhu, Y.; Cai, H. Preparation and characterization of κ-carrageenase immobilized onto magnetic iron oxide nanoparticles. Elect. J. Biotechnol. 2016, 19, 1–7. [Google Scholar]
- Li, C.; Jiang, S.; Zhao, X.; Liang, H. Co-immobilization of enzymes and magnetic nanoparticles by metal-nucleotide hydrogel nanofibers for improving stability and recycling. Molecules 2017, 22, 179. [Google Scholar]
- Kitchener, R.L.; Grunden, A.M. Prolidase function in proline metabolism and its medical and biotechnological applications. J. Appl. Microbiol. 2012, 113, 233–247. [Google Scholar] [PubMed]
- Wong, I.Y.; Bhatia, S.N.; Toner, M. Nanotechnology: Emerging tools for biology and medicine. Genes Dev. 2013, 27, 2397–2408. [Google Scholar] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [PubMed]
- Secundo, F. Conformational changes of enzymes upon immobilization. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [PubMed]
- Misson, M.; Zhang, H.; Jin, B. Nanobiocatalyst advancements and bioprocessing applications. J. R. Soc. Interface 2015, 12, 20140891. [Google Scholar]
- Casida, J.E. Pest toxicology: The primary mechanisms of pesticide action. Chem. Res. Toxicol. 2009, 22, 609–619. [Google Scholar] [PubMed]
- Kanekar, P.P.; Bhadbhade, B.J.; Deshpande, N.M.; Sarnaik, S.S. Biodegradation of organophosphorus pesticides. Natl. Acad. Sci. Proc. USA 2004, 70, 57–70. [Google Scholar]
- Singh, B.K.; Walker, A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [PubMed] [Green Version]
- Ghosh, M.; Grunden, A.M.; Dunn, D.M.; Weiss, R.; Adams, M.W. Characterization of native and recombinant forms of an unusual cobalt-dependent proline dipeptidase (prolidase) from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 1998, 180, 4781–4789. [Google Scholar] [PubMed]








| Enzyme Samples | Methyl Paraoxon | Ethyl Paraoxon | ||
|---|---|---|---|---|
| KM (mM) | Vmax (μM min−1) | KM (mM) | Vmax (μM min−1) | |
| Free His6-EcPepQ Immobilized His6-EcPepQ | 8.49 1.97 | 17.48 52.08 | 3.42 1.05 | 6.31 11.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-F.; Lo, H.-F.; Chi, M.-C.; Lai, K.-L.; Lin, M.-G.; Lin, L.-L. Affinity Immobilization of a Bacterial Prolidase onto Metal-Ion-Chelated Magnetic Nanoparticles for the Hydrolysis of Organophosphorus Compounds. Int. J. Mol. Sci. 2019, 20, 3625. https://doi.org/10.3390/ijms20153625
Wang T-F, Lo H-F, Chi M-C, Lai K-L, Lin M-G, Lin L-L. Affinity Immobilization of a Bacterial Prolidase onto Metal-Ion-Chelated Magnetic Nanoparticles for the Hydrolysis of Organophosphorus Compounds. International Journal of Molecular Sciences. 2019; 20(15):3625. https://doi.org/10.3390/ijms20153625
Chicago/Turabian StyleWang, Tzu-Fan, Huei-Fen Lo, Meng-Chun Chi, Kuan-Ling Lai, Min-Guan Lin, and Long-Liu Lin. 2019. "Affinity Immobilization of a Bacterial Prolidase onto Metal-Ion-Chelated Magnetic Nanoparticles for the Hydrolysis of Organophosphorus Compounds" International Journal of Molecular Sciences 20, no. 15: 3625. https://doi.org/10.3390/ijms20153625
APA StyleWang, T. -F., Lo, H. -F., Chi, M. -C., Lai, K. -L., Lin, M. -G., & Lin, L. -L. (2019). Affinity Immobilization of a Bacterial Prolidase onto Metal-Ion-Chelated Magnetic Nanoparticles for the Hydrolysis of Organophosphorus Compounds. International Journal of Molecular Sciences, 20(15), 3625. https://doi.org/10.3390/ijms20153625