The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response
Abstract
:1. Introduction
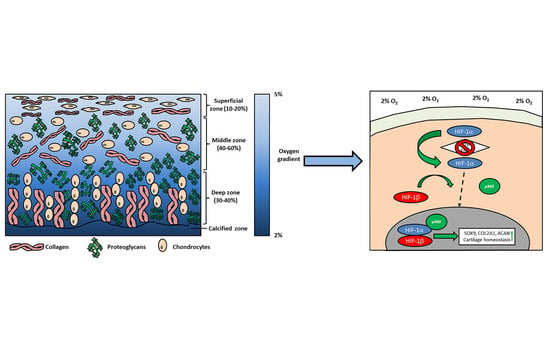
2. Physioxia and Cartilage
3. MSC Isolation and Expansion under Physioxia
4. Physioxia and MSC Chondrogenesis
4.1. Chondrogenic Matrix Formation
4.2. MSC Hypertrophy
4.3. MSC Preconditioning and In Vivo Implantation
4.4. Physioxia Prevents Cytokine Inhibited Chondrogenesis
5. Physioxia Mechanisms in MSC Chondrogenesis
5.1. Hypoxia Inducible Factors (HIF): HIF-1α
5.2. HIF-2α
5.3. HIF-3α
5.4. PI3K/Akt/FOXO Pathway
6. Summary
7. Future Directions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar] [PubMed]
- Goldring, M.B. Osteoarthritis and cartilage: The role of cytokines. Curr. Rheumatol. Rep. 2000, 2, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzi, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cell Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Angele, P.; Fritz, J.; Albrecht, D.; Koh, J.; Zellner, J. Defect type, localization and marker gene expression determines early adverse events of matrix-associated autologous chondrocyte implantation. Injury 2015, 46 (Suppl. 4), S2–S9. [Google Scholar] [CrossRef]
- Niemeyer, P.; Schweigler, K.; Grotejohann, B.; Maurer, J.; Angele, P.; Aurich, M.; Becher, C.; Fay, J.; Feil, R.; Fickert, S.; et al. The German Cartilage Registry (KnorpelRegister DGOU) for evaluation of surgical treatment for cartilage defects: Experience after six months including first demographic data. Z. Orthop. Unfall. 2015, 153, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Kon, E.; Condello, V.; Peretti, G.M.; Steinwachs, M.; Seil, R.; Berruto, M.; Engebretsen, L.; Filardo, G.; Angele, P. Early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1753–1762. [Google Scholar] [CrossRef]
- Luyten, F.P.; Denti, M.; Filardo, G.; Kon, E.; Engebretsen, L. Definition and classification of early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 401–406. [Google Scholar] [CrossRef]
- Madry, H.; Luyten, F.P.; Facchini, A. Biological aspects of early osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 407–422. [Google Scholar] [CrossRef]
- McNulty, A.L.; Rothfusz, N.E.; Leddy, H.A.; Guilak, F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J. Orthop. Res. 2013, 31, 1039–1045. [Google Scholar] [CrossRef] [Green Version]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Andereya, S.; Angele, P.; Ateschrang, A.; Aurich, M.; Baumann, M.; Behrens, P.; Bosch, U.; Erggelet, C.; Fickert, S.; et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: A guideline by the working group “Tissue Regeneration” of the German Society of Orthopaedic Surgery and Traumatology (DGOU). Z. Orthop. Unfall. 2013, 151, 38–47. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Gimble, J.; Guilak, F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy 2003, 5, 362–369. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheumatol. 2005, 52, 2521–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Yoo, J.U.; Barthel, T.S.; Nishimura, K.; Solchaga, L.; Caplan, A.I.; Goldberg, V.M.; Johnstone, B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J. Bone Jt. Surg. Am. 1998, 80, 1745–1757. [Google Scholar] [CrossRef]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheumatol. 2006, 54, 3254–3266. [Google Scholar] [CrossRef] [Green Version]
- Lafont, J.E. Lack of oxygen in articular cartilage: Consequences for chondrocyte biology. Int. J. Exp. Pathol. 2010, 91, 99–106. [Google Scholar] [CrossRef]
- Brighton, C.T.; Heppenstall, R.B. Oxygen tension in zones of the epiphyseal plate, the metaphysis and diaphysis. An in vitro and in vivo study in rats and rabbits. J. Bone Jt. Surg. Am. 1971, 53, 719–728. [Google Scholar] [CrossRef]
- Lund-Olesen, K. Oxygen tension in synovial fluids. Arthritis Rheumatol. 1970, 13, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Grant, J.L.; Smith, B. Bone marrow gas tensions, bone marrow blood flow, and erythropoiesis in man. Ann. Intern. Med. 1963, 58, 801–809. [Google Scholar] [CrossRef]
- Zhou, S.; Cui, Z.; Urban, J.P. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: A modeling study. Arthritis Rheumatol. 2004, 50, 3915–3924. [Google Scholar] [CrossRef] [Green Version]
- Lafont, J.E.; Talma, S.; Hopfgarten, C.; Murphy, C.L. Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J. Biol. Chem. 2008, 283, 4778–4786. [Google Scholar] [CrossRef] [PubMed]
- Lafont, J.E.; Talma, S.; Murphy, C.L. Hypoxia-inducible factor 2α is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheumatol. 2007, 56, 3297–3306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.L.; Sambanis, A. Effect of oxygen tension on chondrocyte extracellular matrix accumulation. Connect. Tissue Res. 2001, 42, 87–96. [Google Scholar] [CrossRef]
- Murphy, C.L.; Sambanis, A. Effect of oxygen tension and alginate encapsulation on restoration of the differentiated phenotype of passaged chondrocytes. Tissue Eng. 2001, 7, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Thoms, B.L.; Dudek, K.A.; Lafont, J.E.; Murphy, C.L. Hypoxia promotes the production and inhibits the destruction of human articular cartilage. Arthritis Rheumatol. 2013, 65, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Strobel, S.; Loparic, M.; Wendt, D.; Schenk, A.D.; Candrian, C.; Lindberg, R.L.; Moldovan, F.; Barbero, A.; Martin, I. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis Res. Ther. 2010, 12, R34. [Google Scholar] [CrossRef] [Green Version]
- Markway, B.D.; Cho, H.; Johnstone, B. Hypoxia promotes redifferentiation and suppresses markers of hypertrophy and degeneration in both healthy and osteoarthritic chondrocytes. Arthritis Res. Ther. 2013, 15, R92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mennan, C.; Garcia, J.; McCarthy, H.; Owen, S.; Perry, J.; Wright, K.; Banerjee, R.; Richardson, J.B.; Roberts, S. Human Articular Chondrocytes Retain Their Phenotype in Sustained Hypoxia While Normoxia Promotes Their Immunomodulatory Potential. Cartilage 2018, 1947603518769714. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Markway, B.D.; Bond, D.; McCarthy, H.E.; Johnstone, B. Responses to altered oxygen tension are distinct between human stem cells of high and low chondrogenic capacity. Stem Cell Res. Ther. 2016, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E. Hypoxia and HIF-1alpha in chondrogenesis. Ann. N. Y. Acad. Sci. 2006, 1068, 66–73. [Google Scholar] [CrossRef]
- Schipani, E.; Ryan, H.E.; Didrickson, S.; Kobayashi, T.; Knight, M.; Johnson, R.S. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001, 15, 2865–2876. [Google Scholar] [CrossRef]
- Taheem, D.K.; Foyt, D.A.; Loaiza, S.; Ferreira, S.A.; Ilic, D.; Auner, H.W.; Grigoriadis, A.E.; Jell, G.; Gentleman, E. Differential Regulation of Human Bone Marrow Mesenchymal Stromal Cell Chondrogenesis by Hypoxia Inducible Factor-1alpha Hydroxylase Inhibitors. Stem Cells 2018, 36, 1380–1392. [Google Scholar] [CrossRef]
- Appelhoff, R.J.; Tian, Y.M.; Raval, R.R.; Turley, H.; Harris, A.L.; Pugh, C.W.; Ratcliffe, P.J.; Gleadle, J.M. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 2004, 279, 38458–38465. [Google Scholar] [CrossRef]
- Tian, Y.M.; Yeoh, K.K.; Lee, M.K.; Eriksson, T.; Kessler, B.M.; Kramer, H.B.; Edelmann, M.J.; Willam, C.; Pugh, C.W.; Schofield, C.J.; et al. Differential sensitivity of hypoxia inducible factor hydroxylation sites to hypoxia and hydroxylase inhibitors. J. Biol. Chem. 2011, 286, 13041–13051. [Google Scholar] [CrossRef] [PubMed]
- Littmann, E.; Autefage, H.; Solanki, A.K.; Kallepitis, C.; Jones, J.R.; Alini, M.; Peroglio, M.; Stevens, M.M. Cobalt-containing bioactive glasses reduce human mesenchymal stem cell chondrogenic differentiation despite HIF-1α stabilisation. J. Eur. Ceram. Soc. 2018, 38, 877–886. [Google Scholar] [CrossRef]
- Teti, G.; Focaroli, S.; Salvatore, V.; Mazzotti, E.; Ingra, L.; Mazzotti, A.; Falconi, M. The Hypoxia-Mimetic Agent Cobalt Chloride Differently Affects Human Mesenchymal Stem Cells in Their Chondrogenic Potential. Stem Cells Int. 2018, 2018, 3237253. [Google Scholar] [CrossRef]
- Krinner, A.; Zscharnack, M.; Bader, A.; Drasdo, D.; Galle, J. Impact of oxygen environment on mesenchymal stem cell expansion and chondrogenic differentiation. Cell Prolif. 2009, 42, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Adesida, A.B.; Mulet-Sierra, A.; Jomha, N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornes, T.D.; Jomha, N.M.; Mulet-Sierra, A.; Adesida, A.B. Hypoxic culture of bone marrow-derived mesenchymal stromal stem cells differentially enhances in vitro chondrogenesis within cell-seeded collagen and hyaluronic acid porous scaffolds. Stem Cell Res. Ther. 2015, 6, 84. [Google Scholar] [CrossRef]
- Boyette, L.B.; Creasey, O.A.; Guzik, L.; Lozito, T.; Tuan, R.S. Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl. Med. 2014, 3, 241–254. [Google Scholar] [CrossRef]
- Sheehy, E.J.; Buckley, C.T.; Kelly, D.J. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012, 417, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Weijers, E.M.; Van Den Broek, L.J.; Waaijman, T.; Van Hinsbergh, V.W.; Gibbs, S.; Koolwijk, P. The influence of hypoxia and fibrinogen variants on the expansion and differentiation of adipose tissue-derived mesenchymal stem cells. Tissue Eng. Part A 2011, 17, 2675–2685. [Google Scholar] [CrossRef]
- Bae, H.C.; Park, H.J.; Wang, S.Y.; Yang, H.R.; Lee, M.C.; Han, H.S. Hypoxic condition enhances chondrogenesis in synovium-derived mesenchymal stem cells. Biomater. Res. 2018, 22, 28. [Google Scholar] [CrossRef]
- Xu, Y.; Malladi, P.; Chiou, M.; Bekerman, E.; Giaccia, A.J.; Longaker, M.T. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007, 13, 2981–2993. [Google Scholar] [CrossRef]
- O′HEireamhoin, S.; Buckley, C.T.; Jones, E.; McGonagle, D.; Mulhall, K.J.; Kelly, D.J. Recapitulating aspects of the oxygen and substrate environment of the damaged joint milieu for stem cell-based cartilage tissue engineering. Tissue Eng. Part C Methods 2013, 19, 117–127. [Google Scholar] [CrossRef]
- Kalpakci, K.N.; Brown, W.E.; Hu, J.C.; Athanasiou, K.A. Cartilage tissue engineering using dermis isolated adult stem cells: The use of hypoxia during expansion versus chondrogenic differentiation. PLoS ONE 2014, 9, e98570. [Google Scholar] [CrossRef]
- Bornes, T.D.; Adesida, A.B.; Jomha, N.M. Articular Cartilage Repair with Mesenchymal Stem Cells After Chondrogenic Priming: A Pilot Study. Tissue Eng. Part A 2018, 24, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Chijimatsu, R.; Hart, D.A.; Koizumi, K.; Sugita, N.; Shimomura, K.; Myoui, A.; Yoshikawa, H.; Nakamura, N. Preparation of Scaffold-Free Tissue-Engineered Constructs Derived from Human Synovial Mesenchymal Stem Cells Under Low Oxygen Tension Enhances Their Chondrogenic Differentiation Capacity. Tissue Eng. Part A 2016, 22, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Muneta, T.; Nakagawa, Y.; Matsukura, Y.; Ichinose, S.; Koga, H.; Tsuji, K.; Sekiya, I. <Original Article>Hypoxia enhances proliferation through increase of colony formation rate with chondrogenic potential in primary synovial mesenchymal stem cells. J. Med. Dent. Sci. 2016, 63, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Pattappa, G.; Thorpe, S.D.; Jegard, N.C.; Heywood, H.K.; de Bruijn, J.D.; Lee, D.A. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng. Part C Methods 2013, 19, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pei, M. Optimization of an in vitro three-dimensional microenvironment to reprogram synovium-derived stem cells for cartilage tissue engineering. Tissue Eng. Part A 2011, 17, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Woods, A.; Sabari, S.; Pagnotta, L.; Stanton, L.A.; Beier, F. RhoA/ROCK signaling suppresses hypertrophic chondrocyte differentiation. J. Biol. Chem. 2004, 279, 13205–13214. [Google Scholar] [CrossRef] [PubMed]
- Pattappa, G.; Heywood, H.K.; de Bruijn, J.D.; Lee, D.A. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J. Cell. Physiol. 2011, 226, 2562–2570. [Google Scholar] [CrossRef]
- Moussavi-Harami, F.; Duwayri, Y.; Martin, J.A.; Moussavi-Harami, F.; Buckwalter, J.A. Oxygen effects on senescence in chondrocytes and mesenchymal stem cells: Consequences for tissue engineering. Iowa Orthop. J. 2004, 24, 15–20. [Google Scholar] [PubMed]
- D′Ippolito, G.; Diabira, S.; Howard, G.A.; Roos, B.A.; Schiller, P.C. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 2006, 39, 513–522. [Google Scholar] [CrossRef]
- Rios, C.; D′Ippolito, G.; Curtis, K.M.; Delcroix, G.J.; Gomez, L.A.; El Hokayem, J.; Rieger, M.; Parrondo, R.; de Las Pozas, A.; Perez-Stable, C.; et al. Low Oxygen Modulates Multiple Signaling Pathways, Increasing Self-Renewal, While Decreasing Differentiation, Senescence, and Apoptosis in Stromal MIAMI Cells. Stem Cells Dev. 2016, 25, 848–860. [Google Scholar] [CrossRef] [Green Version]
- Robins, J.C.; Akeno, N.; Mukherjee, A.; Dalal, R.R.; Aronow, B.J.; Koopman, P.; Clemens, T.L. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone 2005, 37, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Benz, K.; Ahlers, M.; Gaissmaier, C.; Mollenhauer, J. Hypoxic conditions during expansion culture prime human mesenchymal stromal precursor cells for chondrogenic differentiation in three-dimensional cultures. Cell Transplant. 2011, 20, 1589–1602. [Google Scholar] [CrossRef]
- Pilgaard, L.; Lund, P.; Duroux, M.; Lockstone, H.; Taylor, J.; Emmersen, J.; Fink, T.; Ragoussis, J.; Zachar, V. Transcriptional signature of human adipose tissue-derived stem cells (hASCs) preconditioned for chondrogenesis in hypoxic conditions. Exp. Cell Res. 2009, 315, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Merceron, C.; Vinatier, C.; Portron, S.; Masson, M.; Amiaud, J.; Guigand, L.; Cherel, Y.; Weiss, P.; Guicheux, J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am. J. Physiol. Cell Physiol. 2010, 298, C355–C364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betre, H.; Ong, S.R.; Guilak, F.; Chilkoti, A.; Fermor, B.; Setton, L.A. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials 2006, 27, 91–99. [Google Scholar] [CrossRef]
- Malladi, P.; Xu, Y.; Chiou, M.; Giaccia, A.J.; Longaker, M.T. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am. J. Physiol. Cell Physiol. 2006, 290, C1139–C1146. [Google Scholar] [CrossRef]
- Meretoja, V.V.; Dahlin, R.L.; Wright, S.; Kasper, F.K.; Mikos, A.G. The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials 2013, 34, 4266–4273. [Google Scholar] [CrossRef] [Green Version]
- Desance, M.; Contentin, R.; Bertoni, L.; Gomez-Leduc, T.; Branly, T.; Jacquet, S.; Betsch, J.M.; Batho, A.; Legendre, F.; Audigie, F.; et al. Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy. Int. J. Mol. Sci. 2018, 19, 537. [Google Scholar] [CrossRef]
- Gomez-Leduc, T.; Desance, M.; Hervieu, M.; Legendre, F.; Ollitrault, D.; de Vienne, C.; Herlicoviez, M.; Galera, P.; Demoor, M. Hypoxia Is a Critical Parameter for Chondrogenic Differentiation of Human Umbilical Cord Blood Mesenchymal Stem Cells in Type I/III Collagen Sponges. Int. J. Mol. Sci. 2017, 18, 1933. [Google Scholar] [CrossRef]
- Munir, S.; Foldager, C.B.; Lind, M.; Zachar, V.; Soballe, K.; Koch, T.G. Hypoxia enhances chondrogenic differentiation of human adipose tissue-derived stromal cells in scaffold-free and scaffold systems. Cell Tissue Res. 2014, 355, 89–102. [Google Scholar] [CrossRef]
- Wang, D.W.; Fermor, B.; Gimble, J.M.; Awad, H.A.; Guilak, F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J. Cell. Physiol. 2005, 204, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.S.; Adesida, A.B.; Hardingham, T.E. Hypoxic conditions increase hypoxia-inducible transcription factor 2α and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res. Ther. 2007, 9, R55. [Google Scholar] [CrossRef] [PubMed]
- Malladi, P.; Xu, Y.; Chiou, M.; Giaccia, A.J.; Longaker, M.T. Hypoxia inducible factor-1alpha deficiency affects chondrogenesis of adipose-derived adult stromal cells. Tissue Eng. 2007, 13, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Felka, T.; Schafer, R.; Schewe, B.; Benz, K.; Aicher, W.K. Hypoxia reduces the inhibitory effect of IL-1β on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthr. Cartil. 2009, 17, 1368–1376. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, L.; Arnhold, S.; Brixius, K.; Addicks, K.; Bloch, W. Human mesenchymal stem cells: Influence of oxygen pressure on proliferation and chondrogenic differentiation in fibrin glue in vitro. J. Biomed. Mater. Res. A 2010, 93, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.T.; Vinardell, T.; Kelly, D.J. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 1345–1354. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.S.; Adesida, A.B.; Tew, S.R.; Lowe, E.T.; Hardingham, T.E. Bone marrow-derived mesenchymal stem cells express the pericyte marker 3G5 in culture and show enhanced chondrogenesis in hypoxic conditions. J. Orthop. Res. 2010, 28, 834–840. [Google Scholar] [CrossRef]
- Meyer, E.G.; Buckley, C.T.; Thorpe, S.D.; Kelly, D.J. Low oxygen tension is a more potent promoter of chondrogenic differentiation than dynamic compression. J. Biomech. 2010, 43, 2516–2523. [Google Scholar] [CrossRef]
- Stoyanov, J.V.; Gantenbein-Ritter, B.; Bertolo, A.; Aebli, N.; Baur, M.; Alini, M.; Grad, S. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur. Cell Mater. 2011, 21, 533–547. [Google Scholar] [CrossRef]
- Gawlitta, D.; van Rijen, M.H.; Schrijver, E.J.; Alblas, J.; Dhert, W.J. Hypoxia impedes hypertrophic chondrogenesis of human multipotent stromal cells. Tissue Eng. Part A 2012, 18, 1957–1966. [Google Scholar] [CrossRef]
- Portron, S.; Merceron, C.; Gauthier, O.; Lesoeur, J.; Sourice, S.; Masson, M.; Fellah, B.H.; Geffroy, O.; Lallemand, E.; Weiss, P.; et al. Effects of in vitro low oxygen tension preconditioning of adipose stromal cells on their in vivo chondrogenic potential: Application in cartilage tissue repair. PLoS ONE 2013, 8, e62368. [Google Scholar] [CrossRef] [PubMed]
- Leijten, J.; Georgi, N.; Moreira Teixeira, L.; van Blitterswijk, C.A.; Post, J.N.; Karperien, M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc. Natl. Acad. Sci. USA 2014, 111, 13954–13959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Feng, Q.; Bian, L. Differential effect of hypoxia on human mesenchymal stem cell chondrogenesis and hypertrophy in hyaluronic acid hydrogels. Acta Biomater. 2014, 10, 1333–1340. [Google Scholar] [CrossRef]
- Portron, S.; Hivernaud, V.; Merceron, C.; Lesoeur, J.; Masson, M.; Gauthier, O.; Vinatier, C.; Beck, L.; Guicheux, J. Inverse regulation of early and late chondrogenic differentiation by oxygen tension provides cues for stem cell-based cartilage tissue engineering. Cell. Physiol. Biochem. 2015, 35, 841–857. [Google Scholar] [CrossRef]
- Markway, B.D.; Cho, H.; Anderson, D.E.; Holden, P.; Ravi, V.; Little, C.B.; Johnstone, B. Reoxygenation enhances tumour necrosis factor alpha-induced degradation of the extracellular matrix produced by chondrogenic cells. Eur. Cell Mater. 2016, 31, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Galeano-Garces, C.; Camilleri, E.T.; Riester, S.M.; Dudakovic, A.; Larson, D.R.; Qu, W.; Smith, J.; Dietz, A.B.; Im, H.J.; Krych, A.J.; et al. Molecular Validation of Chondrogenic Differentiation and Hypoxia Responsiveness of Platelet-Lysate Expanded Adipose Tissue-Derived Human Mesenchymal Stromal Cells. Cartilage 2017, 8, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Legendre, F.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Gruchy, N.; Mallein-Gerin, F.; Leclercq, S.; Demoor, M.; Galera, P. Enhanced chondrogenesis of bone marrow-derived stem cells by using a combinatory cell therapy strategy with BMP-2/TGF-beta1, hypoxia, and COL1A1/HtrA1 siRNAs. Sci. Rep. 2017, 7, 3406. [Google Scholar] [CrossRef]
- Rodenas-Rochina, J.; Kelly, D.J.; Gomez Ribelles, J.L.; Lebourg, M. Influence of oxygen levels on chondrogenesis of porcine mesenchymal stem cells cultured in polycaprolactone scaffolds. J. Biomed. Mater. Res. A 2017, 105, 1684–1691. [Google Scholar] [CrossRef]
- Martin-Rendon, E.; Hale, S.J.; Ryan, D.; Baban, D.; Forde, S.P.; Roubelakis, M.; Sweeney, D.; Moukayed, M.; Harris, A.L.; Davies, K.; et al. Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells 2007, 25, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Markway, B.D.; Tan, G.K.; Brooke, G.; Hudson, J.E.; Cooper-White, J.J.; Doran, M.R. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 2010, 19, 29–42. [Google Scholar] [CrossRef]
- Ronziere, M.C.; Perrier, E.; Mallein-Gerin, F.; Freyria, A.M. Chondrogenic potential of bone marrow- and adipose tissue-derived adult human mesenchymal stem cells. Biomed. Mater. Eng. 2010, 20, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Duval, E.; Bauge, C.; Andriamanalijaona, R.; Benateau, H.; Leclercq, S.; Dutoit, S.; Poulain, L.; Galera, P.; Boumediene, K. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials 2012, 33, 6042–6051. [Google Scholar] [CrossRef]
- Lee, H.H.; Chang, C.C.; Shieh, M.J.; Wang, J.P.; Chen, Y.T.; Young, T.H.; Hung, S.C. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Sci. Rep. 2013, 3, 2683. [Google Scholar] [CrossRef]
- Ranera, B.; Remacha, A.R.; Alvarez-Arguedas, S.; Castiella, T.; Vazquez, F.J.; Romero, A.; Zaragoza, P.; Martin-Burriel, I.; Rodellar, C. Expansion under hypoxic conditions enhances the chondrogenic potential of equine bone marrow-derived mesenchymal stem cells. Vet. J. 2013, 195, 248–251. [Google Scholar] [CrossRef]
- Henrionnet, C.; Liang, G.; Roeder, E.; Dossot, M.; Wang, H.; Magdalou, J.; Gillet, P.; Pinzano, A. Hypoxia for Mesenchymal Stem Cell Expansion and Differentiation: The Best Way for Enhancing TGFss-Induced Chondrogenesis and Preventing Calcifications in Alginate Beads. Tissue Eng. Part A 2017, 23, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Hudson, K.D.; Bonassar, L.J. Hypoxic Expansion of Human Mesenchymal Stem Cells Enhances Three-Dimensional Maturation of Tissue-Engineered Intervertebral Discs. Tissue Eng. Part A 2017, 23, 293–300. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.K.; Jung, B.J.; Choi, S.B.; Choi, E.Y.; Kim, C.S. Enhancing proliferation and optimizing the culture condition for human bone marrow stromal cells using hypoxia and fibroblast growth factor-2. Stem Cell Res. 2018, 28, 87–95. [Google Scholar] [CrossRef]
- Liu, Y.; Buckley, C.T.; Downey, R.; Mulhall, K.J.; Kelly, D.J. The role of environmental factors in regulating the development of cartilaginous grafts engineered using osteoarthritic human infrapatellar fat pad-derived stem cells. Tissue Eng. Part A 2012, 18, 1531–1541. [Google Scholar] [CrossRef]
- Pattappa, G.S.R.; Hofmeister, I.; Seja, J.; Zellner, J.; Johnstone, B.; Docheva, D.; Angele, P. Physioxia has a beneficial effect on cartilage matrix production in interleukin-1β inhibited mesenchymal stem cell chondrogenesis. Stem Cells Int. 2018. in submit. [Google Scholar]
- Giovannini, S.; Diaz-Romero, J.; Aigner, T.; Mainil-Varlet, P.; Nesic, D. Population doublings and percentage of S100-positive cells as predictors of in vitro chondrogenicity of expanded human articular chondrocytes. J. Cell. Physiol. 2010, 222, 411–420. [Google Scholar] [CrossRef]
- Bauge, C.; Legendre, F.; Leclercq, S.; Elissalde, J.M.; Pujol, J.P.; Galera, P.; Boumediene, K. Interleukin-1β impairment of transforming growth factor β1 signaling by down-regulation of transforming growth factor beta receptor type II and up-regulation of Smad7 in human articular chondrocytes. Arthritis Rheumatol. 2007, 56, 3020–3032. [Google Scholar] [CrossRef]
- Bauge, C.; Attia, J.; Leclercq, S.; Pujol, J.P.; Galera, P.; Boumediene, K. Interleukin-1beta up-regulation of Smad7 via NF-κB activation in human chondrocytes. Arthritis Rheumatol. 2008, 58, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Bauge, C.; Beauchef, G.; Leclercq, S.; Kim, S.J.; Pujol, J.P.; Galera, P.; Boumediene, K. NFκB mediates IL-1β-induced down-regulation of TβRII through the modulation of Sp3 expression. J. Cell. Mol. Med. 2008, 12, 1754–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfander, D.; Cramer, T.; Swoboda, B. Hypoxia and HIF-1α in osteoarthritis. Int. Orthop. 2005, 29, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Myllyharju, J.; Schipani, E. Extracellular matrix genes as hypoxia-inducible targets. Cell Tissue Res. 2010, 339, 19–29. [Google Scholar] [CrossRef]
- Fernandez-Torres, J.; Zamudio-Cuevas, Y.; Martinez-Nava, G.A.; Lopez-Reyes, A.G. Hypoxia-Inducible Factors (HIFs) in the articular cartilage: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2800–2810. [Google Scholar] [PubMed]
- Yang, S.; Kim, J.; Ryu, J.H.; Oh, H.; Chun, C.H.; Kim, B.J.; Min, B.H.; Chun, J.S. Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 2010, 16, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Kawaguchi, H. HIF-2α as a possible therapeutic target of osteoarthritis. Osteoarthr. Cartil. 2010, 18, 1552–1556. [Google Scholar] [CrossRef] [Green Version]
- Coimbra, I.B.; Jimenez, S.A.; Hawkins, D.F.; Piera-Velazquez, S.; Stokes, D.G. Hypoxia inducible factor-1α expression in human normal and osteoarthritic chondrocytes. Osteoarthr. Cartil. 2004, 12, 336–345. [Google Scholar] [CrossRef]
- Saito, T.; Fukai, A.; Mabuchi, A.; Ikeda, T.; Yano, F.; Ohba, S.; Nishida, N.; Akune, T.; Yoshimura, N.; Nakagawa, T.; et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med. 2010, 16, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Yang, S.; Shin, Y.; Rhee, J.; Chun, C.H.; Chun, J.S. Interleukin-6 plays an essential role in hypoxia-inducible factor 2α-induced experimental osteoarthritic cartilage destruction in mice. Arthritis Rheumatol. 2011, 63, 2732–2743. [Google Scholar] [CrossRef] [Green Version]
- Araldi, E.; Khatri, R.; Giaccia, A.J.; Simon, M.C.; Schipani, E. Lack of HIF-2α in limb bud mesenchyme causes a modest and transient delay of endochondral bone development. Nat. Med. 2011, 17, 25–26; author reply 27–29. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Shi, D.; Dai, J.; Tsezou, A.; Zheng, M.; Norman, P.E.; Chou, C.H.; Lee, M.T.; Hwang, J.Y.; Kim, D.H.; et al. A large-scale replication study for the association of rs17039192 in HIF-2α with knee osteoarthritis. J. Orthop. Res. 2012, 30, 1244–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.L. HIF-2α—A mediator of osteoarthritis? Cell Res. 2010, 20, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Schrobback, K.; Klein, T.J.; Crawford, R.; Upton, Z.; Malda, J.; Leavesley, D.I. Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res. 2012, 347, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.Z.; Erez, A.; Guse, K.; Dawson, B.; Bertin, T.; Chen, Y.; Jiang, M.M.; Yustein, J.; Gannon, F.; Lee, B.H. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci. Transl. Med. 2013, 5, 176ra134. [Google Scholar] [CrossRef] [PubMed]
- Markway, B.D.; Cho, H.; Zilberman-Rudenko, J.; Holden, P.; McAlinden, A.; Johnstone, B. Hypoxia-inducible factor 3-α expression is associated with the stable chondrocyte phenotype. J. Orthop. Res. 2015, 33, 1561–1570. [Google Scholar] [CrossRef]
- Aghajanian, P.; Mohan, S. The art of building bone: Emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 2018, 6, 19. [Google Scholar] [CrossRef]
- Ono, N.; Ono, W.; Nagasawa, T.; Kronenberg, H.M. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat. Cell Biol. 2014, 16, 1157–1167. [Google Scholar] [CrossRef] [Green Version]
- Pacifici, M.; Golden, E.B.; Oshima, O.; Shapiro, I.M.; Leboy, P.S.; Adams, S.L. Hypertrophic chondrocytes. The terminal stage of differentiation in the chondrogenic cell lineage? Ann. N. Y. Acad. Sci. 1990, 599, 45–57. [Google Scholar] [CrossRef]
- Kita, K.; Kimura, T.; Nakamura, N.; Yoshikawa, H.; Nakano, T. PI3K/Akt signaling as a key regulatory pathway for chondrocyte terminal differentiation. Genes Cells 2008, 13, 839–850. [Google Scholar] [CrossRef] [Green Version]
- Beier, F.; Loeser, R.F. Biology and pathology of Rho GTPase, PI-3 kinase-Akt, and MAP kinase signaling pathways in chondrocytes. J. Cell. Biochem. 2010, 110, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akasaki, Y.; Hasegawa, A.; Saito, M.; Asahara, H.; Iwamoto, Y.; Lotz, M.K. Dysregulated FOXO transcription factors in articular cartilage in aging and osteoarthritis. Osteoarthr. Cartil. 2014, 22, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, T.; Alvarez-Garcia, O.; Mokuda, S.; Nagira, K.; Olmer, M.; Gamini, R.; Miyata, K.; Akasaki, Y.; Su, A.I.; Asahara, H.; et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Nakae, J.; Oki, M.; Cao, Y. The FoxO transcription factors and metabolic regulation. FEBS Lett. 2008, 582, 54–67. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, A.; Burgering, B.M. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 440–450. [Google Scholar] [CrossRef]
- Carames, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.J.; Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheumatol. 2010, 62, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Lotz, M.K.; Carames, B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat. Rev. Rheumatol. 2011, 7, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.E.; Johnstone, B. Dynamic Mechanical Compression of Chondrocytes for Tissue Engineering: A Critical Review. Front. Bioeng. Biotechnol. 2017, 5, 76. [Google Scholar] [CrossRef]
- Huang, A.H.; Farrell, M.J.; Mauck, R.L. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J. Biomech. 2010, 43, 128–136. [Google Scholar] [CrossRef] [Green Version]
- O′Conor, C.J.; Case, N.; Guilak, F. Mechanical regulation of chondrogenesis. Stem Cell Res. Ther. 2013, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Soltz, M.A.; Ateshian, G.A. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann. Biomed. Eng. 2000, 28, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Soltz, M.A.; Ateshian, G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J. Biomech. 1998, 31, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, M.; Carriero, A.; Shefelbine, S.J.; Nowlan, N.C. Mechanobiological simulations of prenatal joint morphogenesis. J. Biomech. 2014, 47, 989–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, D.R.; Wong, M. Modelling cartilage mechanobiology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 1461–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, A.; Rolfe, R.; Carroll, S.; Kelly, D.J.; Murphy, P. Chondrogenesis of embryonic limb bud cells in micromass culture progresses rapidly to hypertrophy and is modulated by hydrostatic pressure. Cell Tissue Res. 2017, 368, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, T.; Rolfe, R.A.; Buckley, C.T.; Meyer, E.G.; Ahearne, M.; Murphy, P.; Kelly, D.J. Hydrostatic pressure acts to stabilise a chondrogenic phenotype in porcine joint tissue derived stem cells. Eur. Cell Mater. 2012, 23, 121–132; discussion 133–134. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Mizuno, S.; Murphy, G.F.; Orgill, D.P. The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells. Tissue Eng. Part A 2009, 15, 2937–2945. [Google Scholar] [CrossRef]
- Ogawa, R.; Orgill, D.P.; Murphy, G.F.; Mizuno, S. Hydrostatic pressure-driven three-dimensional cartilage induction using human adipose-derived stem cells and collagen gels. Tissue Eng. Part A 2015, 21, 257–266. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Yang, J.; Liu, J.; Wang, J.; Li, X.; Liu, Y. p38 MAPK mediated in compressive stress-induced chondrogenesis of rat bone marrow MSCs in 3D alginate scaffolds. J. Cell. Physiol. 2009, 221, 609–617. [Google Scholar] [CrossRef]
- Liu, Y.; Buckley, C.T.; Almeida, H.V.; Mulhall, K.J.; Kelly, D.J. Infrapatellar fat pad-derived stem cells maintain their chondrogenic capacity in disease and can be used to engineer cartilaginous grafts of clinically relevant dimensions. Tissue Eng. Part A 2014, 20, 3050–3062. [Google Scholar] [CrossRef] [PubMed]
- Steward, A.J.; Thorpe, S.D.; Vinardell, T.; Buckley, C.T.; Wagner, D.R.; Kelly, D.J. Cell-matrix interactions regulate mesenchymal stem cell response to hydrostatic pressure. Acta Biomater. 2012, 8, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.E.; Allon, A.A.; Cheng, T.; Kuo, A.C.; Kim, H.T.; Vail, T.P.; Marcucio, R.S.; Schneider, R.A.; Lotz, J.C.; Alliston, T. Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthr. Cartil. 2011, 19, 1210–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, C.; Adesida, A.; Zajac, P.; Mumme, M.; Riesle, J.; Martin, I.; Barbero, A. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J. Cell. Physiol. 2012, 227, 88–97. [Google Scholar] [CrossRef]
- Wu, L.; Post, J.N.; Karperien, M. Engineering cartilage tissue by pellet coculture of chondrocytes and mesenchymal stromal cells. Methods Mol. Biol. 2015, 1226, 31–41. [Google Scholar] [CrossRef]
- Ionescu, A.; Kozhemyakina, E.; Nicolae, C.; Kaestner, K.H.; Olsen, B.R.; Lassar, A.B. FoxA family members are crucial regulators of the hypertrophic chondrocyte differentiation program. Dev. Cell 2012, 22, 927–939. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Cleary, M.A.; van Osch, G.J.; Brama, P.A.; Hellingman, C.A.; Narcisi, R. FGF, TGFβ and Wnt crosstalk: Embryonic to in vitro cartilage development from mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2015, 9, 332–342. [Google Scholar] [CrossRef]
- Williams, R.; Khan, I.M.; Richardson, K.; Nelson, L.; McCarthy, H.E.; Analbelsi, T.; Singhrao, S.K.; Dowthwaite, G.P.; Jones, R.E.; Baird, D.M.; et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS ONE 2010, 5, e13246. [Google Scholar] [CrossRef]
- McCarthy, H.E.; Bara, J.J.; Brakspear, K.; Singhrao, S.K.; Archer, C.W. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet. J. 2012, 192, 345–351. [Google Scholar] [CrossRef]
- Nelson, L.; McCarthy, H.E.; Fairclough, J.; Williams, R.; Archer, C.W. Evidence of a Viable Pool of Stem Cells within Human Osteoarthritic Cartilage. Cartilage 2014, 5, 203–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.; Haughton, L.; Bayram, Z.; Boyer, S.; Thomson, B.; et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004, 117, 889–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Publication | Cell Source | Pellets or Scaffolds | Oxygen Tension | Results—Physioxia Response Relative to Hyperoxia |
|---|---|---|---|---|
| Robins et al., 2005 [61] | Mouse ST2 stromal cells/C3H10T1/2 cells | Pellet | 1% O2 | Sex-determining region–box 9 (SOX9) expression is upregulated and induces greater glycosaminoglycan (GAG) deposition. Increased hypoxia-inducible factor-1 alpha (HIF-1α) expression; no change in hypoxia-inducible factor-1 beta (HIF-1β) expression |
| Wang et al., 2005 [71] | Human adipose MSCs | Scaffolds (4 × 106 cells/mL, alginate beads) | 5% O2 | Greater anaerobic respiration as measured by lactate production both under expansion and chondrogenesis. Increased GAG and collagen content |
| Betre et al., 2006 [65] | Human adipose MSCs | Scaffolds (2 × 106 cells/scaffold; elastin-like polypeptide scaffold) | 5% O2 | Upregulation in SOX9 and downregulation in collagen X alpha 1 (COL10A1)—reduction in collagen II alpha I (COL2A1) and aggrecan (ACAN). No difference in GAG or collagen content between conditions |
| Malladi et al., 2006 [66] | Murine inguinal fat pad MSCs | Pellets | 2% O2 | Reduced GAG/DNA and collagen content but larger diameter pellets |
| Khan et al., 2007 [72] | Infrapatellar fat pad MSCs | Pellet | 5% O2 | HIF1α, hypoxia inducible factor-2 alpha (HIF2α), SOX5, SOX6, SOX9, ACAN, COL2A1 and COL10A1 increased expression. HIF2α had greater expression than HIF1α. Increased pellet wet weight, GAG content and collagen II staining |
| Malladi et al., 2007 [73] | Murine adipose MSCs (HIF-1α deleted mice) | Pellets | 2% O2 | HIF-1α expressed by adipose MSCs. HIF-1α deleted MSCs significantly decreased SOX9, ACAN and COL2A1 expression. HIF-1α deleted micromasses had reduced GAG and collagen II deposition |
| Felka et al., 2009 [74] | Human bone marrow MSCs | Pellet | 2% O2; 2 ng/mL IL-1β | No difference in gene transcript levels. Larger pellets with more matrix production. Physioxia increase chondrogenic gene (SOX9, COL2A1, ACAN), pellet size and matrix deposition with reduced expression in matrix metalloproteinase (MMP1 and MMP13) in IL-1β inhibited chondrogenesis |
| Pilgaard et al., 2009 [63] | Adipose derived MSCs | Pellets | 15%, 10%, 5%, 1% O2 | SOX9, collagen I alpha I (COL1A1), COL2A1 and ACAN upregulated at 15% oxygen and donwregulated under physioxia. Reduction in COL10A1 expression. Increased matrix staining and GAG synthesis at 15% oxygen—reduced at lower oxygen tension. Increased matrix synthesis in central regions of ambient cultures due to oxygen gradients—central regions increase chondrogenesis in 15% oxygen culture |
| Baumgartner et al., 2010 [75] | Human bone marrow MSCs | Scaffold (20 × 106 cells/ml fibrin hydrogel) | 3% O2 | Greater and earlier expression of COL2A1. Increased alcian blue matrix staining |
| Buckley et al., 2010 [76] | Porcine infrapatellar fat pad MSCs | Scaffold (15 × 106 cells/mL in 2% agarose) | 2% O2 | Greater GAG and collagen II content with increased staining in core region. Superior mechanical properties |
| Khan et al., 2010 [77] | Human bone marrow MSCs | Pellet | 5% O2 | Upregulated SOX6, COL2A1, ACAN, HIF1α and HIF2α expression. Enhancement in pellet wet weight and GAG content |
| Merceron et al., 2010 [64] | Human adipose MSCs | Pellets | 5% O2 | COL2A1 expression enhanced and no difference in ACAN expression. No difference in matrix deposition |
| Meyer et al., 2010 [78] | Porcine bone marrow MSCs | Scaffold (15 × 106 cells/mL in 2% agarose) | 5% O2 | Greater GAG and collagen II content with increased staining in central regions. Increase in dynamic and equilibrium modulus. No synergistic effect with dynamic loading |
| Li and Pei, 2011 [55] | Human synovial fetal fibroblasts | Pellets | 5% O2 | SOX9, ACAN and COL2A1 expression were upregulated. Larger pellets with greater GAG and collagen II content |
| Stoyanov et al., 2011 [79] | Human bone marrow MSCs | Scaffolds (4 × 106 cells/mL in 1.2% (w/v) alginate beads) | 2% O2 | Increase in SOX9 and COL10A1 expression. Greater GAG and collagen II content. In the presence of GDF-5, increased ACAN and COL2A1 expression compared to TGF-β groups with reduced hypertrophy |
| Gawlitta et al., 2012 [80] | Human bone marrow MSCs | Pellets | 5% O2 | Reduced collagen X staining |
| Meretoja et al., 2013 [67] | Bovine bone marrow MSCs | Scaffolds (Poly (ε-caprolactone; 4.5 × 106 cells/mL, monoculture or co-culture (30% articular chondrocytes: 70% MSCs)) | 5% O2 | COL2A1 upregulated—further enhanced in co-culture. No difference in GAG and collagen content in MSCs monocultures—increased alkaline phosphastase (ALP) and calcification under these conditions. MSC-Chondrocyte co-cultures reduced MSC hypertrophy—chondrocytes prevent this process |
| Portron et al., 2013 [81] | Rabbit and human adipose MSCs | Pellets; Scaffolds (2 × 106 cells/mL (rabbit) or 5 × 105 cells/mL (human) in Si-HPMC) | 5% O2 | Upregulation in COL2A1 and ACAN in both cell and culture types. Increased collagen II and GAG deposition. In vivo implantation of physioxia preconditioned scaffolds had higher O’Driscoll scores |
| Leijten et al., 2014 [82] | Human bone marrow MSCs | Pellets | 2.5% O2 | SOX9, COL2A1 and ACAN gene expression upregulated and COL10A1 and MMP13 gene expression downregulated. Increased GAG staining for physioxia chondrogenesis. Physioxic preconditioned chondrogenesis reduced bone-like formation upon in vivo implantation |
| Munir et al., 2014 [70] | Human adipose MSCs | Pellets, Scaffolds (8 × 106 cells/mL in collagen type I/II scaffold–Chondroglide TM) | 5% O2 | SOX9, COL1A1 and COL2A1 expression upregulated with downregulated COL10A1. Increase in COL2A1/COL1A1 and COL2A1/COL10A1 ratios. Increased matrix staining at periphery and more core deposition in hyperoxic pellets—same in scaffolds |
| Zhu et al., 2014 [83] | Human bone marrow MSCs | Scaffold (20 × 106 cells/mL; Hyaluronic acid hydrogel) | 1% O2 | Reduced hypertophic marker (COL10A1, MMP13, ALP) expression in low cross-linking hydrgoels. Increased GAG content. High cross-linking density and hyaluronic acid concentration increased expression of hypertrophy markers |
| Portron et al., 2015 [84] | Human adipose MSCs | Pellets | 5% O2 | SOX9, ACAN and COL2A1 upregulation and downregulation of COL10A1 and MMP13. No difference in matrix staining |
| Markway et al., 2016 [85] | Human bone marrow MSCs | Pellets | 2% O2; 7 days ± TNF-α (1 ng/mL) at 2% or 20% O2 | Reduction in TNF-α generated loss in GAG content. Reduced MMP2, MMP9 and MMP13; ADAMTS4/5 expression. TNF-α inhibited MSC chondrogenesis |
| Galeano-Garces et al., 2017 [86] | Human adipose MSCs | PCL scaffolds and pellets | 2% O2 | HIF1A, SOX9, COL10A1 and indian hedgehog (IHH) were significantly upregulated and COL1A1 downregulated in pellet cultures. SOX9 and ACAN expression had increased PCL scaffolds, whilst COL10A1 expression was higher in hyperoxic cultures |
| Legendre et al., 2017 [87] | Human bone marrow MSCs | Collagen I/III sponges; TGF-β and BMP2 chondrogenic induction | 3% O2 | Significant upregulation in COL2A1 and an increase in COL2A1/COL1A1 and COL2A1/COL10A1 ratio |
| Gomez-Leduc et al., 2017 [69] | Human umbilical cord MSCs | Collagen I/III sponges; TGF-β and BMP2 chondrogenic induction | 5% O2 | Lower expression of chondrogenic genes (SOX9, COL2A1 and ACAN). Downregulation in COL10A1 and MMP13 expression, and reduced collagen X protein expression. Change in oxygen tension from hyperoxia (Day 0–7) followed by physioxia (Day 7–21) helped to stabilise chondrogenic phenotype with reduction in hypertrophic gene expression (COL10A1, MMP13) |
| Rodenas-Rochina et al., 2017 [88] | Porcine bone marrow MSCs | Polycaprolactone (PCL) composite scaffolds and PCL-hyaluronic acid coated scaffolds | 5%O2 | Significant increase in GAG deposition—no difference in collagen content. Greater collagen II staining |
| Bae et al., 2018 [47] | Human synovium MSCs | Pellets | 5% O2 | Significant upregulation in SOX9, COL2A1 and ACAN. Downregulation in COL10A1. Increased GAG deposition and collagen II protein expression with reduced collagen X expression |
| Desance et al., 2018 [68] | Equine umbilical cord MSCs | Collagen I/III sponges; TGF-β and BMP2 chondrogenic induction | 3% O2 | No difference in chondrogenic gene (SOX9, COL2A1 and ACAN) or hypertrophy gene (COL10A1, runt-related transcription factor-2 (RUNX2)) expression. Hypertrophic genes were expressed significantly lower than chondrogenic genes |
| Publication | Cell Source | Pellets or Scaffolds | Oxygen Tension | Physioxia Chondrogenic Response Relative to Hyperoxia |
|---|---|---|---|---|
| Martin-Rendon et al., 2007 [89] | Bone marrow MSCs | Pellets | 1.5% O2 | Upregulated and stabilised HIF-1α expression. Increase in SOX9 gene expression and pellet wet weight |
| Xu et al., 2007 [49] | Murine adipose MSCs | Pellets | 2% O2 | COL2A1 upregulated; no difference in SOX9 and ACAN gene expression. Downregulation in MMPs (MMP2, MMP3, MMP13) and osteogenic genes (RUNX2, ALP). Physioxia preconditioning increased proteoglycan deposition—no influence of reoxygenation |
| Krinner et al., 2009 [41] | Ovine bone marrow MSCs | Pellets | 5% O2 | Enhancement in GAG and collagen II content |
| Markway et al., 2010 [90] | Human bone marrow MSCs | Pellets | 2% O2 | ACAN, COL2A1 and COL10A1 upregulated. Increased GAG content and larger pellets |
| Ronziere et al., 2010 [91] | Human bone marrow MSCs and adipose MSCs (only preconditioned) | Pellets | 2% O2 | No difference in COL2A1 and ACAN expression. Reduction in hypertophic markers (COL10A1 and MMP13) in physioxia preconditioned MSCs |
| Muller et al., 2011 [62] | Human bone marrow MSCs | Pellets, Scaffolds (4 × 105 cells in 10% (w/v) gelatin) | 4% O2 | Upregulation in SOX9, COL2A1, ACAN and COL10A1 in pellets and scaffolds. Larger pellets and increased GAG content |
| Weijers et al., 2011 [46] | Human adipose MSCs | Pellets | 1% O2 | SOX9 and COL2A1 upregulated. Increase in GAG content |
| Adesida et al., 2012 [42] | Human bone marrow MSCs | Pellets | 3% O2 | Upregulation in SOX9, COL2A1 and ACAN; downregulation in COL10A1. Enhanced GAG content and collagen II staining. Increase in transforming growth factor–beta receptor one and two (TGFBR1 and TGFBR2) and HIF-2α expression |
| Duval et al., 2012 [92] | Human bone marrow MSCs | Scaffolds (5 × 106 cells/mL in alginate beads) | 5% O2 | Increase in SOX5, SOX6, SOX9, ACAN and COL2A1 gene expression and decrease in COL10A1, RUNX2 and ALP. Greater GAG and collagen II content upon in vivo implantation. Application of HIF-1α dominant negative plasmid prevents anabolic response |
| Sheehy et al., 2012 [45] | Porcine bone marrow MSCs | Pellets; Scaffold (15 × 106 cells/mL, 2% agaose) | 5% O2 | Increase in GAG and collagen in both pellets and scaffolds (develops a pericellular matrix). Reduction in ALP; suppression in hypertrophy |
| Lee et al., 2013 [93] | Human bone marrow MSCs | Pellets | 2% O2 | SOX9, COL2A1 and ACAN expression upregulated and COL10A1 and RUNX2 downregulated. Matrix staining for GAG, collagen II and collagen X support findings. Reduced staining for apoptotic markers (Caspase -3 and -8). Akt and downstream targets, FOXO1 and FOXO3, were phosphorylated—Inhibition of pathway, increased hypertrophy (COL10A1 and RUNX2) and reduced response. No effect on hyperoxic chondrogenesis |
| O’HEireamhoin et al., 2013 [49] | Human infrapatellar fat pad MSCs | Pellets, scaffolds (20 × 106 cells/mL in 2% agarose or fibrin) | 5% O2 | Increase in GAG and collagen II content in pellets. Only GAG deposition increased within scaffolds—reduced collagen X staining |
| Pattappa et al., 2013 [54] | Human bone marrow MSCs | Pellets | 5% or 2% O2 | No difference in GAG content |
| Ranera et al., 2013 [94] | Equine bone marrow MSCs | Pellets | 5% O2 | SOX9, COL2A1, ACAN, cartilage oligomeric matrix protein (COMP) gene expression upregulated. Physioxia preconditioned cells enhanced GAG content. HIF-1α expression increased with time |
| Boyette et al., 2014 [44] | Ovine bone marrow MSCs | Pellets | 5% O2 | Enhanced chondrogenesis in physioxia differentiated cells but inhibited differentiation for physioxia preconditioned cells |
| Kalpakci et al., 2014 [50] | Dermis isolated MSCs | Pellets | 5% O2 | Increased GAG and collagen content in physioxia preconditioned MSCs; collagen II content was greater under hyperoxia |
| Bornes et al., 2015 [43] | Ovine bone marrow MSCs | Scaffolds (1 × 107 cells/cm2 on either collagen type I and esterified hyaluronic acid scaffolds) | 3% O2 | Upregulated ACAN and COL2A1 gene expression and downregulation in COL10A1. Enhanced GAG and collagen II content in both scaffold types |
| Anderson et al., 2016 [33] | Human bone marrow MSCs | Pellets | 2% O2 | COL2A1 and ACAN upregulated whilst COL10A1 and MMP13 downregulated depending upon chondrogenic capacity. Enhanced GAG production. MSCs with high chondrogenic capacity stained for collagen X inspite of physioxic culture |
| Henrionnet et al., 2016 [95] | Human bone marrow MSCs | Alginate beads | 5% O2 | Upregulated SOX9, COL2A1, ACAN and COMP, downregulated RUNX2 and ALP expression. No change in COL10A1 expression. Sequential hyperoxia then physioxia increased COL2A1 and ACAN expression with reduction in COL10A1 expression. No calcification |
| Hudson et al., 2016 [96] | Human MSCs | Collagen-alginate scaffold | 5% O2 | Greater GAG content and mechanical properties |
| Ohara et al., 2016 [53] | Human synovial derived MSCs | Pellets | 5% O2 | No difference in pellet wet weight or matrix staining |
| Yasui et al., 2016 [52] | Synovium MSCs | Scaffolds (Sheet–like construct, 4 × 105 cells/cm2) | 5% O2 | Increase in SOX9, ACAN and COL2A1 expression. Increased GAG and collagen II content |
| Bornes et al., 2018 [51] | Ovine bone marrow MSCs | HYAFF scaffolds | 3% O2 | No difference in COL2A1 and ACAN gene expression but significant increase in GAG content after 14 days culture. Significant downregulation in COL10A1 with concomitant increase in COL2A1/COL10A1 ratio at day 4 and 14. No difference in cartilaginous tissue formation in preconditioned chondrogenic MSCs upon in vivo implantation |
| Lee et al., 2018 [97] | Human bone marrow MSCs | Pellets | 1% O2 | Upregulation in SOX9, COL2A1 and ACAN expression. Increase in GAG deposition |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattappa, G.; Johnstone, B.; Zellner, J.; Docheva, D.; Angele, P. The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response. Int. J. Mol. Sci. 2019, 20, 484. https://doi.org/10.3390/ijms20030484
Pattappa G, Johnstone B, Zellner J, Docheva D, Angele P. The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response. International Journal of Molecular Sciences. 2019; 20(3):484. https://doi.org/10.3390/ijms20030484
Chicago/Turabian StylePattappa, Girish, Brian Johnstone, Johannes Zellner, Denitsa Docheva, and Peter Angele. 2019. "The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response" International Journal of Molecular Sciences 20, no. 3: 484. https://doi.org/10.3390/ijms20030484
APA StylePattappa, G., Johnstone, B., Zellner, J., Docheva, D., & Angele, P. (2019). The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response. International Journal of Molecular Sciences, 20(3), 484. https://doi.org/10.3390/ijms20030484