Isolation of a Novel Metalloproteinase from Agkistrodon Venom and Its Antithrombotic Activity Analysis
Abstract
:1. Introduction
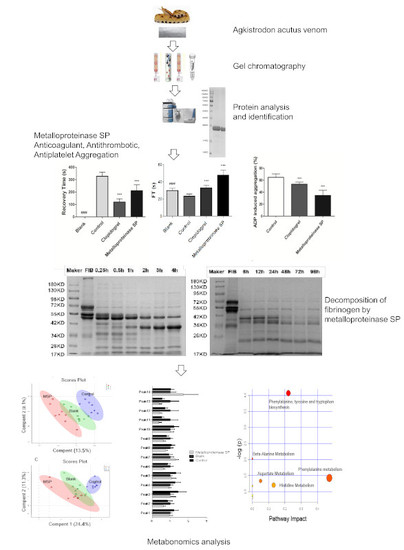
2. Results
2.1. Isolation of Metalloproteinase SP from Agkistrodon Venom
2.2. Protein Identification
2.3. Anti-Thrombotic Activity
2.3.1. Anti-Coagulant Activity
2.3.2. Anti-Thrombotic Activity
2.3.3. Antiplatelet Aggregation Activity
2.4. Fibrinogenolytic Activity
2.5. Effects of Snake Venom Monomer on the Chemical Constituents of Acute Pulmonary Embolism
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Separation and Purification of Metalloproteinase SP
4.3. Protein Quantification and Identification
4.4. Pharmacological Evaluation of Metalloproteinase SP
4.4.1. Anti-Coagulant Activity
Blood Clotting Assays
Blood Parameters Test
Coagulation Function Test
4.4.2. Anti-Thrombotic Activity
ADP-Induced Acute Pulmonary Thrombosis in Mice
Carrageenan-Induced Tail Thrombosis in Mice
4.4.3. Antiplatelet Aggregation Activity
In Vivo Antiplatelet Aggregation Assay
In Vitro Antiplatelet Aggregation Activity
4.4.4. Fibrinogenolytic Activity
Effect of Temperature and pH on Metalloproteinase SP Activity
Effects of Serine Protease and Metalloproteinase Inhibitors on Metalloproteinase SP Activity
4.5. Effects of Snake Venom Monomer on the Chemical Constituents of Acute Pulmonary Embolism Based on UHPLC-Q/TOF-MS Non-Targeted Metabolomics
4.5.1. UHPLC/Q-TOF MS Analysis
4.5.2. Data Processing and Identification of Differential Metabolites
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ADP | Adenosine diphosphate |
| AA | Arachidonic acid |
| RBC | Red blood cell |
| PLT | Platelet |
| PCT | Plateletcrit |
| HCT | Hematocrit |
| MPV | Mean platelet volume |
| PDW | Platelet volume distribution width |
| P-LCR | Platelet -larger cell ratio |
References
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M. Thrombosis: A major contributor to global disease burden. Thromb. Res. 2014, 134, 931–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieswandt, B.; Kleinschnitz, C.; Stoll, G. Ischaemic stroke: A thrombo-inflammatory disease. J. Physiol. 2011, 589, 4115–4123. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Muñoz, G.; Mallavia, B.; Bins, A.; Headley, M.; Krummel, M.F.; Looney, M.R. Aspirin-triggered 15-epi-lipoxin A 4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice. Blood 2014, 124, 2625–2635. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. G. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, Q.D.; Tan, X.L.; Feng, J.; Tang, T.; Xia, J.; Zhang, L.; Huang, L.; Bai, Y.P.; Liu, Y.H. Absence of association between atherosclerotic cerebral infarction and TNFSF4/TNFRSF4 single nucleotide polymorphisms rs1234313, rs1234314 and rs17568 in a Chinese population. J. Int. Med. Res. 2014, 42, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P. Thrombin-like enzymes in snake venoms. In Toxins and Hemostasis, 2nd ed.; Kini, R., Clemetson, K., Eds.; Springer: Dordrecht, Netherlands, 2010; Volume 39, pp. 2–6. [Google Scholar]
- Dempfle, C.E.; Argiriou, S.; Kucher, K.; Müller-Peltzer, H.; Rübsamen, K.; Heene, D.L. Analysis of fibrin formation and proteolysis during intravenous administration of ancrod. Blood 2000, 96, 2793–2802. [Google Scholar]
- Bocian, A.; Urbanik, M.; Hus, K.; Łyskowski, A.; Petrilla, V.; Andrejčáková, Z.; Petrillová, M.; Legáth, J. Proteomic analyses of Agkistrodon contortrix venom using 2D electrophoresis and MS techniques. Toxins 2016, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Tsai, W.C.; Ureña-Diaz, J.M.; Sanz, L.; Mora-Obando, D.; Sánchez, E.E.; Fry, B.G.; Gutiérrez, J.M.; Gibbs, H.L.; Sovic, M.G.; et al. Venomics of new world pit vipers: Genus-wide comparisons of venom proteomes across Agkistrodon. J. Proteomics 2014, 96, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.S.; Wang, X.H.; Zhang, J.H.; Tang, B.S.; Qian, L.; Li, P.Y.; Luo, L.W. Biochemical properties and comparative pharmacology of a coagulant from Deinagkistrodon acutus snake venom. Eur. J. Pharm. Sci. 2013, 49, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Xu, Z.; Qian, C.; Jiang, F.; Ding, X.; Ruan, Y.; Ding, Z.; Fan, Y. Antiplatelet aggregation and antithrombosis efficiency of peptides in the snake venom of Deinagkistrodon acutusr, isolation, identification, and evaluation. Evid-Based Compl. Alt. 2015, 5, 1–6. [Google Scholar]
- Ming, X. A novel direct factor Xa inhibitory peptide with anti-platelet aggregation activity from Agkistrodon acutus venom hydrolysates. Sci. Rep. 2015, 5, 10846. [Google Scholar]
- Zeng, F.; Shen, B.; Zhu, Z.; Zhang, P.; Ji, Y.; Niu, L.; Li, X.; Teng, M. Crystal structure and activating effect on RyRs of AhV_TL-I, a glycosylated thrombin-like enzyme from Agkistrodon halys snake venom. Arch. Toxicol. 2013, 87, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.C.; Zingali, R.B.; Albuquerque, M.G.; Pujol-Luz, M.; Rodrigues, C.R. Snake venom thrombin-like enzymes: From reptilase to now. Cell. Mol. Life Sci. 2004, 61, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.H.; Chen, Y.Y.; Zhou, G.S.; Liu, X.; Tang, Y.P.; Liu, R.; Liu, P.; Li, N.; Yang, J.; Wang, J.; et al. Novel antithrombotic protease from marine worm sipunculus nudus. Int. J. Mol. Sci. 2018, 19, 3023. [Google Scholar] [CrossRef] [PubMed]
- Kini, M.R. Anticoagulant proteins from snake venoms: Structure, function and mechanism. Biochem. J. 2006, 397, 377–387. [Google Scholar] [CrossRef]
- Suntravat, M.; Nuchprayoon, I.; Pérez, J.C. Comparative study of anticoagulant and procoagulant properties of 28 snake venoms from families Elapidae, Viperidae, and purified Russell’s viper venom-factor X activator (RVV-X). Toxicon 2010, 56, 544–553. [Google Scholar] [CrossRef]
- Depta, J.P.; Bhatt, D.L. New approaches to inhibiting platelets and coagulation. Annu. Rev. Pharmacol. 2015, 55, 373–397. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.C.I.; Armugam, A.; Jeyaseelan, K. Snake venom components and their applications in biomedicine. Cell Mol. Life Sci. 2006, 63, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Niall, H.D. Automated edman degradation: The protein sequenator. Method Enzymol. 1973, 27, 942–1010. [Google Scholar]
- Breen, P. Basics of coagulation pathways. Int. Anesthesiol. Clin. 2004, 42, 1–9. [Google Scholar] [CrossRef]
- Corte, A.L.C.L.; Philippou, H.; Ariëns, R.A.S. Role of fibrin structure in thrombosis and vascular disease. Adv. Protein Chem. Str. 2011, 75–127. [Google Scholar]
- Chernysh, I.N.; Nagaswami, C.; Weisel, J.W. Visualization and identification of the structures formed during early stages of fibrin polymerization. Blood 2011, 117, 4609–4614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, G.D.; Lee, A.J.; Rumley, A.; Price, J.F.; Fowkes, F.G. Blood viscosity and risk of cardiovascular events: The Edinburgh Artery Study. Brit. J. Haematol. 2015, 96, 168–173. [Google Scholar] [CrossRef]
- Uitte de Willige, S.; de Visser, M.C.; Houwing-Duistermaat, J.J.; Rosendaal, F.R.; Vos, H.L.; Bertina, R.M. Genetic variation in the fibrinogen gamma gene increases the risk for deep venous thrombosis by reducing plasma fibrinogen gamma’ levels. Blood 2005, 106, 4176–4183. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, G.; Yalcin, O.; Bor-Kucukatay, M.; Ozkaya, G.; Baskurt, O.K. Red blood cell rheological properties in various rat hypertension models. Clin. Hemorheol. Micro. 2002, 26, 27–32. [Google Scholar]
- Bennett, J.S. Platelet-fibrinogen interactions. Ann. N. Y. Acad. Sci. 2010, 936, 340–354. [Google Scholar] [CrossRef]
- Weisel, J.W. The mechanical properties of fibrin for basic scientists and clinicians. Biophys. Chem. 2004, 112, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Allali, Y.; Lesty, C.; Tanguy, M.L.; Silvain, J.; Ankri, A.; Blanchet, B.; Dumaine, R.; Gianetti, J.; Payot, L.; et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscl. Throm. Vas. 2006, 26, 2567–2573. [Google Scholar] [CrossRef]
- Collet, J.P.; Allali, Y.; Lesty, C.; Tanguy, M.L.; Silvain, J.; Ankri, A.; Blanchet, B.; Dumaine, R.; Gianetti, J.; Payot, L.; et al. Fibrinogen Hershey IV: A novel dysfibrinogen with a gammaV411I mutation in the integrin alpha(IIb)beta(3) binding site. J. Thromb. Haemost. 2008, 99, 1008–1012. [Google Scholar]
- Goncalves, I.I.; Hughan, S.C.; Schoenwaelder, S.M.; Yap, C.L.; Yuan, Y.; Jackson, S.P. Integrin alpha IIb beta 3-dependent calcium signals regulate platelet-fibrinogen interactions under flow involvement of phospholipase C gamma 2. J. Biol. Chem. 2003, 278, 34812. [Google Scholar] [CrossRef] [PubMed]
- Wolberg, A.S.; Gabriel, D.A.; Hoffman, M. Analyzing fibrin clot structure using a microplate reader. Blood Coagul Fibrin. 2002, 13, 533–539. [Google Scholar] [CrossRef]
- Mannila, M.N.; Lovely, R.S.; Kazmierczak, S.C.; Eriksson, P.; Samnegård, A.; Farrell, D.H.; Hamsten, A.; Silveira, A. Elevated plasma fibrinogen gamma’ concentration is associated with myocardial infarction: Effects of variation in fibrinogen genes and environmental factors. J. Thromb. Haemost. 2010, 5, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Lowe, G.D. Blood viscosity and cardiovascular disease. J. Thromb. Haemost. 1992, 67, 494–498. [Google Scholar] [CrossRef]
- Koenig, W.; Ernst, E. The possible role of hemorheology in atherothrombogenesis. Atherosclerosis 1992, 94, 93–107. [Google Scholar] [CrossRef]
- Fredenburgh, J.C.; Nesheim, M.E. Lys-plasminogen is a significant intermediate in the activation of Glu-plasminogen during fibrinolysis in vitro. J. Biol. Chem. 1992, 267, 26150–26156. [Google Scholar] [PubMed]
- Hsiao, G.; Shen, M.Y.; Lin, K.H.; Chou, C.Y.; Tzu, N.H.; Lin, C.H.; Chou, D.S.; Chen, T.F.; Sheu, J.R. Inhibitory activity of kinetin on free radical formation of activated platelets in vitro and on thrombus formation in vivo. Eur. J. Pharmacol. 2003, 465, 281–287. [Google Scholar] [CrossRef]
- Chung, T.; Connor, D.; Joseph, J.; Emmett, L.; Mansberg, R.; Peters, M.; Ma, D.; Kritharides, L. Platelet activation in acute pulmonary embolism. J. Thromb. Haemost. 2007, 5, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Miao, H.; Feng, Y.L.; Zhao, Y.Y.; Lin, R.C. Metabolomics in dyslipidemia. Adv. Clin. Chem. 2014, 66, 101–119. [Google Scholar] [PubMed]
- Bujak, R.; García-Álvarez, A.; Rupérez, F.J.; Nuño-Ayala, M.; García, A.; Ruiz-Cabello, J.; Fuster, V.; Ibáñez, B.; Barbas, C. Metabolomics reveals metabolite changes in acute pulmonary embolism. J. Proteome Res. 2014, 13, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Zeleznik, O.A.; Poole, E.M.; Lindstrom, S.; Kraft, P.; Van, H.V.A.; Lasky-Su, J.A.; Harrington, L.B.; Hagan, K.; Kim, J.; Parry, B.A.; et al. Metabolomic analysis of 92 pulmonary embolism patients from a nested case-control study identifies metabolites associated with adverse clinical outcomes. J. Thromb. Haemost. 2017, 16, 500–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkaitis, M.S.; Ackerman, H.C. Tetrahydrobiopterin supplementation improves phenylalanine metabolism in a murine model of severe malaria. ACS Infect. Dis. 2016, 2, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, C.; Zhang, A.; Zhang, J.; Wang, X.; Sun, X.; Sun, Z.; Wang, X. High-throughput metabolomics used to identify potential therapeutic targets of Guizhi Fuling Wan against endometriosis of cold coagulation and blood stasis. RSC Adv. 2018, 8, 19238–19250. [Google Scholar] [CrossRef] [Green Version]
- Schwencke, C.; Schmeisser, A.; Weinbrenner, C.; Braun-Dullaeus, R.C.; Marquetant, R.; Strasser, R.H. Trans regulation of the α2-adrenergic signal transduction pathway by chroni-blockade. J. Cardiovasc. Pharm. 2005, 45, 253–259. [Google Scholar] [CrossRef]
- Li, R.J. The role of histidine in metabolism. Prog. Physiol. Sci. 1985, 2, 80–82. [Google Scholar]
- Duan, J.; Liang, Z.; Yang, C.; Zhang, J.; Zhang, L.; Zhang, W.; Zhang, Y. Rapid protein identification using monolithic enzymatic microreactor and LC-ESI-MS/MS. Proteomics 2006, 6, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, J.; Gao, W.; Liu, Z.; Wu, S.; Jing, S. Study on hemostatic activities of the rhizome of Paris bashanensis. Pharm. Biol. 2013, 51, 1321–1325. [Google Scholar]
- Lu, W.J.; Lee, J.J.; Chou, D.S.; Chou, D.S.; Jayakumar, T.; Fong, T.H.; Hsiao, G.; Sheu, J.R. A novel role of andrographolide, an NF-kappa B inhibitor on inhibition of platelet activation: The pivotal mechanisms of endothelial nitric oxide synthase/cyclic GMP. J. Mol. Med. 2011, 89, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Lemini, C.; Jaimez, R.; Franco, Y. Gender and inter-species influence on coagulation tests of rats and mice. Thromb. Res. 2007, 120, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.C.; Ma, H.P.; Hao, Y.; He, X.R.; Sun, A.J.; Jiang, W.; Li, M.X.; Jing, L.L.; He, L.; Ma, J.; et al. A new anti-fibrinolytic hemostatic compound 8-O-acetyl shanzhiside methylester extracted from Lamiophlomis rotata. J. Ethnopharmacol. 2016, 187, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Vesna, K.; Ivana, F.; Zorica, V. Effects of rutin and hesperidin and their Al (III) and Cu (II) complexes on in vitro plasma coagulation assays. Molecules 2011, 16, 1378–1388. [Google Scholar]
- Sheu, J.R.; Hung, W.C.; Wu, C.H.; Lee, Y.M.; Yen, M.H. Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa on platelet plug formation in in vivo experiments. British J. Haematol. 2000, 110, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Simkhada, J.R.; Cho, S.S.; Mander, P.; Choi, Y.H.; Yoo, J.C. Purification, biochemical properties and antithrombotic effect of a novel Streptomyces enzyme on carrageenan-induced mice tail thrombosis model. J. Thromb. Res. 2012, 129, 170–182. [Google Scholar] [CrossRef]
- Devaraja, S.; Girish, K.S.; Devaraj, V.R.; Kemparaju, K. Factor Xa-like and fibrin (ogen)olytic activities of a serine protease from Hippasa agelenoidesspider venom gland extract. J. Thromb. Thrombolys 2010, 29, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Dutta, S.; Das, T.; Chattopadhyay, P.; Mukherjee, A.K. Antiplatelet and antithrombotic activity of a fibrin (ogen)olytic protease from Bacillus cereus strain FF01. Int. J. Biol. Macromol. 2015, 79, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Mais, E.; Alolga, R.N.; Wang, S.L.; Linus, L.O.; Yin, X.; Qi, L.W. A comparative UPLC-Q/TOF-MS-based metabolomics approach for distinguishing Zingiber officinale Roscoe of two geographical origins. Food Chem. 2017, 240, 239–244. [Google Scholar] [CrossRef]








| Red Blood Cell Count (1012/L) | Hematocrit (%) | Blood Platelet Count (109/L) | Plateletcrit (%) | Mean Platelet Volume (fL) | Platelet Volume Distribution Width (%) | Platelet Larger Cell Ratio (%) | |
|---|---|---|---|---|---|---|---|
| Blank | 5.50 ± 0.29 | 29.4 ± 1.81 | 812 ± 36.7 | 0.440 ± 0.03 | 5.45 ± 0.16 | 4.15 ± 0.20 | 5.93 ± 0.93 |
| Clopidogrel sulfate | 5.61 ± 0.22 | 29.2 ± 0.81 | 783 ± 27.5 | 0.430 ± 0.02 | 5.47 ± 0.41 | 4.22 ± 0.22 | 4.42 ± 0.50 *** |
| Metalloproteinase SP | 5.47 ± 0.20 | 28.9 ± 0.96 | 777 ± 26.5 * | 0.410 ± 0.02 ** | 5.42 ± 0.27 | 4.21 ± 0.30 | 6.45 ± 0.26 |
| Red Blood Cell Count (1012/L) | Hematocrit (%) | Blood Platelet Count (109/L) | Plateletcrit (%) | Mean Platelet Volume (fL) | Platelet Volume Distribution Width (%) | Platelet-Larger Cell Ratio (%) | |
|---|---|---|---|---|---|---|---|
| Blank | 6.20 ± 0.57 | 31.4 ± 2.51 | 701 ± 86.3 | 0.391 ± 0.04 | 5.46 ± 0.19 | 4.37 ± 0.20 | 6.21 ± 0.79 |
| Clopidogrel sulfate | 5.37 ± 0.31 ** | 27.9 ± 1.66 ** | 733 ± 99.5 | 0.382 ± 0.06 | 5.29 ± 0.21 | 4.45 ± 0.25 | 4.60 ± 0.94 ** |
| Metalloproteinase SP | 5.63 ± 0.24 * | 30.1 ± 2.56 | 613 ± 61.6 * | 0.341 ± 0.02 ** | 5.41 ± 0.29 | 4.36 ± 0.60 | 5.39 ± 1.21 |
| Red Blood Cell Count (1012/L) | Hematocrit (%) | Blood Platelet Count (109/L) | Plateletcrit (%) | Mean Platelet Volume (fL) | Platelet Volume Distribution Width (%) | |
|---|---|---|---|---|---|---|
| Blank | 8.01 ± 0.33 | 37.4 ± 2.36 | 865 ± 51.6 | 0.400 ± 0.04 | 4.16 ± 1.83 | 4.15 ± 0.21 |
| Control | 7.54 ± 0.40 | 34.6 ± 1.64 | 720 ± 73.5 | 0.350 ± 0.04 | 4.44 ± 0.65 | 3.99 ± 0.34 |
| Clopidogrel sulfate | 6.93 ± 0.16 ** | 31.3 ± 1.83 ** | 704 ± 56.9 | 0.320 ± 0.01 | 4.57 ± 0.167 | 3.91 ± 0.36 |
| Metalloproteinase SP | 6.92 ± 0.19 ** | 32.0 ± 1.16 ** | 640 ± 64.3 * | 0.300 ± 0.03 * | 4.70 ± 0.20 | 3.84 ± 0.28 |
| Peak NO | Metabolite Name | Formula | Rt (min) | Molecular Weight | p-Value | FD | Control | Metalloproteinase SP | (+) MS | (+) MS/MS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5-Aminopentanoic acid | C5H11NO2 | 0.850 | 117.0790 | *↓ | 0.7883 | 1.205 ± 0.241 | 0.952 ± 0.179 | 118.0861 | 118.086, 100.075, 72.080, 57.033, 56.049, 55.054, 44.049, 43.018 |
| 2 | o-Tyrosine | C9H11NO3 | 1.100 | 181.0739 | *↓ | 0.7218 | 0.857 ± 0.255 | 0.615 ± 0.131 | 182.0812 | 182.081, 165.05, 136.075, 119.040 |
| 3 | Piperidine | C5H11N | 1.467 | 85.0891 | **↓ | 0.7029 | 1.431 ± 0.445 | 1.006 ± 0.295 | 86.0964 | 86.096, 69.070, 57.070, 56.050, 55.054, 52.033, 44.050, 43.054, |
| 4 | L-Isoleucine | C6H13NO2 | 1.483 | 131.0946 | *↓ | 0.7330 | 1.261 ± 0.316 | 0.924 ± 0.138 | 132.1016 | 132.101, 86.096, 69.069, 44.049, 41.038 |
| 5 | L-Phenylalanine | C9H11NO2 | 1.917 | 165.0790 | *↓ | 0.8219 | 1.314 ± 0.249 | 1.083 ± 0.237 | 166.0856 | 166.086, 149.059, 131.049, 120.080, 107.049, 103.054 |
| 6 | L-Norleucine | C6H13NO2 | 2.083 | 131.0946 | **↓ | 0.7168 | 0.860 ± 0.153 | 0.616 ± 0.095 | 132.1009 | 132.10, 97.653, 86.695, 69.069, 44.048, 30.338 |
| 7 | Palmitoyl Ethanolamide | C18H37NO2 | 3.767 | 299.2824 | **↑ | 1.3987 | 0.917 ± 0.208 | 1.283 ± 0.183 | 300.2892 | 300.289, 282.279, 85.100, 83.085, 71.085, 67.054, 62.060, 57.069, 43.054, |
| 8 | beta-Alanine | C3H7NO2 | 0.617 | 89.0477 | *↓ | 0.7330 | 1.071 ± 0.195 | 0.751 ± 0.110 | 88.0406 | 88.040, 71.013, 59.013, 53.003, 44.997, 43.018, 42.034, 41.002 |
| 9 | L-Tyrosine | C9H11NO3 | 0.900 | 181.0739 | *↓ | 0.7772 | 0.795 ± 0.116 | 0.618 ± 0.127 | 180.0664 | 180.066, 163.040, 119.050 72.009, 93.034, 74.024 |
| 10 | Benzyl glycinate | C9H11NO2 | 1.916 | 165.0790 | *↓ | 0.8400 | 1.208 ± 0.269 | 1.015 ± 0.174 | 164.0716 | 164.071, 91.055, 77.039 |
| 11 | p-Cresol glucuronide | C13H16O7 | 2.267 | 284.0896 | **↓ | 0.6891 | 0.540 ± 0.116 | 0.372 ± 0.158 | 283.0818 | 283.081, 265.073, 175.026, 117.019, 107.049, 87.008, 71.014, 43.018 |
| 12 | p-Cresol sulfate | C7H8O4S | 2.633 | 188.0143 | **↓ | 0.5838 | 0.714 ± 0.159 | 0.417 ± 0.128 | 187.0073 | 187.007, 107.50, 105.334, 77.039 |
| 13 | 10-HDoHE | C22H32O3 | 4.800 | 344.2351 | **↓ | 0.6892 | 0.559 ± 0.120 | 0.385 ± 0.164 | 343.2274 | 343.227, 325.210, 281.227, 189.130, 161.130, 153.092, 137.095, 109.102, 59.013 |
| 14 | Octadec-9-enoic Acid | C18H34O2 | 10.150 | 282.4680 | **↑ | 1.6569 | 1.033 ± 0.174 | 1.713 ± 0.826 | 281.2478 | 281.248, 263.238,59.0139 |
| NO | Pathway | Hits | Metabolites |
|---|---|---|---|
| 1 | Phenylalanine, tyrosine and tryptophan biosynthesis | 2 | L-Phenylalanine, L-Tyrosine |
| 2 | Phenylalanine metabolism | 2 | L-Phenylalanine, L-Tyrosine |
| 3 | Beta-Alanine Metabolism | 1 | Beta-Alanine |
| 4 | Aspartate Metabolism | 1 | Beta-Alanine |
| 5 | Histidine Metabolism | 1 | Beta-Alanine |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Fan, H.; Yin, X.; Huang, F. Isolation of a Novel Metalloproteinase from Agkistrodon Venom and Its Antithrombotic Activity Analysis. Int. J. Mol. Sci. 2019, 20, 4088. https://doi.org/10.3390/ijms20174088
Huang J, Fan H, Yin X, Huang F. Isolation of a Novel Metalloproteinase from Agkistrodon Venom and Its Antithrombotic Activity Analysis. International Journal of Molecular Sciences. 2019; 20(17):4088. https://doi.org/10.3390/ijms20174088
Chicago/Turabian StyleHuang, Jin, Hui Fan, Xiaojian Yin, and Fang Huang. 2019. "Isolation of a Novel Metalloproteinase from Agkistrodon Venom and Its Antithrombotic Activity Analysis" International Journal of Molecular Sciences 20, no. 17: 4088. https://doi.org/10.3390/ijms20174088
APA StyleHuang, J., Fan, H., Yin, X., & Huang, F. (2019). Isolation of a Novel Metalloproteinase from Agkistrodon Venom and Its Antithrombotic Activity Analysis. International Journal of Molecular Sciences, 20(17), 4088. https://doi.org/10.3390/ijms20174088