Claudin-19 Is Regulated by Extracellular Osmolality in Rat Kidney Inner Medullary Collecting Duct Cells
Abstract
:1. Introduction
2. Results
2.1. Claudin19 Is Expressed in Inner Medullary Collecting Duct
2.2. AVP Signaling Is Preserved in IMCD Cells at Different Osmotic Culture Conditions
2.3. AVP Treatment Changes IMCD Cell Electrophysiological Properties but not Claudin-19 Localization
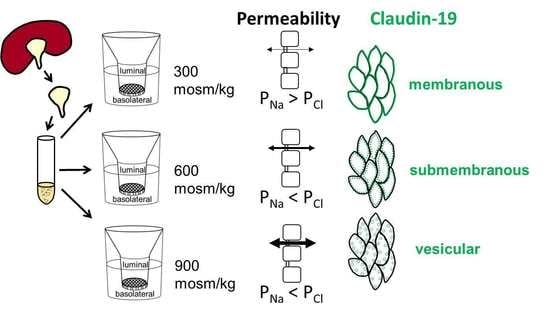
2.4. Differences in Extracellular Osmolality Determine IMCD-Cell Trans- and Paracellular Properties
2.5. Effect of the Different Osmolality on IMCD Transport Properties
2.6. Differences in Extracellular Osmolality Change Claudin-19 Subcellular Localization
2.7. Further Observations
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture
4.3. Immunohistochemistry
4.4. Western Blotting
4.5. Electrophysiology
4.6. FITC Dextran Permeability Measurement
4.7. Electrolyte Measurement
4.8. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IMCD | Inner medullary collecting duct |
| AVP | Arginine vasopressin |
| PG2 | Prostaglandin E2 |
| TJ | Tight junction |
| TAL | Thick ascending limb |
| PT | Proximal tubule |
| PCT | Proximal convoluted tubule |
| PST | Proximal straight |
| DCT | Distal convoluted tubule |
| CNT/CD | Connecting tubule and cortical collecting duct |
| mCD | Medullary collecting duct (outer stripe of outer medulla) |
| SDS | sodium dodecyl sulfate |
| AQP2 | Aquaporin 2 |
| Vte | Transepithelial voltage |
| Rte | Transepithelial resistance |
| I’sc | Equivalent short circuit current |
| TER | Transepithelial resistance (cell culture) |
| DP | Diffusion potential |
References
- Dantzler, W.H.; Pannabecker, T.L.; Layton, A.T.; Layton, H.E. Urine concentrating mechanism in the inner medulla of the mammalian kidney: Role of three-dimensional architecture. Acta Physiol. 2011, 202, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Pannabecker, T.L. Comparative physiology and architecture associated with the mammalian urine concentrating mechanism: Role of inner medullary water and urea transport pathways in the rodent medulla. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R488–R503. [Google Scholar] [CrossRef] [PubMed]
- Sands, J.M.; Layton, H.E. The physiology of urinary concentration: An update. Semin. Nephrol. 2009, 29, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Fenton, R.A.; Knepper, M.A. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol. Rev. 2007, 87, 1083–1112. [Google Scholar] [CrossRef] [PubMed]
- Sheen, M.R.; Kim, J.-A.; Lim, S.W.; Jung, J.-Y.; Han, K.-H.; Jeon, U.S.; Park, S.-H.; Kim, J.; Kwon, H.M. Interstitial tonicity controls TonEBP expression in the renal medulla. Kidney Int. 2009, 75, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Maril, N.; Margalit, R.; Mispelter, J.; Degani, H. Sodium magnetic resonance imaging of diuresis: Spatial and kinetic response. Magn. Reson. Med. 2005, 53, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Berl, T. How Do Kidney Cells Adapt to Survive in Hypertonic Inner Medulla? Trans. Am. Clin. Climatol. Assoc. 2009, 120, 389. [Google Scholar] [PubMed]
- Schulze Blasum, B.; Schröter, R.; Neugebauer, U.; Hofschröer, V.; Pavenstädt, H.; Ciarimboli, G.; Schlatter, E.; Edemir, B. The kidney-specific expression of genes can be modulated by the extracellular osmolality. FASEB J. 2016, 30, 3588–3597. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D.; Yu, A.S.L. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Günzel, D.; Fromm, M. Claudins and other tight junction proteins. Compr. Physiol. 2012, 2, 1819–1852. [Google Scholar]
- González-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Himmerkus, N.; Sunq, A.; Milatz, S.; Merkel, C.; Bleich, M.; Hou, J. ILDR1 is important for paracellular water transport and urine concentration mechanism. Proc. Natl. Acad. Sci. USA 2017, 114, 5271–5276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flamion, B.; Spring, K.R.; Abramow, M. Adaptation of inner medullary collecting duct to dehydration involves a paracellular pathway. Am. J. Physiol. 1995, 268, F53–F63. [Google Scholar] [CrossRef] [PubMed]
- Muto, S. Physiological roles of claudins in kidney tubule paracellular transport. Am. J. Physiol. Ren. Physiol. 2017, 312, F9–F24. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Renigunta, A.; Yang, J.; Waldegger, S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc. Natl. Acad. Sci. USA 2010, 107, 18010–18015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luk, J.M.; Tong, M.-K.; Mok, B.W.; Tam, P.-C.; Yeung, W.S.B.; Lee, K.-F. Sp1 site is crucial for the mouse claudin-19 gene expression in the kidney cells. FEBS Lett. 2004, 578, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konrad, M.; Schaller, A.; Seelow, D.; Pandey, A.V.; Waldegger, S.; Lesslauer, A.; Vitzthum, H.; Suzuki, Y.; Luk, J.M.; Becker, C.; et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am. J. Hum. Genet. 2006, 79, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Angelow, S.; El-Husseini, R.; Kanzawa, S.A.; Yu, A.S.L. Renal localization and function of the tight junction protein, claudin-19. Am. J. Physiol. Ren. Physiol. 2007, 293, F166–F177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Renigunta, A.; Konrad, M.; Gomes, A.S.; Schneeberger, E.E.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J. Clin. Investig. 2008, 118, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Weber, S.; Hoffmann, K.; Jeck, N.; Saar, K.; Boeswald, M.; Kuwertz-Broeking, E.; Meij, I.I.C.; Knoers, N.V.A.M.; Cochat, P.; Suláková, T.; et al. Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur. J. Hum. Genet. 2000, 8, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Renigunta, A.; Gomes, A.S.; Hou, M.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc. Natl. Acad. Sci. USA 2009, 106, 15350–15355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Günzel, D.; Haisch, L.; Pfaffenbach, S.; Krug, S.M.; Milatz, S.; Amasheh, S.; Hunziker, W.; Müller, D. Claudin function in the thick ascending limb of Henle’s loop. Ann. N. Y. Acad. Sci. 2009, 1165, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.P.Y.; Tong, M.K.; Leung, P.P.; Chan, V.W.; Leung, S.; Tam, P.-C.; Chan, K.-W.; Lee, K.-F.; Yeung, W.S.B.; Luk, J.M. Kidney claudin-19: Localization in distal tubules and collecting ducts and dysregulation in polycystic renal disease. FEBS Lett. 2006, 580, 923–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Chou, C.-L.; Knepper, M.A. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J. Am. Soc. Nephrol. 2015, 26, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Maric, K.; Oksche, A.; Rosenthal, W. Aquaporin-2 expression in primary cultured rat inner medullary collecting duct cells. Am. J. Physiol. 1998, 275, F796–F801. [Google Scholar] [CrossRef] [PubMed]
- Schenk, L.K.; Rinschen, M.M.; Klokkers, J.; Kurian, S.M.; Neugebauer, U.; Salomon, D.R.; Pavenstaedt, H.; Schlatter, E.; Edemir, B. Cyclosporin-A Induced Toxicity in Rat Renal Collecting Duct Cells: Interference with Enhanced Hypertonicity Induced Apoptosis. Cell. Physiol. Biochem. 2010, 26, 887–900. [Google Scholar] [CrossRef]
- Sonntag, S.R.; Ziemens, A.; Wulfmeyer, V.C.; Milatz, S.; Bleich, M.; Himmerkus, N. Diuretic state affects ascending thin limb tight junctions. Am. J. Physiol. Renal Physiol. 2018, 314, F190–F195. [Google Scholar] [CrossRef]
- Gong, Y.; Hou, J. Claudins in barrier and transport function-the kidney. Pflug. Arch. 2017, 469, 105–113. [Google Scholar] [CrossRef]
- Yu, A.S.L. Claudins and the kidney. J. Am. Soc. Nephrol. 2015, 26, 11–19. [Google Scholar] [CrossRef]
- Ruhfus, B.; Bauernschmitt, H.G.; Kinne, R.K. Properties of a polarized primary culture from rat renal inner medullary collecting duct (IMCD) cells. Cell. Dev. Biol. Anim. 1998, 34, 227–231. [Google Scholar] [CrossRef]
- Cil, O.; Ertunc, M.; Onur, R. The diuretic effect of urea analog dimethylthiourea in female Wistar rats. Hum. Exp. Toxicol. 2012, 31, 1050–1055. [Google Scholar] [CrossRef]
- Husted, R.F.; Hayashi, M.; Stokes, J.B. Characteristics of papillary collecting duct cells in primary culture. Am. J. Physiol. 1988, 255, F1160–F1169. [Google Scholar] [CrossRef] [PubMed]
- Mongelli-Sabino, B.M.; Canuto, L.P.; Collares-Buzato, C.B. Acute and chronic exposure to high levels of glucose modulates tight junction-associated epithelial barrier function in a renal tubular cell line. Life Sci. 2017, 188, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Canuto, L.P.; Collares-Buzato, C.B. Increased osmolality enhances the tight junction-mediated barrier function in a cultured renal epithelial cell line. Cell Biol. Int. 2019, 43, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Michea, L.; Ferguson, D.R.; Peters, E.M.; Andrews, P.M.; Kirby, M.R.; Burg, M.B. Cell cycle delay and apoptosis are induced by high salt and urea in renal medullary cells. Am. J. Physiol. Ren. Physiol. 2000, 278, F209–F218. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.C.; Chevaile, A.; Hébert, M.J.; Zagajeski, J.; Gullans, S.R. A combination of NaCl and urea enhances survival of IMCD cells to hyperosmolality. Am. J. Physiol. 1998, 274, F1167–F1173. [Google Scholar] [CrossRef] [PubMed]
- Capasso, J.M.; Rivard, C.J.; Berl, T. Long-term adaptation of renal cells to hypertonicity: Role of MAP kinases and Na-K-ATPase. Am. J. Physiol. Ren. Physiol. 2001, 280, F768–F776. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Almeida, N.E.; Andres-Hernando, A.; Rivard, C.J.; Capasso, J.M.; Berl, T. The tight junction protein, MUPP1, is up-regulated by hypertonicity and is important in the osmotic stress response in kidney cells. Proc. Natl. Acad. Sci. USA 2007, 104, 13672–13677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanaspa, M.A.; Andres-Hernando, A.; Rivard, C.J.; Dai, Y.; Berl, T. Hypertonic stress increases claudin-4 expression and tight junction integrity in association with MUPP1 in IMCD3 cells. Proc. Natl. Acad. Sci. USA 2008, 105, 15797–15802. [Google Scholar] [CrossRef] [Green Version]
- Hager, H.; Kwon, T.H.; Vinnikova, A.K.; Masilamani, S.; Brooks, H.L.; Frøkiaer, J.; Knepper, M.A.; Nielsen, S. Immunocytochemical and immunoelectron microscopic localization of alpha-, beta-, and gamma-ENaC in rat kidney. Am. J. Physiol. Ren. Physiol. 2001, 280, F1093–F1106. [Google Scholar] [CrossRef]
- Ronzaud, C.; Loffing, J.; Bleich, M.; Gretz, N.; Gröne, H.-J.; Schütz, G.; Berger, S. Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J. Am. Soc. Nephrol. 2007, 18, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Leviel, F.; Hübner, C.A.; Houillier, P.; Morla, L.; El Moghrabi, S.; Brideau, G.; Hatim, H.; Parker, M.D.; Kurth, I.; Kougioumtzes, A.; et al. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J. Clin. Investig. 2010, 120, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Crambert, G.; Ernandez, T.; Lamouroux, C.; Roth, I.; Dizin, E.; Martin, P.-Y.; Féraille, E.; Hasler, U. Epithelial sodium channel abundance is decreased by an unfolded protein response induced by hyperosmolality. Physiol. Rep. 2014, 2, e12169. [Google Scholar] [CrossRef] [PubMed]
- Milatz, S.; Himmerkus, N.; Wulfmeyer, V.C.; Drewell, H.; Mutig, K.; Hou, J.; Breiderhoff, T.; Müller, D.; Fromm, M.; Bleich, M.; et al. Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc. Natl. Acad. Sci. USA 2017, 114, E219–E227. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Renigunta, V.; Himmerkus, N.; Zhang, J.; Renigunta, A.; Bleich, M.; Hou, J. Claudin-14 regulates renal Ca(+)(+) transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. 2012, 31, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Nusrat, A.; Parkos, C.A.; Verkade, P.; Foley, C.S.; Liang, T.W.; Innis-Whitehouse, W.; Eastburn, K.K.; Madara, J.L. Tight junctions are membrane microdomains. J. Cell Sci. 2000, 113, 1771–1781. [Google Scholar] [PubMed]
- Mitic, L.L. Expression, solubilization, and biochemical characterization of the tight junction transmembrane protein claudin-4. Protein Sci. 2003, 12, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugibayashi, K.; Onuki, Y.; Takayama, K. Displacement of tight junction proteins from detergent-resistant membrane domains by treatment with sodium caprate. Eur. J. Pharm. Sci. 2009, 36, 246–253. [Google Scholar] [CrossRef]
- Wilson, J.L.L.; Miranda, C.A.; Knepper, M.A. Vasopressin and the regulation of aquaporin-2. Clin. Exp. Nephrol. 2013, 17, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Krug, S.M.; Amasheh, S.; Richter, J.F.; Milatz, S.; Günzel, D.; Westphal, J.K.; Huber, O.; Schulzke, J.D.; Fromm, M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell 2009, 20, 3713–3724. [Google Scholar] [CrossRef]
- Tokuda, S.; Yu, A.S.L. Regulation of Epithelial Cell Functions by the Osmolality and Hydrostatic Pressure Gradients: A Possible Role of the Tight Junction as a Sensor. Int. J. Mol. Sci. 2019, 20, 3513. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, S.; Hirai, T.; Furuse, M. Effects of osmolality on paracellular transport in MDCK II cells. PLoS ONE 2016, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Himmerkus, N.; Plain, A.; Marques, R.D.; Sonntag, S.R.; Paliege, A.; Leipziger, J.; Bleich, M. AVP dynamically increases paracellular Na+ permeability and transcellular NaCl transport in the medullary thick ascending limb of Henle’s loop. Pflug. Arch. 2017, 469, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Plain, A.; Wulfmeyer, V.C.; Milatz, S.; Klietz, A.; Hou, J.; Bleich, M.; Himmerkus, N. Corticomedullary difference in the effects of dietary Ca2+ on tight junction properties in thick ascending limbs of Henle’s loop. Pflug. Arch. 2016, 468, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.; Kaminski, H.; Castrop, H.; Bader, M.; Himmerkus, N.; Bleich, M.; Bachmann, S.; Theilig, F. Intrarenal renin angiotensin system revisited: Role of megalin-dependent endocytosis along the proximal nephron. J. Biol. Chem. 2010, 285, 41935–41946. [Google Scholar] [CrossRef] [PubMed]
- Barry, P.H.; Lynch, J.W. Liquid junction potentials and small cell effects in patch-clamp analysis. J. Membr. Biol. 1991, 121, 101–117. [Google Scholar] [CrossRef]
- Kimizuka, H.; Koketsu, K. Ion transport through cell membrane. J. Theor. Biol. 1964, 6, 290–305. [Google Scholar] [CrossRef]









| Control Solution | Low NaCl Solution | |||||
|---|---|---|---|---|---|---|
| ‘300‘ | ‘600‘ | ‘900‘ | ‘300‘ | ‘600‘ | ‘900‘ | |
| NaCl | 145 | 245 | 345 | 30 | 50 | 70 |
| KH2PO4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| K2HPO4 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 |
| MgCl2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ca-gluconate | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| glucose | 5 | 5 | 5 | 5 | 5 | 5 |
| urea | - | 100 | 200 | - | 100 | 200 |
| mannitol | - | - | - | 230 | 390 | 550 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziemens, A.; Sonntag, S.R.; Wulfmeyer, V.C.; Edemir, B.; Bleich, M.; Himmerkus, N. Claudin-19 Is Regulated by Extracellular Osmolality in Rat Kidney Inner Medullary Collecting Duct Cells. Int. J. Mol. Sci. 2019, 20, 4401. https://doi.org/10.3390/ijms20184401
Ziemens A, Sonntag SR, Wulfmeyer VC, Edemir B, Bleich M, Himmerkus N. Claudin-19 Is Regulated by Extracellular Osmolality in Rat Kidney Inner Medullary Collecting Duct Cells. International Journal of Molecular Sciences. 2019; 20(18):4401. https://doi.org/10.3390/ijms20184401
Chicago/Turabian StyleZiemens, Annalisa, Svenja R. Sonntag, Vera C. Wulfmeyer, Bayram Edemir, Markus Bleich, and Nina Himmerkus. 2019. "Claudin-19 Is Regulated by Extracellular Osmolality in Rat Kidney Inner Medullary Collecting Duct Cells" International Journal of Molecular Sciences 20, no. 18: 4401. https://doi.org/10.3390/ijms20184401
APA StyleZiemens, A., Sonntag, S. R., Wulfmeyer, V. C., Edemir, B., Bleich, M., & Himmerkus, N. (2019). Claudin-19 Is Regulated by Extracellular Osmolality in Rat Kidney Inner Medullary Collecting Duct Cells. International Journal of Molecular Sciences, 20(18), 4401. https://doi.org/10.3390/ijms20184401