Molecular Mechanisms of Dermal Aging and Antiaging Approaches
Abstract
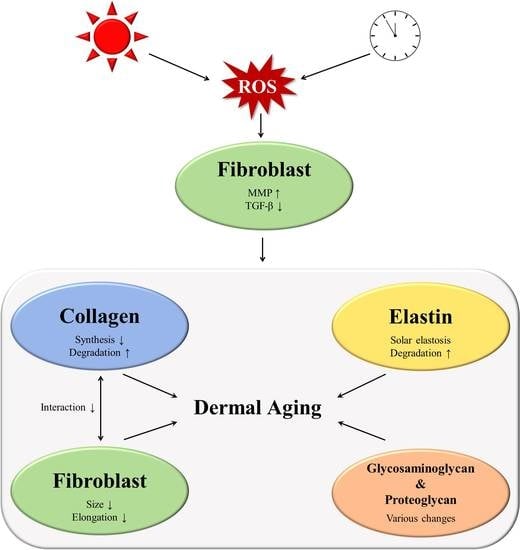
:1. Introduction
2. Composition of the Dermis
3. Changes in Dermal Components with Aging
3.1. Collagen
3.1.1. Increased Matrix Metalloproteinase (MMP) Levels
3.1.2. Impaired Transforming Growth Factor-β Signaling during Aging
3.1.3. Interaction between Fibroblasts and the ECM
3.1.4. Summary
3.2. Changes to other ECM Components
3.2.1. Elastic Fiber Remodeling
3.2.2. Changes in Glycosaminoglycans
3.2.3. Changes to Proteoglycans
3.2.4. Summary
4. Antiaging Approaches
4.1. Topicals
4.1.1. Topical Retinoids
4.1.2. Other Cosmeceuticals
4.2. Energy Based Dermal Rejuvenation
4.2.1. Fractional Lasers
4.2.2. Non-Laser-Based Approaches
4.3. Filler as an ECM Microenvironment Modulator
5. Conclusions
Funding
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Zeng, J.P.; Bi, B.; Chen, L.; Yang, P.; Guo, Y.; Zhou, Y.Q.; Liu, T.Y. Repeated exposure of mouse dermal fibroblasts at a sub-cytotoxic dose of uvb leads to premature senescence: A robust model of cellular photoaging. J. Dermatol. Sci. 2014, 73, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Purohit, T.; He, T.; Qin, Z.; Li, T.; Fisher, G.J.; Yan, Y.; Voorhees, J.J.; Quan, T. Smad3-dependent regulation of type i collagen in human dermal fibroblasts: Impact on human skin connective tissue aging. J. Dermatol. Sci. 2016, 83, 80–83. [Google Scholar] [CrossRef]
- Xia, W.; Quan, T.; Hammerberg, C.; Voorhees, J.J.; Fisher, G.J. A mouse model of skin aging: Fragmentation of dermal collagen fibrils and reduced fibroblast spreading due to expression of human matrix metalloproteinase-1. J. Dermatol. Sci. 2015, 78, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E. The clinical identification and quantification of photodamage. Br. J. Dermatol. 1992, 127 (Suppl. 41), 37–42. [Google Scholar] [CrossRef]
- Lapiere, C.M. The ageing dermis: The main cause for the appearance of ‘old’ skin. Br. J. Dermatol. 1990, 122 (Suppl. 35), 5–11. [Google Scholar] [CrossRef] [PubMed]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Talwar, H.S.; Griffiths, C.E.; Fisher, G.J.; Hamilton, T.A.; Voorhees, J.J. Reduced type i and type iii procollagens in photodamaged adult human skin. J. Investig. Dermatol. 1995, 105, 285–290. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. Photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Yonetsu, M.; Tanaka, R.; Tanaka, Y.; Fukushima, S.; Yamashita, T.; Ogura, Y.; Hirao, T.; Murota, H.; Araki, T. In vivo observation of age-related structural changes of dermal collagen in human facial skin using collagen-sensitive second harmonic generation microscope equipped with 1250-nm mode-locked cr:Forsterite laser. J. Biomed. Opt. 2013, 18, 31108. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking older: Fibroblast collapse and therapeutic implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Shao, Y.; He, T.; Voorhees, J.J.; Fisher, G.J. Reduced expression of connective tissue growth factor (ctgf/ccn2) mediates collagen loss in chronologically aged human skin. J. Investig. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.H.; Wang, Z.Q.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin a antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (mmps): Chemical-biological functions and (q)sars. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in uv-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Newby, A.C. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol. Rev. 2005, 85, 1–31. [Google Scholar] [CrossRef]
- Kobayashi, Y. Langerhans’ cells produce type iv collagenase (mmp-9) following epicutaneous stimulation with haptens. Immunology 1997, 90, 496–501. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and timps. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef]
- Qin, Z.; Balimunkwe, R.M.; Quan, T. Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br. J. Dermatol. 2017, 177, 1337–1348. [Google Scholar] [CrossRef]
- Tewari, A.; Grys, K.; Kollet, J.; Sarkany, R.; Young, A.R. Upregulation of mmp12 and its activity by uva1 in human skin: Potential implications for photoaging. J. Investig. Dermatol. 2014, 134, 2598–2609. [Google Scholar] [CrossRef]
- Parkinson, L.G.; Toro, A.; Zhao, H.; Brown, K.; Tebbutt, S.J.; Granville, D.J. Granzyme b mediates both direct and indirect cleavage of extracellular matrix in skin after chronic low-dose ultraviolet light irradiation. Aging Cell 2015, 14, 67–77. [Google Scholar] [CrossRef]
- Yokose, U.; Hachiya, A.; Sriwiriyanont, P.; Fujimura, T.; Visscher, M.O.; Kitzmiller, W.J.; Bello, A.; Tsuboi, R.; Kitahara, T.; Kobinger, G.P.; et al. The endogenous protease inhibitor timp-1 mediates protection and recovery from cutaneous photodamage. J. Investig. Dermatol. 2012, 132, 2800–2809. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation and aging. Science 1992, 257, 1220–1224. [Google Scholar] [CrossRef] [Green Version]
- Golden, T.R.; Hinerfeld, D.A.; Melov, S. Oxidative stress and aging: Beyond correlation. Aging Cell 2002, 1, 117–123. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.W.; Kim, E.K.; Lee, S.J.; Park, N.H.; Kim, H.S.; Kim, H.K.; Char, K.; Jang, Y.P.; Kim, J.W. Inhibition effect of gynura procumbens extract on uv-b-induced matrix-metalloproteinase expression in human dermal fibroblasts. J. Ethnopharmacol. 2011, 137, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.M.; Chen, H.C.; Chiu, H.H.; Chen, C.W.; Wang, S.M.; Wen, K.C. Neonauclea reticulata (havil.) merr stimulates skin regeneration after uvb exposure via ros scavenging and modulation of the mapk/mmps/collagen pathway. Evid Based Complement. Altern. Med. 2013, 2013, 324864. [Google Scholar] [CrossRef]
- Park, J.E.; Pyun, H.B.; Woo, S.W.; Jeong, J.H.; Hwang, J.K. The protective effect of kaempferia parviflora extract on uvb-induced skin photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2014, 30, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. Ap-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Choi, Y.J.; Moon, K.M.; Chung, K.W.; Jeong, J.W.; Park, D.; Kim, D.H.; Yu, B.P.; Chung, H.Y. The underlying mechanism of proinflammatory nf-kappab activation by the mtorc2/akt/ikkalpha pathway during skin aging. Oncotarget 2016, 7, 52685–52694. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.; He, T.; Shao, Y.; Fonseca, M.J.; Verri, W.A., Jr.; Fisher, G.J.; Xu, Y. Quercetin inhibits uv irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing nf-kappab pathway. J. Dermatol. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Noh, E.M.; Han, J.H.; Kim, J.M.; Hwang, J.K.; Hwang, B.M.; Chung, E.Y.; Kim, B.S.; Lee, S.H.; Lee, S.J.; et al. Brazilin inhibits uvb-induced mmp-1/3 expressions and secretions by suppressing the nf-kappab pathway in human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Rosenbloom, J.; Jimenez, S.A. Transforming growth factor beta (tgf beta) causes a persistent increase in steady-state amounts of type i and type iii collagen and fibronectin mrnas in normal human dermal fibroblasts. Biochem. J. 1987, 247, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.W.; Hwang, E.; Lee, H.J.; Lee, T.Y.; Song, H.G.; Park, S.Y.; Shin, H.S.; Lee, D.G.; Yi, T.H. Effects of galla chinensis extracts on uvb-irradiated mmp-1 production in hairless mice. J. Nat. Med. 2015, 69, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Lee, D.G.; Park, S.H.; Oh, M.S.; Kim, S.Y. Coriander leaf extract exerts antioxidant activity and protects against uvb-induced photoaging of skin by regulation of procollagen type i and mmp-1 expression. J. Med. Food 2014, 17, 985–995. [Google Scholar] [CrossRef]
- Chen, B.; Li, R.; Yan, N.; Chen, G.; Qian, W.; Jiang, H.L.; Ji, C.; Bi, Z.G. Astragaloside iv controls collagen reduction in photoaging skin by improving transforming growth factor-beta/smad signaling suppression and inhibiting matrix metalloproteinase-1. Mol. Med. Rep. 2015, 11, 3344–3348. [Google Scholar] [CrossRef]
- He, T.; Quan, T.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Oxidative exposure impairs tgf-beta pathway via reduction of type ii receptor and smad3 in human skin fibroblasts. Age (Dordr) 2014, 36, 9623. [Google Scholar] [CrossRef]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef]
- Fisher, G.J.; Shao, Y.; He, T.; Qin, Z.; Perry, D.; Voorhees, J.J.; Quan, T. Reduction of fibroblast size/mechanical force down-regulates tgf-beta type ii receptor: Implications for human skin aging. Aging Cell 2016, 15, 67–76. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef]
- Quan, C.; Cho, M.K.; Perry, D.; Quan, T. Age-associated reduction of cell spreading induces mitochondrial DNA common deletion by oxidative stress in human skin dermal fibroblasts: Implication for human skin connective tissue aging. J. Biomed. Sci. 2015, 22, 62. [Google Scholar] [CrossRef]
- Quan, T.; Wang, F.; Shao, Y.; Rittie, L.; Xia, W.; Orringer, J.S.; Voorhees, J.J.; Fisher, G.J. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J. Investig. Dermatol. 2013, 133, 658–667. [Google Scholar] [CrossRef]
- Amano, S. Characterization and mechanisms of photoageing-related changes in skin. Damages of basement membrane and dermal structures. Exp. Dermatol. 2016, 25 (Suppl. 3), 14–19. [Google Scholar] [CrossRef] [Green Version]
- Doubal, S.; Klemera, P. Visco-elastic response of human skin and aging. J. Am. Aging Assoc. 2002, 25, 115–117. [Google Scholar] [CrossRef] [Green Version]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin structure and its involvement in skin photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef]
- Rossetti, D.; Kielmanowicz, M.G.; Vigodman, S.; Hu, Y.P.; Chen, N.; Nkengne, A.; Oddos, T.; Fischer, D.; Seiberg, M.; Lin, C.B. A novel anti-ageing mechanism for retinol: Induction of dermal elastin synthesis and elastin fibre formation. Int. J. Cosmet. Sci. 2011, 33, 62–69. [Google Scholar] [CrossRef]
- Noblesse, E.; Cenizo, V.; Bouez, C.; Borel, A.; Gleyzal, C.; Peyrol, S.; Jacob, M.P.; Sommer, P.; Damour, O. Lysyl oxidase-like and lysyl oxidase are present in the dermis and epidermis of a skin equivalent and in human skin and are associated to elastic fibers. J. Investig. Dermatol. 2004, 122, 621–630. [Google Scholar] [CrossRef]
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. J. Cell Sci. 2002, 115, 2817–2828. [Google Scholar]
- Sherratt, M.J. Tissue elasticity and the ageing elastic fibre. Age (Dordr) 2009, 31, 305–325. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, F.; Carta, L.; Lee-Arteaga, S.; Liu, C.; Nistala, H.; Smaldone, S. Fibrillin-rich microfibrils—Structural and instructive determinants of mammalian development and physiology. Connect. Tissue Res. 2008, 49, 1–6. [Google Scholar] [CrossRef]
- Dahlback, K.; Ljungquist, A.; Lofberg, H.; Dahlback, B.; Engvall, E.; Sakai, L.Y. Fibrillin immunoreactive fibers constitute a unique network in the human dermis: Immunohistochemical comparison of the distributions of fibrillin, vitronectin, amyloid p component, and orcein stainable structures in normal skin and elastosis. J. Investig. Dermatol. 1990, 94, 284–291. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Davis, E.C.; Starcher, B.C.; Ouchi, T.; Yanagisawa, M.; Richardson, J.A.; Olson, E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 2002, 415, 168–171. [Google Scholar] [CrossRef]
- Langton, A.K.; Sherratt, M.J.; Griffiths, C.E.; Watson, R.E. Differential expression of elastic fibre components in intrinsically aged skin. Biogerontology 2012, 13, 37–48. [Google Scholar] [CrossRef]
- Ashworth, J.L.; Murphy, G.; Rock, M.J.; Sherratt, M.J.; Shapiro, S.D.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin degradation by matrix metalloproteinases: Implications for connective tissue remodelling. Biochem. J. 1999, 340, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Mandal, M.; Das, S.; Mandal, A.; Chakraborti, T. Regulation of matrix metalloproteinases: An overview. Mol. Cell Biochem. 2003, 253, 269–285. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on uva-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef]
- Chung, J.H.; Seo, J.Y.; Lee, M.K.; Eun, H.C.; Lee, J.H.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Ultraviolet modulation of human macrophage metalloelastase in human skin in vivo. J. Investig. Dermatol. 2002, 119, 507–512. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging i: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef]
- Cenizo, V.; Andre, V.; Reymermier, C.; Sommer, P.; Damour, O.; Perrier, E. Loxl as a target to increase the elastin content in adult skin: A dill extract induces the loxl gene expression. Exp. Dermatol. 2006, 15, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.R.; Gallo, R.L. Glycosaminoglycans and their proteoglycans: Host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006, 20, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, Y.K.; Jung, J.Y.; Shin, J.E.; Chung, J.H. Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo. Exp. Dermatol. 2011, 20, 454–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Oh, J.H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- Anderegg, U.; Simon, J.C.; Averbeck, M. More than just a filler—The role of hyaluronan for skin homeostasis. Exp. Dermatol. 2014, 23, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Humbert, P.G.; Haftek, M.; Creidi, P.; Lapiere, C.; Nusgens, B.; Richard, A.; Schmitt, D.; Rougier, A.; Zahouani, H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. Placebo. Exp. Dermatol. 2003, 12, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, Y.K.; Jung, J.Y.; Shin, J.E.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Intrinsic aging- and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J. Dermatol. Sci. 2011, 62, 192–201. [Google Scholar] [CrossRef]
- Maytin, E.V. Hyaluronan: More than just a wrinkle filler. Glycobiology 2016, 26, 553–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobiishi, M.; Sayo, T.; Yoshida, H.; Kusaka, A.; Kawabata, K.; Sugiyama, Y.; Ishikawa, O.; Inoue, S. Changes in epidermal hyaluronan metabolism following uvb irradiation. J. Dermatol. Sci. 2011, 64, 31–38. [Google Scholar] [CrossRef]
- Tzellos, T.G.; Klagas, I.; Vahtsevanos, K.; Triaridis, S.; Printza, A.; Kyrgidis, A.; Karakiulakis, G.; Zouboulis, C.C.; Papakonstantinou, E. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp. Dermatol. 2009, 18, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Xia, W.; Lei, D.; Voorhees, J.J.; Fisher, G.J. Age-dependent alterations of decorin glycosaminoglycans in human skin. Sci. Rep. 2013, 3, 2422. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.F.; Fisher, L.W.; Li, K.; LeBaron, R.G.; Tan, E.M.; Uitto, J. Differential expression of the versican and decorin genes in photoaged and sun-protected skin. Comparison by immunohistochemical and northern analyses. Lab. Investig. 1995, 72, 662–669. [Google Scholar]
- Kang, S. The mechanism of action of topical retinoids. Cutis 2005, 75, 10–13; discussion 13. [Google Scholar]
- Woodley, D.T.; Zelickson, A.S.; Briggaman, R.A.; Hamilton, T.A.; Weiss, J.S.; Ellis, C.N.; Voorhees, J.J. Treatment of photoaged skin with topical tretinoin increases epidermal-dermal anchoring fibrils. A preliminary report. JAMA 1990, 263, 3057–3059. [Google Scholar] [CrossRef]
- Kim, H.J.; Bogdan, N.J.; D’Agostaro, L.J.; Gold, L.I.; Bryce, G.F. Effect of topical retinoic acids on the levels of collagen mrna during the repair of uvb-induced dermal damage in the hairless mouse and the possible role of tgf-beta as a mediator. J. Investig. Dermatol. 1992, 98, 359–363. [Google Scholar] [CrossRef]
- Berardesca, E.; Gabba, P.; Farinelli, N.; Borroni, G.; Rabbiosi, G. In vivo tretinoin-induced changes in skin mechanical properties. Br. J. Dermatol. 1990, 122, 525–529. [Google Scholar] [CrossRef]
- Hubbard, B.A.; Unger, J.G.; Rohrich, R.J. Reversal of skin aging with topical retinoids. Plast. Reconstr. Surg. 2014, 133, 481e–490e. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, G.D.; Nigra, T.P.; Pochi, P.E.; Savin, R.C.; Allan, A.; Benik, K.; Jeffes, E.; Lufrano, L.; Thorne, E.G. Topical tretinoin for treatment of photodamaged skin. A multicenter study. Arch. Dermatol. 1991, 127, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Myllyla, R.; Majamaa, K.; Gunzler, V.; Hanauske-Abel, H.M.; Kivirikko, K.I. Ascorbate is consumed stoichiometrically in the uncoupled reactions catalyzed by prolyl 4-hydroxylase and lysyl hydroxylase. J. Biol. Chem. 1984, 259, 5403–5405. [Google Scholar] [PubMed]
- Yamamoto, I.; Muto, N.; Murakami, K.; Akiyama, J. Collagen synthesis in human skin fibroblasts is stimulated by a stable form of ascorbate, 2-o-alpha-d-glucopyranosyl-l-ascorbic acid. J. Nutr. 1992, 122, 871–877. [Google Scholar] [CrossRef]
- Ohshima, H.; Mizukoshi, K.; Oyobikawa, M.; Matsumoto, K.; Takiwaki, H.; Kanto, H.; Itoh, M. Effects of vitamin c on dark circles of the lower eyelids: Quantitative evaluation using image analysis and echogram. Skin Res. Technol. 2009, 15, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Colven, R.M.; Pinnell, S.R. Topical vitamin c in aging. Clin. Dermatol. 1996, 14, 227–234. [Google Scholar] [CrossRef]
- Ditre, C.M.; Griffin, T.D.; Murphy, G.F.; Sueki, H.; Telegan, B.; Johnson, W.C.; Yu, R.J.; Van Scott, E.J. Effects of alpha-hydroxy acids on photoaged skin: A pilot clinical, histologic, and ultrastructural study. J. Am. Acad. Dermatol. 1996, 34, 187–195. [Google Scholar] [CrossRef]
- Katayama, K.; Seyer, J.M.; Raghow, R.; Kang, A.H. Regulation of extracellular matrix production by chemically synthesized subfragments of type i collagen carboxy propeptide. Biochemistry 1991, 30, 7097–7104. [Google Scholar] [CrossRef] [PubMed]
- Langholz, O.; Rockel, D.; Mauch, C.; Kozlowska, E.; Bank, I.; Krieg, T.; Eckes, B. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by alpha 1 beta 1 and alpha 2 beta 1 integrins. J. Cell Biol. 1995, 131, 1903–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, L.R.; Fitzgerald, N.C.; Doughty, D.G.; Dawes, N.C.; Berge, C.A.; Bissett, D.L. Topical palmitoyl pentapeptide provides improvement in photoaged human facial skin. Int. J. Cosmet. Sci. 2005, 27, 155–160. [Google Scholar] [CrossRef]
- Husein El Hadmed, H.; Castillo, R.F. Cosmeceuticals: Peptides, proteins, and growth factors. J. Cosmet. Dermatol. 2016, 15, 514–519. [Google Scholar] [CrossRef]
- Orringer, J.S.; Sachs, D.L.; Shao, Y.; Hammerberg, C.; Cui, Y.; Voorhees, J.J.; Fisher, G.J. Direct quantitative comparison of molecular responses in photodamaged human skin to fractionated and fully ablative carbon dioxide laser resurfacing. Dermatol. Surg. 2012, 38, 1668–1677. [Google Scholar] [CrossRef]
- Kim, J.E.; Won, C.H.; Bak, H.; Kositratna, G.; Manstein, D.; Dotto, G.P.; Chang, S.E. Gene profiling analysis of the early effects of ablative fractional carbon dioxide laser treatment on human skin. Dermatol. Surg. 2013, 39, 1033–1043. [Google Scholar] [CrossRef]
- Tierney, E.P.; Hanke, C.W. Fractionated carbon dioxide laser treatment of photoaging: Prospective study in 45 patients and review of the literature. Dermatol. Surg. 2011, 37, 1279–1290. [Google Scholar] [CrossRef]
- Rhie, J.W.; Shim, J.S.; Choi, W.S. A pilot study of skin resurfacing using the 2,790-nm erbium:Ysgg laser system. Arch. Plast. Surg. 2015, 42, 52–58. [Google Scholar] [CrossRef]
- Wanner, M.; Tanzi, E.L.; Alster, T.S. Fractional photothermolysis: Treatment of facial and nonfacial cutaneous photodamage with a 1,550-nm erbium-doped fiber laser. Dermatol. Surg. 2007, 33, 23–28. [Google Scholar] [CrossRef]
- Zelickson, B.D.; Kist, D.; Bernstein, E.; Brown, D.B.; Ksenzenko, S.; Burns, J.; Kilmer, S.; Mehregan, D.; Pope, K. Histological and ultrastructural evaluation of the effects of a radiofrequency-based nonablative dermal remodeling device: A pilot study. Arch. Dermatol. 2004, 140, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Kist, D.; Burns, A.J.; Sanner, R.; Counters, J.; Zelickson, B. Ultrastructural evaluation of multiple pass low energy versus single pass high energy radio-frequency treatment. Lasers Surg. Med. 2006, 38, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Bogle, M.A.; Ubelhoer, N.; Weiss, R.A.; Mayoral, F.; Kaminer, M.S. Evaluation of the multiple pass, low fluence algorithm for radiofrequency tightening of the lower face. Lasers Surg. Med. 2007, 39, 210–217. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; El-Ammawi, T.S.; Medhat, W.; Moawad, O.; Brennan, D.; Mahoney, M.G.; Uitto, J. Radiofrequency facial rejuvenation: Evidence-based effect. J. Am. Acad. Dermatol. 2011, 64, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Bloom, B.S.; Emer, J.; Goldberg, D.J. Assessment of safety and efficacy of a bipolar fractionated radiofrequency device in the treatment of photodamaged skin. J. Cosmet. Laser Ther. 2012, 14, 208–211. [Google Scholar] [CrossRef]
- Taub, A.F.; Tucker, R.D.; Palange, A. Facial tightening with an advanced 4-mhz monopolar radiofrequency device. J. Drugs Dermatol. 2012, 11, 1288–1294. [Google Scholar]
- Ruiz-Esparza, J.; Gomez, J.B. Nonablative radiofrequency for active acne vulgaris: The use of deep dermal heat in the treatment of moderate to severe active acne vulgaris (thermotherapy): A report of 22 patients. Dermatol. Surg. 2003, 29, 333–339; discussion 339. [Google Scholar] [CrossRef]
- Suh, D.H.; Choi, J.H.; Lee, S.J.; Jeong, K.H.; Song, K.Y.; Shin, M.K. Comparative histometric analysis of the effects of high-intensity focused ultrasound and radiofrequency on skin. J. Cosmet. Laser Ther. 2015, 17, 230–236. [Google Scholar] [CrossRef]
- Oni, G.; Hoxworth, R.; Teotia, S.; Brown, S.; Kenkel, J.M. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet. Surg. J. 2014, 34, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Fabi, S.G.; Goldman, M.P. Retrospective evaluation of micro-focused ultrasound for lifting and tightening the face and neck. Dermatol. Surg. 2014, 40, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Courderot-Masuyer, C.; Robin, S.; Tauzin, H.; Humbert, P. Evaluation of lifting and antiwrinkle effects of calcium hydroxylapatite filler. In vitro quantification of contractile forces of human wrinkle and normal aged fibroblasts treated with calcium hydroxylapatite. J. Cosmet. Dermatol. 2016, 15, 260–268. [Google Scholar] [CrossRef]
- Kligman, L.H.; Duo, C.H.; Kligman, A.M. Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect. Tissue Res. 1984, 12, 139–150. [Google Scholar] [CrossRef]
- Darlenski, R.; Surber, C.; Fluhr, J.W. Topical retinoids in the management of photodamaged skin: From theory to evidence-based practical approach. Br. J. Dermatol. 2010, 163, 1157–1165. [Google Scholar] [CrossRef]
- Sorg, O.; Antille, C.; Kaya, G.; Saurat, J.H. Retinoids in cosmeceuticals. Dermatol. Ther. 2006, 19, 289–296. [Google Scholar] [CrossRef]
- Caputo, R.; Monti, M.; Motta, S.; Barbareschi, M.; Tosti, A.; Serri, R.; Rigoni, C. The treatment of visible signs of senescence: The italian experience. Br. J. Dermatol. 1990, 122 (Suppl. 35), 97–103. [Google Scholar] [CrossRef]
- Olsen, E.A.; Katz, H.I.; Levine, N.; Shupack, J.; Billys, M.M.; Prawer, S.; Gold, J.; Stiller, M.; Lufrano, L.; Thorne, E.G. Tretinoin emollient cream: A new therapy for photodamaged skin. J. Am. Acad. Dermatol. 1992, 26, 215–224. [Google Scholar] [CrossRef]
- McCook, J.P. Topical products for the aging face. Clin. Plast. Surg. 2016, 43, 597–604. [Google Scholar] [CrossRef]
- Kafi, R.; Kwak, H.S.; Schumacher, W.E.; Cho, S.; Hanft, V.N.; Hamilton, T.A.; King, A.L.; Neal, J.D.; Varani, J.; Fisher, G.J.; et al. Improvement of naturally aged skin with vitamin a (retinol). Arch. Dermatol. 2007, 143, 606–612. [Google Scholar] [CrossRef]
- Kang, S.; Duell, E.A.; Fisher, G.J.; Datta, S.C.; Wang, Z.Q.; Reddy, A.P.; Tavakkol, A.; Yi, J.Y.; Griffiths, C.E.; Elder, J.T.; et al. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J. Investig. Dermatol. 1995, 105, 549–556. [Google Scholar] [CrossRef]
- Alster, T.S.; West, T.B. Effect of topical vitamin c on postoperative carbon dioxide laser resurfacing erythema. Dermatol. Surg. 1998, 24, 331–334. [Google Scholar] [CrossRef]
- Van Scott, E.J.; Yu, R.J. Alpha hydroxy acids: Procedures for use in clinical practice. Cutis 1989, 43, 222–228. [Google Scholar] [CrossRef]
- Ratner, D.; Tse, Y.; Marchell, N.; Goldman, M.P.; Fitzpatrick, R.E.; Fader, D.J. Cutaneous laser resurfacing. J. Am. Acad. Dermatol. 1999, 41, 365–389; quiz 390-362. [Google Scholar] [CrossRef]
- Nanni, C.A.; Alster, T.S. Complications of carbon dioxide laser resurfacing. An evaluation of 500 patients. Dermatol. Surg. 1998, 24, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Manstein, D.; Herron, G.S.; Sink, R.K.; Tanner, H.; Anderson, R.R. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg. Med. 2004, 34, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Hantash, B.M.; Bedi, V.P.; Chan, K.F.; Zachary, C.B. Ex vivo histological characterization of a novel ablative fractional resurfacing device. Lasers Surg. Med. 2007, 39, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Britt, C.J.; Marcus, B. Energy-based facial rejuvenation: Advances in diagnosis and treatment. JAMA Facial Plast. Surg. 2017, 19, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Luebberding, S.; Alexiades-Armenakas, M.R. Fractional, nonablative q-switched 1,064-nm neodymium yag laser to rejuvenate photoaged skin: A pilot case series. J. Drugs Dermatol. 2012, 11, 1300–1304. [Google Scholar]
- Sukal, S.A.; Geronemus, R.G. Thermage: The nonablative radiofrequency for rejuvenation. Clin. Dermatol. 2008, 26, 602–607. [Google Scholar] [CrossRef]
- de Felipe, I.; Redondo, P. Animal model to explain fat atrophy using nonablative radiofrequency. Dermatol. Surg. 2007, 33, 141–145. [Google Scholar]
- Boisnic, S.; Divaris, M.; Branchet, M.C.; Nelson, A.A. Split-face histological and biochemical evaluation of tightening efficacy using temperature- and impedance-controlled continuous non-invasive radiofrequency energy. J. Cosmet. Laser Ther. 2017, 19, 128–132. [Google Scholar] [CrossRef]
- Lee, H.S.; Jang, W.S.; Cha, Y.J.; Choi, Y.H.; Tak, Y.; Hwang, E.; Kim, B.J.; Kim, M.N. Multiple pass ultrasound tightening of skin laxity of the lower face and neck. Dermatol. Surg. 2012, 38, 20–27. [Google Scholar] [CrossRef]
- Chiang, Y.Z.; Pierone, G.; Al-Niaimi, F. Dermal fillers: Pathophysiology, prevention and treatment of complications. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 405–413. [Google Scholar] [CrossRef]

| Photoaging | Components | Intrinsic Aging |
|---|---|---|
| Decreased and fragmented | Collagen | Decreased and fragmented |
| Abnormally accumulated (SE) | Elastic fiber | Decreased |
| Increased in SE region | Hyaluronic acid | Not changed |
| Increased | Total sulfated GAGs | Decreased |
| Increased in SE region | Versican | Not changed? |
| Not changed | Biglycan | Decreased |
| Decreased in SE region | Decorin | Not changed? |
| Modalities | Mechanisms of Action | Clinical Efficacies |
|---|---|---|
| Topicals | ||
| Retinoid acid (RA) | Acts through RARs and RXRs [78] Increases type I, III, and VII collagens [79] Decreases MMPs [78,80] Reorganizes elastic fiber [81] Normalizes GAG deposition [82] | Application of 0.05% RA for 6 months improved fine and coarse wrinkles, roughness, and skin laxity [83]. Application of 0.025% RA for 3 months improved rough and fine wrinkles, skin firmness, and roughness (Ho ET) |
| Ascorbic acid | Reduces ROS [84] Acts as a cofactor in the biosynthesis of procollagen and elastin [85] Induces collagen synthesis in human skin fibroblasts and increase dermal thickness [86,87] | Application of 5% ascorbic acid for 6 months led to a clinical improvement of the photodamaged skin [88]. |
| Glycolic acid | Stimulates the production of GAGs and collagen in the dermis [89] Improves histologic quality of elastic fibers [89] | Application of 25% glycolic acid for 6 months increased skin thickness [89]. |
| Peptides | Regulate fibroblasts and control the production of ECM [90,91]. | Application of Pal-KTTKS for 3 months reduced wrinkles [92]. Application of copper–GHK reduced the depth and length of wrinkles and made skin smoother [93]. |
| Energy-based devices | ||
| Fractional lasers (FL) | Heat the dermis and stimulate matrix remodeling by deeply penetrating columns of laser energy [94] Induce biosynthesis of type I and III protocollagens [94,95] | Two or three treatments with CO2 fractional laser improved skin texture, laxity, and overall cosmetic outcome [96]. Two treatments with 2790 nm Er:YSGG laser improved wrinkle and skin texture [97]. Three treatments of fractional 1550 nm erbium-doped fiber laser improved wrinkles [98]. |
| Ablative FL | ||
| Nonablative FL | ||
| Radiofrequency (RF) | Causes direct collagen contraction and immediate skin tightening [99,100] Reorganizes collagen bundles [101] Induces increase in types I and III collagens [102] Improves the quality of elastic fibers and solar elastosis [102] | Three treatments with fractional bipolar RF improved wrinkles and skin texture [103]. Six treatments with monopolar RF improved laxity, texture, and wrinkles [104]. |
| High-intensity focused ultrasound (HIFU) | Creates precision microwounds in the dermis [105] Induces the higher level of neocollagenesis and neoelastogenesis in the deep reticular dermis [106] | Single treatment with HIFU improved skin laxity of lower face and neck [107]. Single treatment with HIFU improved skin laxity of face and upper neck [108]. |
| Fillers | ||
| Restore the contractile properties and elongation of aged fibroblasts [48,109] Induce type I collagen synthesis [48] | Further investigation is needed. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. https://doi.org/10.3390/ijms20092126
Shin J-W, Kwon S-H, Choi J-Y, Na J-I, Huh C-H, Choi H-R, Park K-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. International Journal of Molecular Sciences. 2019; 20(9):2126. https://doi.org/10.3390/ijms20092126
Chicago/Turabian StyleShin, Jung-Won, Soon-Hyo Kwon, Ji-Young Choi, Jung-Im Na, Chang-Hun Huh, Hye-Ryung Choi, and Kyung-Chan Park. 2019. "Molecular Mechanisms of Dermal Aging and Antiaging Approaches" International Journal of Molecular Sciences 20, no. 9: 2126. https://doi.org/10.3390/ijms20092126
APA StyleShin, J. -W., Kwon, S. -H., Choi, J. -Y., Na, J. -I., Huh, C. -H., Choi, H. -R., & Park, K. -C. (2019). Molecular Mechanisms of Dermal Aging and Antiaging Approaches. International Journal of Molecular Sciences, 20(9), 2126. https://doi.org/10.3390/ijms20092126