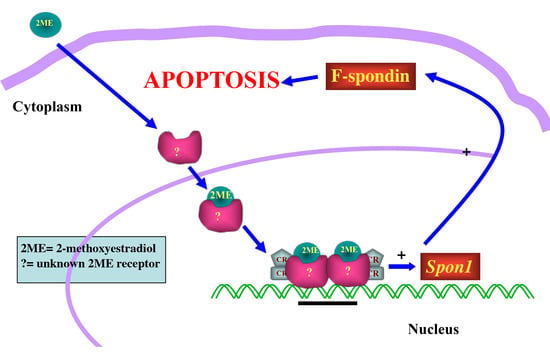
F-Spondin Is the Signal by Which 2-Methoxyestradiol Induces Apoptosis in the Endometrial Cancer Cell Line Ishikawa
Abstract
:1. Introduction
2. Results
2.1. Spon1, but not Scd2 and Snx6, Increased with an Apoptotic Concentration of 2ME
2.2. ME Increased mRNA and Protein Levels of F-Spondin in a Time–Course Manner
2.3. ME Did not Change the Intracellular Distribution of F-Spondin in Ishikawa Cells
2.4. ME-Induced Spon1 Increase Did not Require ER Activation in Ishikawa Cells
2.5. Neutralizing F-Spondin Antibody Blocked the Effect of 2ME on Cell Viability
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Treatments
4.3. Measurement of Cell Viability
4.4. Real-Time Polymerase Chain Reaction
4.5. Immunoblotting
4.6. Immunofluorescence
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Mooberry, S.L. New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Curr. Opin. Oncol. 2003, 15, 425–430. [Google Scholar] [CrossRef]
- Parada-Bustamante, A.; Valencia, C.; Reuquen, P.; Diaz, P.; Rincón-Rodriguez, R.; Orihuela, P. Role of 2-methoxyestradiol, an endogenous estrogen metabolite, in health and disease. Mini Rev. Med. Chem. 2015, 15, 427–438. [Google Scholar] [CrossRef]
- Lee, A.J.; Cai, M.X.; Thomas, P.E.; Conney, A.H.; Zhu, B.T. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology 2003, 14, 3382–3398. [Google Scholar] [CrossRef]
- Sutherland, T.E.; Schuliga, M.; Harris, T.; Eckhardt, B.L.; Anderson, R.L.; Quan, L.; Stewart, A.G. 2-methoxyestradiol is an estrogen receptor agonist that supports tumor growth in murine xenograft models of breast cancer. Clin. Cancer Res. 2005, 11, 1722–1732. [Google Scholar] [CrossRef]
- Mueck, A.O.; Seeger, H. 2-Methoxyestradiol--biology and mechanism of action. Steroids 2010, 75, 625–631. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; van Linbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Carter, J.; Pather, S. An overview of uterine cancer and its management. Expert Rev. Anticancer Ther. 2006, 6, 33–42. [Google Scholar] [CrossRef]
- Saso, S.; Chatterjee, J.; Georgiou, E.; Ditri, A.M.; Smith, J.R.; Ghaem-Maghami, S. Endometrial cancer. BMJ 2011, 6, 343–354. [Google Scholar] [CrossRef]
- Basu, A.; Castle, V.P.; Bouziane, M.; Bhalla, K.; Haldar, S. Crosstalk between extrinsic and intrinsic cell death pathways in pancreatic cancer: Synergistic action of estrogen metabolite and ligands of death receptor family. Cancer Res. 2006, 66, 4309–4318. [Google Scholar] [CrossRef]
- D’Amato, R.J.; Lin, C.M.; Flynn, E.; Folkman, J.; Hamel, E. 2-Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine site. Proc. Natl. Acad. Sci. USA 1994, 91, 3964–3968. [Google Scholar] [CrossRef]
- Nishida, M.; Kasahara, K.; Kaneko, M.; Iwasaki, H.; Hayashi, K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi 1985, 37, 1103–1111. [Google Scholar]
- Kato, S.; Sadarangani, A.; Lange, S.; Villalón, M.; Brañes, J.; Brosens, J.J.; Owen, G.I.; Cuello, M. The oestrogen metabolite 2-methoxyoestradiol alone or in combination with tumour necrosis factor-related apoptosis-inducing ligand mediates apoptosis in cancerous but not healthy cells of the human endometrium. Endocr. Relat. Cancer 2007, 14, 351–368. [Google Scholar] [CrossRef]
- Li, L.; Yu, F.; Wu, X.; Cheng, J.; Ulmsten, U.; Fu, X. Effects of 2-methoxyestradiol on endometrial carcinoma xenografts. J. Cancer Res. Clin. Oncol. 2007, 133, 315–320. [Google Scholar] [CrossRef]
- Parada-Bustamante, A.; Orihuela, P.A.; Molina, C.; Cardenas, H.; Reuquen, P.; Valencia, C.; Rincon, R. Hydroxyestradiols and Methoxyestradiols as Endogenous Factors Associated to Physiological and Physiopathological Conditions. In Estradiol: Synthesis, Health Effects and Drug Interactions; Palmieri, R., Grimaudo, S., Eds.; Nova Science Publishers: New York, NY, USA, 2013; Volume 1, pp. 121–142. [Google Scholar]
- Miyazaki, M.; Gomez, F.E.; Ntambi, J.M. Lack of stearoyl-CoA desaturase-1 function induces a palmitoyl-CoAΔ6 desaturase and represses the stearoyl-CoA desaturase-3 gene in the preputial glands of the mouse. J. Lipid Res. 2002, 43, 2146–2154. [Google Scholar] [CrossRef]
- Parks, W.T.; Frank, D.B.; Huff, C.; Haft, C.F.; Martin, J.; Meng, X.; Caestecker, M.P.; McNally, J.G.; Reddi, A.; Taylor, S.I.; et al. Sorting Nexin 6, a Novel SNX, Interacts with the Transforming Growth Factor-β Family of Receptor Serine-Threonine Kinases. J. Biol. Chem. 2001, 276, 19332–19339. [Google Scholar] [CrossRef]
- Cavet, M.E.; Pang, J.; Yin, G.; Berk, B.C. An epidermal growth factor (EGF)-dependent interaction between GIT1 and sorting nexin 6 promotes degradation of the EGF receptor. FASEB J. 2008, 22, 3607–3616. [Google Scholar] [CrossRef]
- Sibonga, J.D.; Lotinun, S.; Evans, G.L.; Pribluda, V.S.; Green, S.J.; Turner, R.T. Dose-Response Effects of 2-Methoxyestradiol on Estrogen Target Tissues in the Ovariectomized Rat. Endocrinology 2003, 144, 785–792. [Google Scholar] [CrossRef]
- Feinstein, Y.; Klar, A. The neuronal class 2 TSR proteins F-spondin and Mindin: A small family with divergent biological activities. Int. J. Biochem. Cell Biol. 2004, 36, 975–980. [Google Scholar] [CrossRef]
- Tsai, C.L.; Wu, H.M.; Lin, C.Y.; Lin, Y.J.; Chao, A.; Wang, T.H.; Hsueh, S.; Lai, C.H.; Wang, H.S. Estradiol and tamoxifen induce cell migration through GPR30 and activation of focal adhesion kinase (FAK) in endometrial cancers with low or without nuclear estrogen receptor α (ERα). PLoS ONE 2013, 8, e72999. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.A.; Sabo, E. Association of the membrane estrogen receptor, GPR30, with breast tumor metastasis and transactivation of the epidermal growth factor receptor. Steroids 2008, 73, 870–873. [Google Scholar] [CrossRef]
- Kang, S.; Liu, Y.; Sun, D.; Zhou, C.; Liu, A.; Xu, C.; Hao, Y.; Li, D.; Yan, C.; Sun, H. Chronic activation of the G protein-coupled receptor 30 with agonist G-1 attenuates heart failure. PLoS ONE 2012, 7, e48185. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, L.; Mao, X.; Tong, C.; Chen, X.; Zhao, D.; Baker, P.N.; Xia, Y.; Zhang, H. Association of a reduction of G-protein coupled receptor 30 expression and the pathogenesis of preeclampsia. Mol. Med. Rep. 2017, 16, 5997–6003. [Google Scholar] [CrossRef] [Green Version]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001, 27, 93–805. [Google Scholar] [CrossRef]
- Terai, Y.; Abe, M.; Miyamoto, K.; Koike, M.; Yamasaki, M.; Ueda, M.; Ueki, M.; Sato, Y. Vascular smooth muscle cellgrowth-promoting factor/F-spondin inhibits angiogenesis via the blockade of integrin avb3 on vascular endothelial cells. J. Cell Physiol. 2001, 188, 394–402. [Google Scholar] [CrossRef]
- Xiong, S.; Klausen, C.; Cheng, J.C.; Leung, P.C.K. TGFβ1 induces endometrial cancer cell adhesion and migration by up-regulating integrin αvβ3 via SMAD-independent MEK-ERK1/2 signaling. Cell Signal. 2017, 34, 92–101. [Google Scholar] [CrossRef]
- LaVallee, T.M.; Zhan, X.H.; Johnson, M.S.; Herbstritt, C.J.; Swartz, G.; Williams, M.S.; Hembrough, W.A.; Green, S.J.; Pribluda, V.S. 2-methoxyestradiol up-regulates death receptor 5 and induces apoptosis through activation of the extrinsic pathway. Cancer Res. 2003, 63, 468–475. [Google Scholar]
- Thews, O.; Lambert, C.; Kelleher, D.K.; Biesalski, H.K.; Vaupel, P.; Frank, J. Possible protective effects of alpha-tocopherol on enhanced induction of reactive oxygen species by 2-methoxyestradiol in tumors. Adv. Exp. Med. Biol. 2005, 566, 349–355. [Google Scholar]
- Vetvicka, V.; Laganá, A.S.; Salmeri, F.M.; Triolo, O.; Palmara, V.I.; Vitale, S.G.; Sofo, V.; Králíčková, M. Regultaion of apoptotic pathways during endometriosis: From the molecular basis to the future perspectives. Arch. Gynecol. Obstet. 2016, 294, 897–904. [Google Scholar] [CrossRef]
- Kanasaki, K.; Palmsten, K.; Sugimoto, H.; Ahmad, S.; Hamano, Y.; Xie, L.; Parry, S.; Augustin, H.G.; Gattone, V.H.; Folkman, J.; et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 2008, 453, 1117–1121. [Google Scholar] [CrossRef]
- Samartzis, E.P.; Imesch, P.; Twiehaus, A.; Dubey, R.K.; Leeners, B. The estrogen metabolites 2-methoxyestradiol and 2-hydroxyestradiol inhibit endometriotic cell proliferation in estrogen-receptor-independent manner. Gynecol. Endocrinol. 2016, 32, 529–533. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rincón-Rodriguez, R.; Mena, D.; Mena, J.; Díaz-Saldivar, P.; Guajardo-Correa, E.; Godoy-Guzman, C.; Cardenas, H.; Orihuela, P.A. F-Spondin Is the Signal by Which 2-Methoxyestradiol Induces Apoptosis in the Endometrial Cancer Cell Line Ishikawa. Int. J. Mol. Sci. 2019, 20, 3850. https://doi.org/10.3390/ijms20163850
Rincón-Rodriguez R, Mena D, Mena J, Díaz-Saldivar P, Guajardo-Correa E, Godoy-Guzman C, Cardenas H, Orihuela PA. F-Spondin Is the Signal by Which 2-Methoxyestradiol Induces Apoptosis in the Endometrial Cancer Cell Line Ishikawa. International Journal of Molecular Sciences. 2019; 20(16):3850. https://doi.org/10.3390/ijms20163850
Chicago/Turabian StyleRincón-Rodriguez, Ramiro, Dennise Mena, Javier Mena, Patricia Díaz-Saldivar, Emanuel Guajardo-Correa, Carlos Godoy-Guzman, Hugo Cardenas, and Pedro A. Orihuela. 2019. "F-Spondin Is the Signal by Which 2-Methoxyestradiol Induces Apoptosis in the Endometrial Cancer Cell Line Ishikawa" International Journal of Molecular Sciences 20, no. 16: 3850. https://doi.org/10.3390/ijms20163850
APA StyleRincón-Rodriguez, R., Mena, D., Mena, J., Díaz-Saldivar, P., Guajardo-Correa, E., Godoy-Guzman, C., Cardenas, H., & Orihuela, P. A. (2019). F-Spondin Is the Signal by Which 2-Methoxyestradiol Induces Apoptosis in the Endometrial Cancer Cell Line Ishikawa. International Journal of Molecular Sciences, 20(16), 3850. https://doi.org/10.3390/ijms20163850