The Role of Angiogenesis and Pro-Angiogenic Exosomes in Regenerative Dentistry
Abstract
:1. Introduction
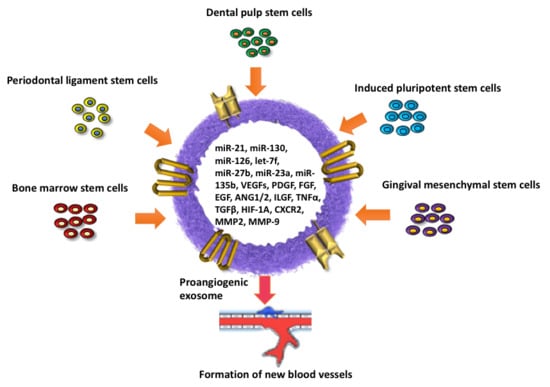
2. Stem Cells Present in the Oral Cavity—Their Regenerative and Pro-Angiogenic Capacity
2.1. General Considerations
2.2. Bone Marrow Mesenchymal Stem Cells (BM-MSC)
2.3. Periodontal Ligament Stem Cells (PDLSCs)
2.4. Dental Pulp Stem Cells (DPSC)
2.5. Gingival Mesenchymal Stem Cells (GMSCs)
2.6. Induced Pluripotent Stem Cells (iPSCs)
3. Characteristics of Pro-Angiogenic Exosomes in Regenerative Dentistry
3.1. Definition and General Characteristics of Exosomes
3.2. Cargo of Pro-Angiogenic Exosomes
4. Key miRNAs with Pro-Angiogenic Effects
5. Key miRNAs with Anti-Angiogenic Effects
| Type of Stem Cells | Source | Differentiation Potential | Alteration of Angiogenesis Related Genes | Ref. |
|---|---|---|---|---|
| BM-MSC | Non-hematopoietic components of the BM | osteoblasts, adipocytes, chondrocytes, smooth muscle, sarcomeric muscle, endothelial, neural and hepatocytic lineages | Upregulation of BCL-2, CXCL1 and CXCR2 | [166,167,168] |
| PDLSC | Mature periodontal ligaments | adipocytes and osteoblasts, cementoblasts, fibroblasts, adipocytes, and chondroblasts, | Upregulation of VEGF and FGF-2 | [59,66] |
| DPSC | Human exfoliated deciduous teeth, apical papilla, periodontal ligament and dental follicle tissue. | osteoblasts, rare pancreatic islands, endothelial and smooth muscle cells | Upregulation of BMP2, BMP6, TGFB1, VEGFA, FGF2 | [112,169,170] |
| GMSC | Gingival connective tissue as gingival mesenchymal stem/progenitor cells | osteoblasts, adipocytes, chondrocytes, endothelial and neural cells | N/A | [75,171] |
| iPSC | Different types of adult cells | fibroblast, adipocytes, cardiomyocytes, pancreatic cells, neural cells | Upregulation of VEGF, TGFB1 and ANG | [78,172] |
6. Current Challenges
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 2010, 11, 710–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, M.Z. (Ed.) Adult Stem Cell Therapies: Alternatives to Plasticity; Springer: New York, NY, USA, 2014. [Google Scholar]
- Jopling, C.; Boue, S.; Izpisua Belmonte, J.C. Dedifferentiation, transdifferentiation and reprogramming: Three routes to regeneration. Nat. Rev. Mol. Cell Biol. 2011, 12, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Amrollahi, P.; Shah, B.; Seifi, A.; Tayebi, L. Recent advancements in regenerative dentistry: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Saghiri, M.A.; Asatourian, A.; Sheibani, N. Angiogenesis in regenerative dentistry. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saghiri, M.A.; Asatourian, A.; Sorenson, C.M.; Sheibani, N. Role of angiogenesis in endodontics: Contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. J. Endod. 2015, 41, 797–803. [Google Scholar] [CrossRef]
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef] [Green Version]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Yin, K.J.; Hamblin, M.; Chen, Y.E. Angiogenesis-regulating microRNAs and Ischemic Stroke. Curr. Vasc. Pharmacol. 2015, 13, 352–365. [Google Scholar] [CrossRef]
- Cochain, C.; Channon, K.M.; Silvestre, J.S. Angiogenesis in the infarcted myocardium. Antioxid. Redox Signal. 2013, 18, 1100–1113. [Google Scholar] [CrossRef]
- Madeddu, P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp. Physiol. 2005, 90, 315–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Kim, K.E.; Choi, D.K.; Jang, J.Y.; Jung, J.J.; Kiyonari, H.; Shioi, G.; Chang, W.; Suda, T.; Mochizuki, N.; et al. Angiopoietin-1 guides directional angiogenesis through integrin alphavbeta5 signaling for recovery of ischemic retinopathy. Sci. Transl. Med. 2013, 5, 203ra127. [Google Scholar] [CrossRef] [PubMed]
- Welch-Reardon, K.M.; Wu, N.; Hughes, C.C. A role for partial endothelial-mesenchymal transitions in angiogenesis? Arterioscler. Thromb. Vasc. Biol. 2015, 35, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Lyu, X.; Wang, Q.; Hu, M.; Zhang, X. Endothelial to mesenchymal transition in the cardiovascular system. Life Sci. 2017, 184, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Medici, D. Endothelial-Mesenchymal Transition in Regenerative Medicine. Stem Cells Int. 2016, 2016, 6962801. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Lee, A.; Chang, W.; Lee, M.S.; Kim, J. Endothelial to Mesenchymal Transition Represents a Key Link in the Interaction between Inflammation and Endothelial Dysfunction. Front. Immunol. 2018, 9, 294. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular mediators of angiogenesis. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2010, 31, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [Green Version]
- Luttun, A.; Tjwa, M.; Carmeliet, P. Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1): Novel therapeutic targets for angiogenic disorders. Ann. N. Y. Acad. Sci. 2002, 979, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Van Cruijsen, H.; Giaccone, G.; Hoekman, K. Epidermal growth factor receptor and angiogenesis: Opportunities for combined anticancer strategies. Int. J. Cancer 2005, 117, 883–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckouche, N.; Bignon, M.; Lelarge, V.; Mathivet, T.; Pichol-Thievend, C.; Berndt, S.; Hardouin, J.; Garand, M.; Ardidie-Robouant, C.; Barret, A.; et al. The interaction of heparan sulfate proteoglycans with endothelial transglutaminase-2 limits VEGF165-induced angiogenesis. Sci. Signal. 2015, 8, ra70. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Fuster, M.M.; Lawrence, R.; Esko, J.D. Heparan sulfate regulates VEGF165- and VEGF121-mediated vascular hyperpermeability. J. Biol. Chem. 2011, 286, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Huo, Y.; Chen, C.; Zeng, H.; Lu, X.; Wei, C.; Ruan, C.; Zhang, X.; Hu, Z.; Shibuya, M.; et al. Vascular endothelial growth factor (VEGF) receptor-2 tyrosine 1175 signaling controls VEGF-induced von Willebrand factor release from endothelial cells via phospholipase C-gamma 1- and protein kinase A-dependent pathways. J. Biol. Chem. 2009, 284, 23217–23224. [Google Scholar] [CrossRef] [PubMed]
- Goertz, L.; Schneider, S.W.; Desch, A.; Mayer, F.T.; Koett, J.; Nowak, K.; Karampinis, I.; Bohlmann, M.K.; Umansky, V.; Bauer, A.T. Heparins that block VEGF-A-mediated von Willebrand factor fiber generation are potent inhibitors of hematogenous but not lymphatic metastasis. Oncotarget 2016, 7, 68527–68545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, D.O. Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res. 2010, 87, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Sacchi, V.; Mittermayr, R.; Hartinger, J.; Martino, M.M.; Lorentz, K.M.; Wolbank, S.; Hofmann, A.; Largo, R.A.; Marschall, J.S.; Groppa, E. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc. Natl. Acad. Sci. USA 2014, 111, 6952–6957. [Google Scholar] [CrossRef] [Green Version]
- Jozkowicz, A.; Fugl, A.; Nanobashvili, J.; Neumayer, C.; Dulak, J.; Valentini, D.; Funovics, P.; Polterauer, P.; Redl, H.; Huk, I. Delivery of high dose VEGF plasmid using fibrin carrier does not influence its angiogenic potency. Int. J. Artif. Organs 2003, 26, 161–169. [Google Scholar] [CrossRef]
- Sobhani, A.; Khanlarkhani, N.; Baazm, M.; Mohammadzadeh, F.; Najafi, A.; Mehdinejadiani, S.; Sargolzaei Aval, F. Multipotent Stem Cell and Current Application. Acta Med. Iran. 2017, 55, 6–23. [Google Scholar] [PubMed]
- Tabansky, I.; Stern, J.N.H. Basics of Stem Cell Biology as Applied to the Brain. In Stem Cells in Neuroendocrinology; Pfaff, D., Christen, Y., Eds.; Spring: Cham, Switzerland, 2016; pp. 11–24. [Google Scholar] [Green Version]
- Behr, B.; Ko, S.H.; Wong, V.W.; Gurtner, G.C.; Longaker, M.T. Stem cells. Plast. Reconstr. Surg. 2010, 126, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Szade, K.; Szade, A.; Nowak, W.; Jozkowicz, A. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim. Pol. 2015, 62, 329–337. [Google Scholar] [CrossRef]
- Fischbach, G.D.; Fischbach, R.L. Stem cells: Science, policy, and ethics. J. Clin. Investig. 2004, 114, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falanga, V. Stem cells in tissue repair and regeneration. J. Investig. Dermatol. 2012, 132, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Tataria, M.; Perryman, S.V.; Sylvester, K.G. Stem cells: Tissue regeneration and cancer. Semin. Pediatr. Surg. 2006, 15, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Passier, R.; Mummery, C. Origin and use of embryonic and adult stem cells in differentiation and tissue repair. Cardiovasc. Res. 2003, 58, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Mali, R.; Lele, P. Vishakha. Guided tissue regeneration in communicating periodontal and endodontic lesions—A hope for the hopeless! J. Indian Soc. Periodontol. 2011, 15, 410–413. [Google Scholar] [CrossRef]
- Moschouris, K.; Firoozi, N.; Kang, Y. The application of cell sheet engineering in the vascularization of tissue regeneration. Regen. Med. 2016, 11, 559–570. [Google Scholar] [CrossRef]
- Ono, M.; Oshima, M.; Ogawa, M.; Sonoyama, W.; Hara, E.S.; Oida, Y.; Shinkawa, S.; Nakajima, R.; Mine, A.; Hayano, S.; et al. Practical whole-tooth restoration utilizing autologous bioengineered tooth germ transplantation in a postnatal canine model. Sci. Rep. 2017, 7, 44522. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, R.M.; Drissi, H. Differentiation of Human Induced Pluripotent Stem Cells to Chondrocytes. Methods Mol. Biol. 2015, 1340, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Kanke, K.; Masaki, H.; Saito, T.; Komiyama, Y.; Hojo, H.; Nakauchi, H.; Lichtler, A.C.; Takato, T.; Chung, U.I.; Ohba, S. Stepwise differentiation of pluripotent stem cells into osteoblasts using four small molecules under serum-free and feeder-free conditions. Stem Cell Rep. 2014, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Heino, T.J.; Hentunen, T.A. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2008, 3, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.H.; Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Clark, E.A.; Kalomoiris, S.; Nolta, J.A.; Fierro, F.A. Concise review: MicroRNA function in multipotent mesenchymal stromal cells. Stem Cells 2014, 32, 1074–1082. [Google Scholar] [CrossRef]
- Tome, M.; Lopez-Romero, P.; Albo, C.; Sepulveda, J.C.; Fernandez-Gutierrez, B.; Dopazo, A.; Bernad, A.; Gonzalez, M.A. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ. 2011, 18, 985–995. [Google Scholar] [CrossRef]
- Wang, T.; Shi, S.B.; Sha, H.Y. MicroRNAs in regulation of pluripotency and somatic cell reprogramming: Small molecule with big impact. RNA Biol. 2013, 10, 1255–1261. [Google Scholar] [CrossRef]
- Bork, S.; Horn, P.; Castoldi, M.; Hellwig, I.; Ho, A.D.; Wagner, W. Adipogenic differentiation of human mesenchymal stromal cells is down-regulated by microRNA-369-5p and up-regulated by microRNA-371. J. Cell. Physiol. 2011, 226, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, H.; Tran, K.; Civini, S.; Jin, P.; Castiello, L.; Feng, J.; Kuznetsov, S.A.; Robey, P.G.; Sabatino, M.; et al. Human bone marrow stromal cell confluence: Effects on cell characteristics and methods of assessment. Cytotherapy 2015, 17, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, L.; Porter, C.; Alexander, G.; Patel, D.; Vines, J.; Zhang, X.; Chasteen-Boyd, D.; Sung, H.J.; Li, Y.P.; et al. Angiogenic and Osteogenic Synergy of Human Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells Cocultured on a Nanomatrix. Sci. Rep. 2018, 8, 15749. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.W.; Wang, J.; Lee, C.J.; Liu, M.; Neelamegham, S.; Canty, J.M.; Nguyen, J. The microRNA regulatory landscape of MSC-derived exosomes: A systems view. Sci. Rep. 2018, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Pejcic, A.; Kojovic, D.; Mirkovic, D.; Minic, I. Stem cells for periodontal regeneration. Balkan J. Med. Genet. 2013, 16, 7–12. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, M. Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem Cells Int. 2015, 2015, 972313. [Google Scholar] [CrossRef]
- Yang, S.; Guo, L.; Su, Y.; Wen, J.; Du, J.; Li, X.; Liu, Y.; Feng, J.; Xie, Y.; Bai, Y.; et al. Nitric oxide balances osteoblast and adipocyte lineage differentiation via the JNK/MAPK signaling pathway in periodontal ligament stem cells. Stem Cell Res. Ther. 2018, 9, 118. [Google Scholar] [CrossRef]
- Choi, S.; Cho, T.J.; Kwon, S.K.; Lee, G.; Cho, J. Chondrogenesis of periodontal ligament stem cells by transforming growth factor-beta3 and bone morphogenetic protein-6 in a normal healthy impacted third molar. Int. J. Oral Sci. 2013, 5, 7–13. [Google Scholar] [CrossRef]
- Martinez-Sarra, E.; Montori, S.; Gil-Recio, C.; Nunez-Toldra, R.; Costamagna, D.; Rotini, A.; Atari, M.; Luttun, A.; Sampaolesi, M. Human dental pulp pluripotent-like stem cells promote wound healing and muscle regeneration. Stem Cell Res. Ther. 2017, 8, 175. [Google Scholar] [CrossRef]
- Goorha, S.; Reiter, L.T. Culturing and Neuronal Differentiation of Human Dental Pulp Stem Cells. Curr. Protoc. Hum. Genet. 2017, 92, 21.6.1–21.6.10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Ratajczak, J.; Hilkens, P.; Gervois, P.; Wolfs, E.; Jacobs, R.; Lambrichts, I.; Bronckaers, A. Angiogenic Capacity of Periodontal Ligament Stem Cells Pretreated with Deferoxamine and/or Fibroblast Growth Factor-2. PLoS ONE 2016, 11, e0167807. [Google Scholar] [CrossRef] [PubMed]
- Yellowley, C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. BoneKEy Rep. 2013, 2, 300. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mamillapalli, R.; Mutlu, L.; Du, H.; Taylor, H.S. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015, 15, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Qiu, L.; Zhang, Y.; Xu, D.; Zheng, J.C.; Jiang, L. CXCL12 enhances angiogenesis through CXCR7 activation in human umbilical vein endothelial cells. Sci. Rep. 2017, 7, 8289. [Google Scholar] [CrossRef]
- Liekens, S.; Schols, D.; Hatse, S. CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell mobilization. Current Pharm. Des. 2010, 16, 3903–3920. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Sun, X.; Zhou, J.; Yang, P. CXCL12 overexpression promotes the angiogenesis potential of periodontal ligament stem cells. Sci. Rep. 2017, 7, 10286. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, J.; Bronckaers, A.; Dillen, Y.; Gervois, P.; Vangansewinkel, T.; Driesen, R.B.; Wolfs, E.; Lambrichts, I.; Hilkens, P. The Neurovascular Properties of Dental Stem Cells and Their Importance in Dental Tissue Engineering. Stem Cells Int. 2016, 2016, 9762871. [Google Scholar] [CrossRef]
- Xian, X.; Gong, Q.; Li, C.; Guo, B.; Jiang, H. Exosomes with Highly Angiogenic Potential for Possible Use in Pulp Regeneration. J. Endod. 2018, 44, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dissanayaka, W.L.; Zhang, C. Dental pulp stem cells overexpressing stromal-derived factor-1alpha and vascular endothelial growth factor in dental pulp regeneration. Clin. Oral Investig. 2018. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, D.; Kumar, K.P.M.; Alur, J.B. Gingival mesenchymal stem cells. J. Oral Maxillofac. Pathol. 2017, 21, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Dorfer, C.E. Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem Cells Int. 2016, 2016, 7154327. [Google Scholar] [CrossRef] [PubMed]
- Beltrao-Braga, P.C.; Pignatari, G.C.; Maiorka, P.C.; Oliveira, N.A.; Lizier, N.F.; Wenceslau, C.V.; Miglino, M.A.; Muotri, A.R.; Kerkis, I. Feeder-free derivation of induced pluripotent stem cells from human immature dental pulp stem cells. Cell Transplant. 2011, 20, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.W.; Li, Q.; Niu, X.; Hu, B.; Liu, J.; Zhou, S.M.; Guo, S.C.; Lang, H.L.; Zhang, C.Q.; Wang, Y.; et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 2015, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezenah, J.R.; Rioja, A.Y.; Juliar, B.; Friend, N.; Putnam, A.J. Assessing the ability of human endothelial cells derived from induced pluripotent stem cells to form functional microvasculature in vivo. Biotechnol. Bioeng. 2018. [Google Scholar] [CrossRef]
- Zhang, G.; Shang, B.; Yang, P.; Cao, Z.; Pan, Y.; Zhou, Q. Induced pluripotent stem cell consensus genes: Implication for the risk of tumorigenesis and cancers in induced pluripotent stem cell therapy. Stem Cells Dev. 2012, 21, 955–964. [Google Scholar] [CrossRef]
- Roux, B.M.; Akar, B.; Zhou, W.; Stojkova, K.; Barrera, B.; Brankov, J.; Brey, E.M. Preformed Vascular Networks Survive and Enhance Vascularization in Critical Sized Cranial Defects. Tissue Eng. Part A 2018, 24, 1603–1615. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Pant, S.; Hilton, H.; Burczynski, M.E. The multifaceted exosome: Biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem. Pharmacol. 2012, 83, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thery, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Jing, H.; He, X.; Zheng, J. Exosomes and regenerative medicine: State of the art and perspectives. Transl. Res. 2018, 196, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Meckes, D.G., Jr.; Raab-Traub, N. Microvesicles and viral infection. J. Virol. 2011, 85, 12844–12854. [Google Scholar] [CrossRef] [PubMed]
- Janas, T.; Janas, M.M.; Sapon, K.; Janas, T. Mechanisms of RNA loading into exosomes. FEBS Lett. 2015, 589, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Bhome, R.; Del Vecchio, F.; Lee, G.H.; Bullock, M.D.; Primrose, J.N.; Sayan, A.E.; Mirnezami, A.H. Exosomal microRNAs (exomiRs): Small molecules with a big role in cancer. Cancer Lett. 2018, 420, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Halvaei, S.; Daryani, S.; Eslami, S.Z.; Samadi, T.; Jafarbeik-Iravani, N.; Bakhshayesh, T.O.; Majidzadeh, A.K.; Esmaeili, R. Exosomes in Cancer Liquid Biopsy: A Focus on Breast Cancer. Mol. Ther. Nucleic Acids 2018, 10, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Perez-Boza, J.; Lion, M.; Struman, I. Exploring the RNA landscape of endothelial exosomes. RNA 2018, 24, 423–435. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. Discovery of Double-Stranded Genomic DNA in Circulating Exosomes. Cold Spring Harbor. Symp. Quant. Biol. 2016, 81, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Han, C.; Labuz, J.M.; Kim, J.; Kim, J.; Cho, S.; Gho, Y.S.; Takayama, S.; Park, J. High-yield isolation of extracellular vesicles using aqueous two-phase system. Sci. Rep. 2015, 5, 13103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.G.; Grizzle, W.E. Exosomes: A novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am. J. Pathol. 2014, 184, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.R.; Jiang, C.; Krzyzanowski, G.D.; Ryan, W.L. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS ONE 2017, 12, e0183915. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Vannberg, F.O.; Dixon, J.B. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci. Rep. 2016, 6, 24436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braicu, C.; Tomuleasa, C.; Monroig, P.; Cucuianu, A.; Berindan-Neagoe, I.; Calin, G.A. Exosomes as divine messengers: Are they the Hermes of modern molecular oncology? Cell Death Differ. 2015, 22, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Castellana, D.; Klingbeil, P.; Cuesta Hernandez, I.; Vitacolonna, M.; Orlicky, D.J.; Roffler, S.R.; Brodt, P.; Zoller, M. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia 2009, 11, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Psaila, B.; Lyden, D. The metastatic niche: Adapting the foreign soil. Nat. Rev. Cancer 2009, 9, 285–293. [Google Scholar] [CrossRef]
- Gulei, D.; Irimie, A.I.; Cojocneanu-Petric, R.; Schultze, J.L.; Berindan-Neagoe, I. Exosomes-Small Players, Big Sound. Bioconjugate Chem. 2018, 29, 635–648. [Google Scholar] [CrossRef]
- Zlotogorski-Hurvitz, A.; Dayan, D.; Chaushu, G.; Korvala, J.; Salo, T.; Sormunen, R.; Vered, M. Human saliva-derived exosomes: Comparing methods of isolation. J. Histochem. Cytochem. 2015, 63, 181–189. [Google Scholar] [CrossRef]
- Michael, A.; Bajracharya, S.D.; Yuen, P.S.; Zhou, H.; Star, R.A.; Illei, G.G.; Alevizos, I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, A.; Simons, M. Exosomes: Vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013, 352, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef] [Green Version]
- Baquir, B.; Hancock, R.E. Exosomes, your body’s answer to immune health. Ann. Transl. Med. 2017, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yeo, R.W.Y.; Lai, R.C.; Sim, E.W.K.; Chin, K.C.; Lim, S.K. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy 2018, 20, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Wu, S.; Cui, M.; Xu, T. Focus on Mesenchymal Stem Cell-Derived Exosomes: Opportunities and Challenges in Cell-Free Therapy. Stem Cells Int. 2017, 2017, 6305295. [Google Scholar] [CrossRef] [PubMed]
- Inukai, T.; Katagiri, W.; Yoshimi, R.; Osugi, M.; Kawai, T.; Hibi, H.; Ueda, M. Novel application of stem cell-derived factors for periodontal regeneration. Biochem. Biophys. Res. Commun. 2013, 430, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, S.; Hara, H.; Hirono, H.; Watanabe, K.; Hasegawa, K. Regenerative medicine using dental pulp stem cells for liver diseases. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Jarmalavičiūtė, A.; Tunaitis, V.; Pivoraitė, U.; Venalis, A.; Pivoriūnas, A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine–induced apoptosis. Cytotherapy 2015, 17, 932–939. [Google Scholar] [CrossRef]
- Huang, C.C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.F.; Zhu, H.; Millard, R.W.; Fan, G.C. Exosomes Function in Pro- and Anti-Angiogenesis. Curr. Angiogenes 2013, 2, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, H.; Heikamp, E.; Turley, H.; Dragovic, R.; Thomas, P.; Oon, C.E.; Leek, R.; Edelmann, M.; Kessler, B.; Sainson, R.C.; et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood 2010, 116, 2385–2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Choi, D.Y.; Yun, S.J.; Choi, S.M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef] [PubMed]
- Alcayaga-Miranda, F.; Varas-Godoy, M.; Khoury, M. Harnessing the Angiogenic Potential of Stem Cell-Derived Exosomes for Vascular Regeneration. Stem Cells Int. 2016, 2016, 3409169. [Google Scholar] [CrossRef]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.-C. Hypoxic exosomes promote angiogenesis. Blood 2014, 124, 3669–3670. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-King, H.; Garcia, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepulveda, P. Hypoxia Inducible Factor-1alpha Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef]
- Shi Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, S.P.; Elkahloun, A.G.; Wu, W.; Abu-Asab, M.S.; Roberts, D.D. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 37, 49–59. [Google Scholar] [CrossRef]
- Tomasoni, S.; Longaretti, L.; Rota, C.; Morigi, M.; Conti, S.; Gotti, E.; Capelli, C.; Introna, M.; Remuzzi, G.; Benigni, A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013, 22, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Lin Lin, S.; Zhang, Q.; Shao, X.; Zhang, T.; Xue, C.; Shi, S.; Zhao, D.; Lin, Y. IGF-1 promotes angiogenesis in endothelial cells/adipose-derived stem cells co-culture system with activation of PI3K/Akt signal pathway. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef]
- McBride, J.D.; Rodriguez-Menocal, L.; Guzman, W.; Candanedo, A.; Garcia-Contreras, M.; Badiavas, E.V. Bone Marrow Mesenchymal Stem Cell-Derived CD63(+) Exosomes Transport Wnt3a Exteriorly and Enhance Dermal Fibroblast Proliferation, Migration, and Angiogenesis In Vitro. Stem Cells Dev. 2017, 26, 1384–1398. [Google Scholar] [CrossRef] [PubMed]
- Pivoraite, U.; Jarmalaviciute, A.; Tunaitis, V.; Ramanauskaite, G.; Vaitkuviene, A.; Kaseta, V.; Biziuleviciene, G.; Venalis, A.; Pivoriunas, A. Exosomes from Human Dental Pulp Stem Cells Suppress Carrageenan-Induced Acute Inflammation in Mice. Inflammation 2015, 38, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Redis, R.S.; Berindan-Neagoe, I.; Pop, V.I.; Calin, G.A. Non-coding RNAs as theranostics in human cancers. J. Cell. Biochem. 2012, 113, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Zeller, T. microRNA-based diagnostics and therapy in cardiovascular disease-Summing up the facts. Cardiovasc. Diagn. Ther. 2015, 5, 17–36. [Google Scholar] [CrossRef]
- Nelson, P.T.; Wang, W.X.; Rajeev, B.W. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol. 2008, 18, 130–138. [Google Scholar] [CrossRef]
- Kim, J. MicroRNAs as critical regulators of the endothelial to mesenchymal transition in vascular biology. BMB Rep. 2018, 51, 65–72. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Volkmann, I.; Jazbutyte, V.; Dangwal, S.; Park, D.H.; Thum, T. Transforming growth factor-beta-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 361–369. [Google Scholar] [CrossRef]
- Shi, B.; Wang, Y.; Zhao, R.; Long, X.; Deng, W.; Wang, Z. Bone marrow mesenchymal stem cell-derived exosomal miR-21 protects C-kit+ cardiac stem cells from oxidative injury through the PTEN/PI3K/Akt axis. PLoS ONE 2018, 13, e0191616. [Google Scholar] [CrossRef]
- Zhou, W.; Su, L.; Duan, X.; Chen, X.; Hays, A.; Upadhyayula, S.; Shivde, J.; Wang, H.; Li, Y.; Huang, D.; et al. MicroRNA-21 down-regulates inflammation and inhibits periodontitis. Mol. Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Y.; Yu, Y.; Zhao, J.; Ou, Y.; Chao, Y.; Yang, B.; Yu, X. MicroRNA-378 Promotes Osteogenesis-Angiogenesis Coupling in BMMSCs for Potential Bone Regeneration. Anal. Cell. Pathol. 2018, 2018, 8402390. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gorski, D.H. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood 2008, 111, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Cantaluppi, V.; Gatti, S.; Medica, D.; Figliolini, F.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia–reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012, 82, 412–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.K.; Nagpal, V.; Covington, J.W.; Michaels, M.A.; Vaughan, D.E. Molecular basis of cardiac endothelial-to-mesenchymal transition (EndMT): Differential expression of microRNAs during EndMT. Cell. Signal. 2012, 24, 1031–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuehbacher, A.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ. Res. 2007, 101, 59–68. [Google Scholar] [CrossRef]
- Pirotte, S.; Lamour, V.; Lambert, V.; Alvarez Gonzalez, M.L.; Ormenese, S.; Noel, A.; Mottet, D.; Castronovo, V.; Bellahcene, A. Dentin matrix protein 1 induces membrane expression of VE-cadherin on endothelial cells and inhibits VEGF-induced angiogenesis by blocking VEGFR-2 phosphorylation. Blood 2011, 117, 2515–2526. [Google Scholar] [CrossRef]
- Yue, J.; Wu, B.; Gao, J.; Huang, X.; Li, C.; Ma, D.; Fang, F. DMP1 is a target of let-7 in dental pulp cells. Int. J. Mol. Med. 2012, 30, 295–301. [Google Scholar] [CrossRef]
- van Balkom, B.W.; de Jong, O.G.; Smits, M.; Brummelman, J.; den Ouden, K.; de Bree, P.M.; van Eijndhoven, M.A.; Pegtel, D.M.; Stoorvogel, W.; Wurdinger, T.; et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013, 121, 3997–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnerty, J.R.; Wang, W.X.; Hebert, S.S.; Wilfred, B.R.; Mao, G.; Nelson, P.T. The miR-15/107 group of microRNA genes: Evolutionary biology, cellular functions, and roles in human diseases. J. Mol. Biol. 2010, 402, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Caporali, A.; Emanueli, C. MicroRNA-503 and the extended microRNA-16 family in angiogenesis. Trends Cardiovasc. Med. 2011, 21, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, R.; Zhu, C.; Qiao, H.; Liu, Y.; Ji, H.; Miao, J.; Chen, L.; Liu, X.; Yang, Y. MiR-503 suppresses hypoxia-induced proliferation, migration and angiogenesis of endothelial progenitor cells by targeting Apelin. Peptides 2018. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Jorganes, A.; Araldi, E.; Penalva, L.O.; Sandhu, D.; Fernandez-Hernando, C.; Suarez, Y. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Fafián-Labora, J.; Lesende-Rodriguez, I.; Fernández-Pernas, P.; Sangiao-Alvarellos, S.; Monserrat, L.; Arntz, O.J.; van de Loo, F.A.J.; Mateos, J.; Arufe, M.C. Effect of age on pro-inflammatory miRNAs contained in mesenchymal stem cell-derived extracellular vesicles. Sci. Rep. 2017, 7, 43923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasanthan, P.; Govindasamy, V.; Gnanasegaran, N.; Kunasekaran, W.; Musa, S.; Abu Kasim, N.H. Differential expression of basal microRNAs’ patterns in human dental pulp stem cells. J. Cell. Mol. Med. 2015, 19, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zgheib, C.; Hu, J.; Wu, W.; Zhang, L.; Liechty, K.W. The role of microRNA-15b in the impaired angiogenesis in diabetic wounds. Wound Repair Regen. 2014, 22, 671–677. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, Y.; Lan, B.; Wang, J.; Zhang, Z.; Zhang, L.; Xiao, P.; Meng, Q.; Geng, Y.J.; Yu, X.Y.; et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. BioMed Res. Int. 2017, 2017, 4150705. [Google Scholar] [CrossRef]
- Ott, C.E.; Grunhagen, J.; Jager, M.; Horbelt, D.; Schwill, S.; Kallenbach, K.; Guo, G.; Manke, T.; Knaus, P.; Mundlos, S.; et al. MicroRNAs differentially expressed in postnatal aortic development downregulate elastin via 3′ UTR and coding-sequence binding sites. PLoS ONE 2011, 6, e16250. [Google Scholar] [CrossRef]
- Patel, A.; Fine, B.; Sandig, M.; Mequanint, K. Elastin biosynthesis: The missing link in tissue-engineered blood vessels. Cardiovasc. Res. 2006, 71, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.P.; Wang, X.H.; Zhang, H.X.; Shang, M.M.; Liu, X.X.; Sun, H.M.; Song, Y.P. MiR-103 regulates the angiogenesis of ischemic stroke rats by targeting vascular endothelial growth factor (VEGF). Iran. J. Basic Med. Sci. 2018, 21, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, R.; Ferracin, M.; Wojcik, S.E.; Garzon, R.; Alder, H.; Agosto-Perez, F.J.; Davuluri, R.; Liu, C.G.; Croce, C.M.; Negrini, M.; et al. A microRNA signature of hypoxia. Mol. Cell. Biol. 2007, 27, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Papangeli, I.; Park, Y.; Jeong, H.N.; Choi, J.; Kang, H.; Jo, H.N.; Kim, J.; Chun, H.J. A PPARgamma-dependent miR-424/503-CD40 axis regulates inflammation mediated angiogenesis. Sci. Rep. 2017, 7, 2528. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Jinnin, M.; Etoh, T.; Fukushima, S.; Masuguchi, S.; Maruo, K.; Inoue, Y.; Ishihara, T.; Ihn, H. Down-regulation of mir-424 contributes to the abnormal angiogenesis via MEK1 and cyclin E1 in senile hemangioma: Its implications to therapy. PLoS ONE 2010, 5, e14334. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gong, Q.; Ling, J.; Zhang, W.; Liu, Z.; Quan, J. Role of miR-424 on angiogenic potential in human dental pulp cells. J. Endod. 2014, 40, 76–82. [Google Scholar] [CrossRef]
- Chan, Y.C.; Khanna, S.; Roy, S.; Sen, C.K. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J. Biol. Chem. 2011, 286, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Roy, S.; Khanna, S.; Sen, C.K. Downregulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Pecot, C.V.; Rupaimoole, R.; Yang, D.; Akbani, R.; Ivan, C.; Lu, C.; Wu, S.; Han, H.D.; Shah, M.Y.; Rodriguez-Aguayo, C.; et al. Tumour angiogenesis regulation by the miR-200 family. Nat. Commun. 2013, 4, 2427. [Google Scholar] [CrossRef]
- Choi, Y.C.; Yoon, S.; Jeong, Y.; Yoon, J.; Baek, K. Regulation of vascular endothelial growth factor signaling by miR-200b. Mol. Cells 2011, 32, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Korpal, M.; Lee, E.S.; Hu, G.; Kang, Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008, 283, 14910–14914. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Galfo, F.; Pallio, G.; Mannino, F.; D’Amore, A.; Pellegrino, E.; Ieni, A.; Russo, G.T.; Calapai, M.; et al. Effects of the antagomiRs 15b and 200b on the altered healing pattern of diabetic mice. Br. J. Pharmacol. 2018, 175, 644–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumann, P.; Lindhorst, D.; Kampmann, A.; Gellrich, N.C.; Krone-Wolf, S.; Meyer-Lindenberg, A.; von See, C.; Gander, T.; Lanzer, M.; Rucker, M.; et al. Decelerated vascularization in tissue-engineered constructs in association with diabetes mellitus in vivo. J. Diabetes Its Complicat. 2015, 29, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontikoglou, C.; Deschaseaux, F.; Sensebe, L.; Papadaki, H.A. Bone marrow mesenchymal stem cells: Biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev. 2011, 7, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Charbord, P. Bone marrow mesenchymal stem cells: Historical overview and concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Sueyama, Y.; Kaneko, T.; Ito, T.; Kaneko, R.; Okiji, T. Implantation of Endothelial Cells with Mesenchymal Stem Cells Accelerates Dental Pulp Tissue Regeneration/Healing in Pulpotomized Rat Molars. J. Endod. 2017, 43, 943–948. [Google Scholar] [CrossRef]
- Potdar, P.D.; Jethmalani, Y.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 7, 839–851. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L.J. Dental pulp stem cells: Function, isolation and applications in regenerative medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1205–1216. [Google Scholar] [CrossRef]
- Angelopoulos, I.; Brizuela, C.; Khoury, M. Gingival Mesenchymal Stem Cells Outperform Haploidentical Dental Pulp-derived Mesenchymal Stem Cells in Proliferation Rate, Migration Ability, and Angiogenic Potential. Cell Transplant. 2018, 27, 967–978. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Takenaka, C.; Yoda, Y.; Oshima, Y.; Kagawa, K.; Miyajima, H.; Sasaki, T.; Kawamata, S. Differentiation potential of Pluripotent Stem Cells correlates to the level of CHD7. Sci. Rep. 2018, 8, 241. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef] [PubMed]
- Komaki, M.; Numata, Y.; Morioka, C.; Honda, I.; Tooi, M.; Yokoyama, N.; Ayame, H.; Iwasaki, K.; Taki, A.; Oshima, N.; et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res. Ther. 2017, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, C.; Shen, Y.; Li, X.; Li, B.; Zhao, S.; Gu, J.; Chen, Y.; Ma, B.; Wei, J.; Han, Q.; et al. Exosomes Derived from Hypoxia-Treated Human Adipose Mesenchymal Stem Cells Enhance Angiogenesis Through the PKA Signaling Pathway. Stem Cells Dev. 2018, 27, 456–465. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef]



| Type of Secreting Cell | Up/Down Level | Cargo/Targeted Molecules | Biological Changes of the Targeted Cell (EC) | Ref. |
|---|---|---|---|---|
| DP- MSC. Lentiviral transfected with HIF-1A |  | miR-15/16, miR-17, miR-31, miR-221/222, miR-320a, miR-424 miR-126, miR-145 | Stimulation of angiogenesis | [120] |
| MSC |  | STAT3, C-MYC, CYCLIN A1, CYCLIN D2 | Induced vascular tube formation, Activate several intracellular signalling pathways | [173] |
 | miR-10a/b, miR-21, miR-19a/b miR-126, | Promote angiogenesis | [174] | |
| Pla-MSC and BM-MSC |  (BMMSC) | VEGF, HGF, IGFBP2, IGFBP6 | Increased endothelial tube formation and migration | [175] |
 (Pla-MSC) | HGF, IGFBP2, IGFBP3, IGFBP6 | |||
| iMSC |  | VEGF, TGFB1, ANG | Promotes EC migration, proliferation Increased tube formation | [78] |
| AD-MSC |  | miR-125a | EC pro-angiogenic activity | [176] |
 | ANGPT1, FLK1, HIF-1A VEGF | Increased tube formation | [177] | |
 (Nrf2+ ADSC) | SMP30, VEGF, VEGFR2 phosphorylation, | Inhibited ROS and inflammatory cytokine expression Inhibited EC senescence | [178] | |
| HucMSC |  | PCNA, CYCLIN D3, N-CADHERIN | Proliferation and Migration of EC Improved the tube-formation ability of EC | [179] |
| DPC |  | FGF-2, VEGF-A, KDR, MMP-9 | Promoted increased tube formation EC proliferation | [67] |
| GMSC | N/A | N/A | A higher number of newly formed microvesicles | [121] |
| MSC |  | miR-424, miR-30c, miR-30b, and let-7f | Promote angiogenesis | [174] |
| AD-MSC |  | DLL4 | EC pro-angiogenic activity | [176] |
| AD-MSC |  | VASH1 | Increased tube formation | [177] |
| AD-MSC, Nrf2+AD-MSC |  (ADSC) | SMP30, VEGF | Inhibited ROS and inflammatory cytokine expression Inhibited EC senescence | [178] |
| HucMSC |  | E-CADHERIN | Proliferation and Migration of EC Improved the tube-formation ability of EC | [179] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimta, A.-A.; Baru, O.; Badea, M.; Buduru, S.D.; Berindan-Neagoe, I. The Role of Angiogenesis and Pro-Angiogenic Exosomes in Regenerative Dentistry. Int. J. Mol. Sci. 2019, 20, 406. https://doi.org/10.3390/ijms20020406
Zimta A-A, Baru O, Badea M, Buduru SD, Berindan-Neagoe I. The Role of Angiogenesis and Pro-Angiogenic Exosomes in Regenerative Dentistry. International Journal of Molecular Sciences. 2019; 20(2):406. https://doi.org/10.3390/ijms20020406
Chicago/Turabian StyleZimta, Alina-Andreea, Oana Baru, Mandra Badea, Smaranda Dana Buduru, and Ioana Berindan-Neagoe. 2019. "The Role of Angiogenesis and Pro-Angiogenic Exosomes in Regenerative Dentistry" International Journal of Molecular Sciences 20, no. 2: 406. https://doi.org/10.3390/ijms20020406
APA StyleZimta, A. -A., Baru, O., Badea, M., Buduru, S. D., & Berindan-Neagoe, I. (2019). The Role of Angiogenesis and Pro-Angiogenic Exosomes in Regenerative Dentistry. International Journal of Molecular Sciences, 20(2), 406. https://doi.org/10.3390/ijms20020406