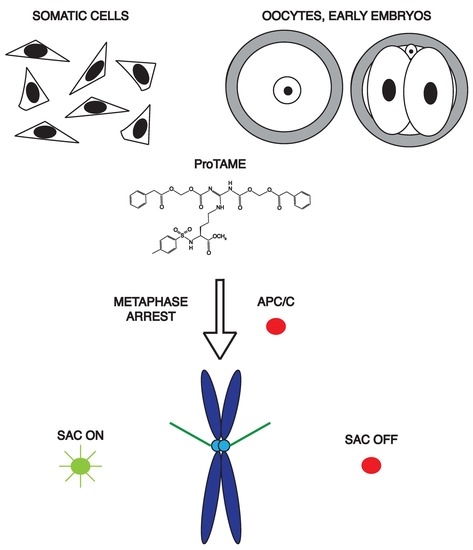
ProTAME Arrest in Mammalian Oocytes and Embryos Does Not Require Spindle Assembly Checkpoint Activity
Abstract
:1. Introduction
2. Results
2.1. ProTAME Prevents Anaphase Entry in Mouse and Bovine Oocytes and also in Mouse 2-Cell Embryos
2.2. ProTAME Arrest of Meiosis I in Mouse Oocytes is Due to the Inhibition of APC/C
2.3. ProTAME-Induced Arrest of Oocytes in Meiosis I Is Irreversible
2.4. ProTAME Affects Spindle Morphology in Oocytes and Embryos
2.5. ProTAME-Induced Arrest of Mouse Oocytes and Embryos Does Not Require SAC
3. Discussion
4. Materials and Methods
4.1. Animals, Isolation of Oocytes and Embryos and Cell Cultivation
4.2. Microinjection of GV Oocytes and Embryos
4.3. Treatment with Inhibitors
4.4. Immunodetection
4.5. Microscopy and Live Cell Imaging
4.6. Image Analysis and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APC/C | Anaphase promoting complex/cyclosome |
| BUBR1 | BUB1 related kinase 1 |
| BUB3 | Budding uninhibited by benzimidazole 3 |
| CDC20 | Cell division cycle 20 |
| CDH1 | Cadherin 1 |
| COCs | Cumulus–oocyte complexes |
| GV | Germinal vesicle |
| GVBD | Germinal vesicle breakdown |
| IBMX | 3-isobutyl-1-methylxanthine |
| MAD1 | Mitotic arrest deficient 1 |
| MAD2 | Mitotic arrest deficient 2 |
| MCC | Mitotic checkpoint complex |
| MI | Metaphase I |
| MPS1 | Monopolar spindle 1 |
| MTOCs | Microtubule organizing centers |
| NEBD | Nuclear envelope breakdown |
| ON | Overnight |
| PBE | Polar body extrusion |
| PLK1 | Polo-like kinase 1 |
| ProTAME | Prodrug tosyl-l-arginine methyl ester |
| RT | Room temperature |
| SAC | Spindle assembly checkpoint |
References
- Peters, J.M. The anaphase promoting complex/cyclosome: A machine designed to destroy. Nat. Rev. Mol. Cell Biol. 2006, 7, 644–656. [Google Scholar] [CrossRef]
- Musacchio, A. The Molecular Biology of Spindle Assembly Checkpoint Signaling Dynamics. Curr. Biol. 2015, 25, R1002–R1018. [Google Scholar] [CrossRef] [Green Version]
- Marston, A.L.; Wassmann, K. Multiple Duties for Spindle Assembly Checkpoint Kinases in Meiosis. Front. Cell Dev. Biol. 2017, 5, 109. [Google Scholar] [CrossRef]
- McGuinness, B.E.; Anger, M.; Kouznetsova, A.; Gil-Bernabé, A.M.; Helmhart, W.; Kudo, N.R.; Wuensche, A.; Taylor, S.; Hoog, C.; Novak, B.; et al. Regulation of APC/C activity in oocytes by a BUB1-dependent spindle assembly checkpoint. Curr. Biol. 2009, 19, 369–380. [Google Scholar] [CrossRef]
- Verma, R.; Peters, N.R.; D’Onofrio, M.; Tochtrop, G.P.; Sakamoto, K.M.; Varadan, R.; Zhang, M.; Coffino, P.; Fushman, D.; Deshaies, R.J.; et al. Ubistatins inhibit proteasome-dependent degradation by binding the ubiquitin chain. Science 2004, 306, 117–120. [Google Scholar] [CrossRef]
- Zeng, X.; Sigoillot, F.; Gaur, S.; Choi, S.; Pfaff, K.L.; Oh, D.C.; Hathaway, N.; Dimova, N.; Cuny, G.D.; King, R.W. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell 2010, 18, 382–395. [Google Scholar] [CrossRef]
- Zeng, X.; King, R.W. An APC/C inhibitor stabilizes CYCLIN B1 by prematurely terminating ubiquitination. Nat. Chem. Biol. 2012, 8, 383–392. [Google Scholar] [CrossRef]
- Lara-Gonzalez, P.; Taylor, S.S. Cohesion fatigue explains why pharmacological inhibition of the APC/C induces a spindle checkpoint-dependent mitotic arrest. PLoS ONE 2012, 7, e49041. [Google Scholar] [CrossRef]
- De, K.; Grubb, T.M.; Zalenski, A.A.; Pfaff, K.E.; Pal, D.; Majumder, S.; Summers, M.K.; Venere, M. Hyperphosphorylation of CDH1 in glioblastoma cancer stem cells attenuates APC/CCDH1 activity and pharmacological inhibition of APC/CCDH1/CDC20 compromises viability. Mol. Cancer Res. 2019. [Google Scholar] [CrossRef]
- Raab, M.; Sanhaji, M.; Zhou, S.; Rödel, F.; El-Balat, A.; Becker, S.; Strebhardt, K. Blocking Mitotic Exit of Ovarian Cancer Cells by Pharmaceutical Inhibition of the Anaphase-Promoting Complex Reduces Chromosomal Instability. Neoplasia 2019, 21, 363–375. [Google Scholar] [CrossRef]
- Maes, A.; Maes, K.; De Raeve, H.; De Smedt, E.; Vlummens, P.; Szablewski, V.; Devin, J.; Faict, S.; De Veirman, K.; Menu, E.; et al. The anaphase-promoting complex/cyclosome: A new promising target in diffuse large B-cell lymphoma and mantle cell lymphoma. Br. J. Cancer 2019. [Google Scholar] [CrossRef]
- Akera, T.; Trimm, E.; Lampson, M.A. Molecular and Evolutionary Strategies of Meiotic Cheating by Selfish Centromeres. Available online: https://www.biorxiv.org/content/biorxiv/early/2018/08/30/405068.full.pdf (accessed on 31 August 2018).
- Vázquez-Diez, C.; Paim, L.M.G.; FitzHarris, G. Cell-Size-Independent Spindle Checkpoint Failure Underlies Chromosome Segregation Error in Mouse Embryos. Curr. Biol. 2019, 29, 865–873. [Google Scholar] [CrossRef]
- Reis, A.; Chang, H.Y.; Levasseur, M.; Jones, K.T. APC CDH1 activity in mouse oocytes prevents entry into the first meiotic division. Nat. Cell Biol. 2006, 8, 539–540. [Google Scholar] [CrossRef]
- Rattani, A.; Ballesteros Mejia, R.; Roberts, K.; Roig, M.B.; Godwin, J.; Hopkins, M.; Eguren, M.; Sanchez-Pulido, L.; Okaz, E.; Ogushi, S.; et al. APC/C (CDH1) Enables Removal of SHUGOSHIN-2 from the Arms of Bivalent Chromosomes by Moderating Cyclin-Dependent Kinase Activity. Curr. Biol. 2017, 27, 1462–1476. [Google Scholar] [CrossRef]
- Bennabi, I.; Terret, M.E.; Verlhac, M.H. Meiotic spindle assembly and chromosome segregation in oocytes. J. Cell Biol. 2016, 215, 611–619. [Google Scholar] [CrossRef]
- Mogessie, B.; Scheffler, K.; Schuh, M. Assembly and Positioning of the Oocyte Meiotic Spindle. Ann. Rev. Cell Dev. Biol. 2018, 34, 381–403. [Google Scholar] [CrossRef] [Green Version]
- Holubcová, Z.; Blayney, M.; Elder, K.; Schuh, M. Human oocytes. Error-prone chromosome-mediated spindle assembly favors chromosome segregation defects in human oocytes. Science 2015, 348, 1143–1147. [Google Scholar] [CrossRef]
- Haverfield, J.; Dean, N.L.; Nöel, D.; Rémillard-Labrosse, G.; Paradis, V.; Kadoch, I.J.; FitzHarris, G. Tri-directional anaphases as a novel chromosome segregation defect in human oocytes. Hum. Reprod. 2017, 32, 1293–1303. [Google Scholar] [CrossRef]
- Lane, S.I.R.; Morgan, S.L.; Wu, T.; Collins, J.K.; Merriman, J.A.; ElInati, E.; Turner, J.M.; Jones, K.T. DNA damage induces a kinetochore-based ATM/ATR-independent SAC arrest unique to the first meiotic division in mouse oocytes. Development 2017, 144, 3475–3486. [Google Scholar] [CrossRef] [Green Version]
- Hached, K.; Xie, S.Z.; Buffin, E.; Cladière, D.; Rachez, C.; Sacras, M.; Sorger, P.K.; Wassmann, K. MPS1 at kinetochores is essential for female mouse meiosis I. Development 2011, 138, 2261–2271. [Google Scholar] [CrossRef]
- Kolano, A.; Brunet, S.; Silk, A.D.; Cleveland, D.W.; Verlhac, M.H. Error-prone mammalian female meiosis from silencing the spindle assembly checkpoint without normal interkinetochore tension. Proc. Natl. Acad. Sci. USA 2012, 109, E1858–E1867. [Google Scholar] [CrossRef] [Green Version]
- Kudo, N.R.; Wassmann, K.; Anger, M.; Schuh, M.; Wirth, K.G.; Xu, H.; Helmhart, W.; Kudo, H.; McKay, M.; Maro, B.; et al. Resolution of chiasmata in oocytes requires separase-mediated proteolysis. Cell 2006, 126, 135–146. [Google Scholar] [CrossRef]
- Kudo, N.R.; Anger, M.; Peters, A.H.; Stemmann, O.; Theussl, H.C.; Helmhart, W.; Kudo, H.; Heyting, C.; Nasmyth, K. Role of cleavage by separase of the REC8 kleisin subunit of cohesin during mammalian meiosis I. J. Cell Sci. 2009, 122, 2686–2698. [Google Scholar] [CrossRef]
- Danylevska, A.; Kovacovicova, K.; Awadova, T.; Anger, M. The frequency of precocious segregation of sister chromatids in mouse female meiosis I is affected by genetic background. Chromosome Res. 2014, 22, 365–373. [Google Scholar] [CrossRef]
- Kovacovicova, K.; Awadova, T.; Mikel, P.; Anger, M. In Vitro Maturation of Mouse Oocytes Increases the Level of KIF11/EG5 on Meiosis II Spindles. Biol. Reprod. 2016, 95, 18. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radonova, L.; Svobodova, T.; Skultety, M.; Mrkva, O.; Libichova, L.; Stein, P.; Anger, M. ProTAME Arrest in Mammalian Oocytes and Embryos Does Not Require Spindle Assembly Checkpoint Activity. Int. J. Mol. Sci. 2019, 20, 4537. https://doi.org/10.3390/ijms20184537
Radonova L, Svobodova T, Skultety M, Mrkva O, Libichova L, Stein P, Anger M. ProTAME Arrest in Mammalian Oocytes and Embryos Does Not Require Spindle Assembly Checkpoint Activity. International Journal of Molecular Sciences. 2019; 20(18):4537. https://doi.org/10.3390/ijms20184537
Chicago/Turabian StyleRadonova, Lenka, Tereza Svobodova, Michal Skultety, Ondrej Mrkva, Lenka Libichova, Paula Stein, and Martin Anger. 2019. "ProTAME Arrest in Mammalian Oocytes and Embryos Does Not Require Spindle Assembly Checkpoint Activity" International Journal of Molecular Sciences 20, no. 18: 4537. https://doi.org/10.3390/ijms20184537
APA StyleRadonova, L., Svobodova, T., Skultety, M., Mrkva, O., Libichova, L., Stein, P., & Anger, M. (2019). ProTAME Arrest in Mammalian Oocytes and Embryos Does Not Require Spindle Assembly Checkpoint Activity. International Journal of Molecular Sciences, 20(18), 4537. https://doi.org/10.3390/ijms20184537