Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review
Abstract
:1. Introduction
2. Pathogenesis of Type 2 Diabetes Mellitus
3. Polyphenol Classes and Their Structures
3.1. Flavonoids
3.2. Non-Flavonoids
3.3. Bioavailability of Polyphenols
3.4. The Proposed Mechanisms of Phenolic Action
3.4.1. Interaction with Cell Membrane and Receptors
3.4.2. Metal Chelating Antioxidant Properties
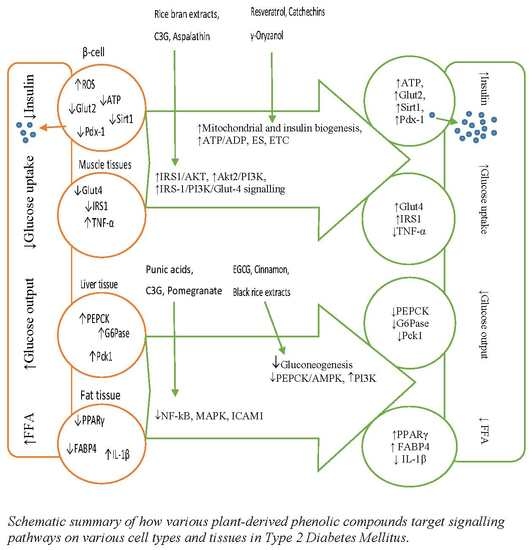
4. Effects of Polyphenols on Gene Modulations in T2DM
4.1. Polyphenols and Gene Modulations on β-Cell Dysfunction
4.2. Polyphenols and Gene Modulations on Insulin Signalling Pathways
4.3. Polyphenols and Gene Modulations on Gluconeogenesis Pathways
4.4. Effects of Polyphenols on Lipid Peroxidation Pathways
4.5. Effect of Polyphenols on Inflammatory Pathways
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 6PGD | 6-phosphogluconate dehydrogenase |
| ACC2 | Acetyl CoA carboxylase 2 |
| ACO | acyl-CoA oxidase |
| ADP | Adenosine diphosphate |
| Akt | Protein kinase B |
| AMPK | Adenine monophosphate activated protein kinase |
| ATP | Adenosine triphosphate |
| BIP | Binding immunoglobulin protein |
| C3G | Cyanidin 3-glucoside |
| CAD | Caspase-activated DNase |
| CAMFs | Methanolic fraction of C. anthelminticum seeds |
| CaMKK | Ca2+/calmodulin-dependent protein kinase kinase |
| cAMP | Cyclic adenosine 3′,5′-monophosphate |
| CAT | Catalase |
| Cdkn1a | Cyclin-dependent kinase inhibitor 1A |
| cDNA | Complementary deoxyribonucleic acid |
| CHOP | C/EBP-homologous protein |
| COX-2 | cyclooxygenase-2 |
| CPT-1 | carnitine palmitoyltransferase-1 |
| Dnajb9 | DnaJ homolog subfamily B member 9 |
| DPP-4 | Dipeptidyl-peptidase-4 |
| EGCG | Epigallocatechin gallate |
| eIF2α | Inositol-requiring kinase 2α |
| ENOS | Endothelial nitric oxide synthase |
| ER | Endoplasmic reticulum |
| ERdj4 | ER resident DNAJ 4 |
| FABP4 | fatty acid binding protein 4 |
| FADH | Flavin adenine dinucleotide |
| Fbp | Fructose-1,6-bisphosphatase |
| FBS | Fetal bovine serum |
| FFAs | Free fatty acids |
| FFP | Fermented food paste |
| FOXO1 | Forkhead box O1 |
| FRB | Fermented rice bran |
| G6Pase | Glucose-6-phosphatase |
| GABPA | GA binding protein transcription factor alpha subunit |
| Glut2 | Glucose transporter 2 |
| Glut4 | Glucose transporter 4 |
| Gpx | Glutathione Peroxidase |
| GRP78 | 78-kDa glucose-regulated protein |
| GSH | Glutathione |
| GSIS | Glucose stimulated insulin secretion |
| HDACs | histone deacetylases |
| Hmox1 | hemeoxygenase 1 |
| ICAM1 | intracellular adhesion molecules-1 |
| IGFR | Insulin-like growth factor receptor |
| IL-1β | Interleukin 1β |
| IL-6 | Interleukin 6 |
| iNOS | Inducible nitric oxide synthase |
| Ins1 | Insulin 1 |
| IR-β | Insulin receptor β |
| IRS2 | Insulin receptor substrates 2 |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| JNK | c-Jun N-kinase |
| LPH | Lactase phloridzin hydrolase |
| LXRα | Liver X receptor alpha |
| Mafa | MAF BZIP Transcription Factor A |
| MAPK | Mitogen-activated protein kinase |
| MEF2a | Myocyte enhance factor 2A |
| NADH | Nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-kB | Nuclear factor kappa light chain enhancer of activated B cells |
| Ngn3 | Neorog-3 |
| NO | Nitric oxide |
| Nqo1 | NAD(P)H quinone oxidoreductase 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| p22phox | Neutrophil cytochrome b 22 kDa polypeptide |
| P3G | Peonidin 3-glucoside |
| pAkt | Phosphorylated protein kinase A |
| PARP | Poly ADP-ribose polymerase |
| Pck | Phosphoenolpyruvate carboxykinase |
| Pck1 | Phosphoenolpyruvate carboxykinase 1 |
| Pde | Phosphodiesterase |
| Pdx-1 | Pancreatic andduodenal homeobox 1 |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PERK | Protein kinase-like endoplasmic reticulum kinase |
| PFK | Phosphofructokinase |
| PI3K | Phosphatidylinositol 3-kinase |
| pIRS-1 | Phosphorylated insulin receptor substrate 1 |
| PKA | Protein kinase A |
| PPAR | peroxisome proliferator-activated receptor |
| RNA | Ribonucleic acid |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RTK | Receptor tyrosine kinases |
| Sirt1 | Sirtuin 1 |
| Socs3 | Suppressor of cytokine signalling 3 |
| SOD2 | Superoxide dismutase 2 |
| SREBP-1c | sterol regulatory element-binding protein-1c |
| T2DM | Type 2 diabetes mellitus |
| TCA | Tricarboxylic acid |
| Tfam | Mitochondrial transcription factor A |
| TGFβ1 | Transforming growth factor-β1 |
| TNF-α | Tumour necrosis factor alpha |
| Tnfsf | Tumour necrosis factor receptor superfamily |
| TXNIP | Thioredoxin interacting protein |
| UPR | Unfolded protein response |
| Xbp1s | X box binding protein 1 |
References
- NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [Green Version]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Holman, N.; Young, B.; Gadsby, R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet. Med. 2015, 32, 1119–1120. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Migdal, C.; Serres, M. Reactive oxygen species and oxidative stress. Med. Sci. 2011, 27, 405–412. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, E.J.; LeRoith, D.; Karnieli, E. Insulin Resistance in Obesity as the Underlying Cause for the Metabolic Syndrome. Mt. Sinai J. Med. J. Transl. Pers. Med. 2010, 77, 511–523. [Google Scholar] [CrossRef]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Repub. Iran 2017, 31, 134. [Google Scholar] [CrossRef] [Green Version]
- Vetterli, L.; Brun, T.; Giovannoni, L.; Bosco, D.; Maechler, P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through a SIRT1-dependent mechanism. J. Biol. Chem. 2011, 286, 6049–6060. [Google Scholar] [CrossRef] [Green Version]
- Scazzocchio, B.; Vari, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Li Volti, G.; Galvano, F.; et al. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARgamma activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-L.; Lin, J.-K. Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human hepG2 hepatoma cells. Mol. Nutr. Food Res. 2008, 52, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Liu, X.; Yan, X.; Liu, D.; Yang, C.; Liu, B.; Huang, Y.; Zhao, C. Role of Green Macroalgae Enteromorpha Prolifera Polyphenols in the Modulation of Gene Expression and Intestinal Microflora Profiles in Type 2 Diabetic Mice. Int. J. Mol. Sci. 2018, 20, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, M.B.; Hu, F.B. Primary prevention of diabetes: What can be done and how much can be prevented? Annu. Rev. Public Health 2005, 26, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Erukainure, O.L.; Ijomone, O.M.; Oyebode, O.A.; Chukwuma, C.I.; Aschner, M.; Islam, M.S. Hyperglycemia-induced oxidative brain injury: Therapeutic effects of Cola nitida infusion against redox imbalance, cerebellar neuronal insults, and upregulated Nrf2 expression in type 2 diabetic rats. Food Chem. Toxicol. 2019, 127, 206–217. [Google Scholar] [CrossRef]
- Jiang, J.; Briedé, J.J.; Jennen, D.G.J.; Van Summeren, A.; Saritas-Brauers, K.; Schaart, G.; Kleinjans, J.C.S.; de Kok, T.M.C.M. Increased mitochondrial ROS formation by acetaminophen in human hepatic cells is associated with gene expression changes suggesting disruption of the mitochondrial electron transport chain. Toxicol. Lett. 2015, 234, 139–150. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Oxidative Stress in Pancreatic Beta Cell Regeneration. Oxidative Med. Cell. Longev. 2017, 2017, 1930261. [Google Scholar] [CrossRef] [Green Version]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Boden, G.; Chen, X.; Stein, T.P. Gluconeogenesis in moderately and severely hyperglycemic patients with type 2 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E23–E30. [Google Scholar] [CrossRef]
- Defronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Santhakumar, A.B.; Bulmer, A.C.; Singh, I. A review of the mechanisms and effectiveness of dietary polyphenols in reducing oxidative stress and thrombotic risk. J. Hum. Nutr. Diet. 2014, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radical Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Frydman, A.; Liberman, R.; Huhman, D.V.; Carmeli-Weissberg, M.; Sapir-Mir, M.; Ophir, R.W.; Sumner, L.; Eyal, Y. The molecular and enzymatic basis of bitter/non-bitter flavor of citrus fruit: Evolution of branch-forming rhamnosyltransferases under domestication. Plant J. 2013, 73, 166–178. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2018, 59, 2040–2051. [Google Scholar] [CrossRef]
- Smith, C.; Lombard, K.A.; Peffley, E.B.; Liu, W. Genetic analysis of quercetin in onion (Allium cepa L.) ‘Lady Raider’. Texas J. Agri. Nat. Resour. 2016, 16, 24–28. [Google Scholar]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Szeja, W.; Grynkiewicz, G.; Rusin, A. Isoflavones, their glycosides and glycoconjugates. Synthesis and biological activity. Curr. Org. Chem. 2017, 21, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Borges, G.; Ryan, D. Phenolic and polyphenolic constituents and the beneficial effects of moderate red wine consumption: The glass that cheers. Biochemist 2010, 32, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, H.; Xiao, J. Polyphenols: Absorption, Bioavailability, and Metabolomics; Woodhead Publishing: Cambridge, UK, 2018; pp. 45–67. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Danielsen, E.T.; Danielsen, E.M. Glycol chitosan: A stabilizer of lipid rafts in the intestinal brush border. Biochim. Biophys. Acta Biomembr. 2017, 1859, 360–367. [Google Scholar] [CrossRef]
- Bowey, E.; Adlercreutz, H.; Rowland, I. Metabolism of isoflavones and lignans by the gut microflora: A study in germ-free and human flora associated rats. Food Chem. Toxicol. 2003, 41, 631–636. [Google Scholar] [CrossRef]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.-K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and Bioavailability of Quercetin Glycosides in Humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef]
- Lee, M.J.; Wang, Z.Y.; Li, H.; Chen, L.; Sun, Y.; Gobbo, S.; Balentine, D.A.; Yang, C.S. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol. Biomark. 1995, 4, 393–399. [Google Scholar]
- Wang, J.F.; Schramm, D.D.; Holt, R.R.; Ensunsa, J.L.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J. Nutr. 2000, 130, 2115S–2119S. [Google Scholar] [CrossRef]
- Donovan, J.L.; Bell, J.R.; Kasim-Karakas, S.; German, J.B.; Walzem, R.L.; Hansen, R.J.; Waterhouse, A.L. Catechin is present as metabolites in human plasma after consumption of red wine. J. Nutr. 1999, 129, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Remesy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Muccitelli, H.U.; Sanchez-Moreno, C.; Prior, R.L. Anthocyanins are absorbed in glycated forms in elderly women: A pharmacokinetic study. Am. J. Clin. Nutr. 2001, 73, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Nesbitt, P.D.; Lam, Y.; Thompson, L.U. Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am. J. Clin. Nutr. 1999, 69, 549–555. [Google Scholar] [CrossRef]
- Watanabe, S.; Yamaguchi, M.; Sobue, T.; Takahashi, T.; Miura, T.; Arai, Y.; Mazur, W.; Wahala, K.; Adlercreutz, H. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako). J. Nutr. 1998, 128, 1710–1715. [Google Scholar] [CrossRef] [Green Version]
- Holt, R.R.; Lazarus, S.A.; Sullards, M.C.; Zhu, Q.Y.; Schramm, D.D.; Hammerstone, J.F.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. Procyanidin dimer B2 [epicatechin-(4β-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr. 2002, 76, 798–804. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019, 10, 6999–7021. [Google Scholar] [CrossRef]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. 2002, 11, 1025–1032. [Google Scholar]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlejman, A.G.; Fraga, C.G.; Oteiza, P.I. Procyanidins protect Caco-2 cells from bile acid-and oxidant-induced damage. Free Radic. Biol. Med. 2006, 41, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G.; Oteiza, P.I. Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radic. Biol. Med. 2003, 34, 84–92. [Google Scholar] [CrossRef]
- Shimizu, M.; Deguchi, A.; Hara, Y.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 334, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Waltner-Law, M.E.; Wang, X.L.; Law, B.K.; Hall, R.K.; Nawano, M.; Granner, D.K. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J. Biol. Chem. 2002, 277, 34933–34940. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar]
- Colin, D.; Limagne, E.; Jeanningros, S.; Jacquel, A.; Lizard, G.; Athias, A.; Gambert, P.; Hichami, A.; Latruffe, N.; Solary, E.; et al. Endocytosis of Resveratrol via Lipid Rafts and Activation of Downstream Signaling Pathways in Cancer Cells. Cancer Prev. Res. 2011, 4, 1095–1106. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Mu, B.; Song, Z.; Ma, Z.; Mu, T. The In Vitro Antioxidant Activity and Inhibition of Intracellular Reactive Oxygen Species of Sweet Potato Leaf Polyphenols. Oxid. Med. Cell. Longev. 2018, 2018, 9017828. [Google Scholar] [CrossRef] [Green Version]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Abdilla, N.; Tormo, M.; Fabia, M.; Chaves, F.; Saez, G.; Redon, J. Impact of the components of metabolic syndrome on oxidative stress and enzymatic antioxidant activity in essential hypertension. J. Hum. Hypertens. 2007, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Brun, T.; Maechler, P. Beta-cell mitochondrial carriers and the diabetogenic stress response. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2540–2549. [Google Scholar] [CrossRef] [PubMed]
- Saraste, M. Oxidative Phosphorylation at the fin de siècle. Science 1999, 283, 1488. [Google Scholar] [CrossRef] [PubMed]
- Maechler, P.; Carobbio, S.; Rubi, B. In beta-cells, mitochondria integrate and generate metabolic signals controlling insulin secretion. Int. J. Biochem. Cell Biol. 2006, 38, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury. J. Diabetes Res. 2017, 2017, 8379327. [Google Scholar] [CrossRef]
- Yang, S.N.; Berggren, P.O. Beta-cell CaV channel regulation in physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E16–E28. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; Dludla, P.; Joubert, E.; February, F.; Mazibuko, S.; Ghoor, S.; Muller, C.; Louw, J. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol. Nutr. Food Res. 2016, 60, 922–934. [Google Scholar] [CrossRef]
- Ortega, Á.; Berná, G.; Rojas, A.; Martín, F.; Soria, B. Gene-Diet Interactions in Type 2 Diabetes: The Chicken and Egg Debate. Int. J. Mol. Sci. 2017, 18, 1188. [Google Scholar] [CrossRef]
- Rouse, M.; Younes, A.; Egan, J.M. Resveratrol and curcumin enhance pancreatic beta-cell function by inhibiting phosphodiesterase activity. J. Endocrinol. 2014, 223, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Millán, E.; Cordero-Herrera, I.; Ramos, S.; Escrivá, F.; Alvarez, C.; Goya, L.; Martín, M.A. Cocoa-rich diet attenuates beta cell mass loss and function in young Zucker diabetic fatty rats by preventing oxidative stress and beta cell apoptosis. Mol. Nutr. Food Res. 2015, 59, 820–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowley, T.J.; Bitner, B.F.; Ray, J.D.; Lathen, D.R.; Smithson, A.T.; Dallon, B.W.; Plowman, C.J.; Bikman, B.T.; Hansen, J.M.; Dorenkott, M.R.; et al. Monomeric cocoa catechins enhance β-cell function by increasing mitochondrial respiration. J. Nuatr. Biochem. 2017, 49, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Im, S.W.; Jung, C.H.; Jang, Y.J.; Ha, T.Y.; Ahn, J. Tyrosol, an olive oil polyphenol, inhibits ER stress-induced apoptosis in pancreatic β-cell through JNK signaling. Biochem. Biophys. Res. Commun. 2016, 469, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, S.; Hentati, O.; Hamdaoui, G.; Fakhreddine, K.; Maillard, E.; Dal, S.; Sigrist, S. Beneficial Effect of Jojoba Seed Extracts on Hyperglycemia-Induced Oxidative Stress in RINm5f Beta Cells. Nutrients 2018, 10, 384. [Google Scholar] [CrossRef] [Green Version]
- Kozuka, C.; Sunagawa, S.; Ueda, R.; Higa, M.; Tanaka, H.; Shimizu-Okabe, C.; Ishiuchi, S.; Takayama, C.; Matsushita, M.; Tsutsui, M.; et al. gamma-Oryzanol protects pancreatic beta-cells against endoplasmic reticulum stress in male mice. Endocrinology 2015, 156, 1242–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, E.P.; Lin, J.-K. Epigallocatechin Gallate (EGCG) and Rutin Suppress the Glucotoxicity through Activating IRS2 and AMPK Signaling in Rat Pancreatic β Cells. J. Agric. Food Chem. 2009, 57, 9817. [Google Scholar] [CrossRef]
- Arya, A.; Yeng Looi, C.; Chuen Cheah, S.; Rais Mustafa, M.; Ali Mohd, M. Anti-diabetic effects of Centratherum anthelminticum seeds methanolic fraction on pancreatic cells, β-TC6 and its alleviating role in type 2 diabetic rats. J. Ethnopharmacol. 2012, 144, 22–32. [Google Scholar] [CrossRef] [PubMed]
- De la Puerta, R.O.; Domínguez, M.E.M.N.; Ruíz-Gutíerrez, V.; Flavill, J.A.; Hoult, J.R.S. Effects of virgin olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci. 2001, 69, 1213–1222. [Google Scholar] [CrossRef]
- Kara, Y. Phenolic Contents and Antioxidant Activity of Jojoba (Simmondsia chinensis (Link). Schindler. Int. J. Sec. Metab. 2017, 4, 142–147. [Google Scholar] [CrossRef]
- Brandes, R.P.; Kreuzer, J. Vascular NADPH oxidases: Molecular mechanisms of activation. Cardiovasc. Res. 2005, 65, 16–27. [Google Scholar] [CrossRef]
- Wu, T.Y.; Khor, T.O.; Saw, C.L.; Loh, S.C.; Chen, A.I.; Lim, S.S.; Park, J.H.; Cai, L.; Kong, A.N. Anti-inflammatory/Anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 2011, 13, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerma-García, M.; Herrero-Martínez, J.; Simó-Alfonso, E.; Mendonça, C.R.; Ramis-Ramos, G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009, 115, 389–404. [Google Scholar] [CrossRef]
- Farese, R.V.; Sajan, M.P.; Standaert, M.L. Insulin-sensitive protein kinases (atypical protein kinase C and protein kinase B/Akt): Actions and defects in obesity and type II diabetes. Exp. Biol. Med. 2005, 230, 593–605. [Google Scholar] [CrossRef]
- Choi, K.; Kim, Y.-B. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J. Int. Med. 2010, 25, 119–129. [Google Scholar] [CrossRef]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I., Jr.; Newsholme, P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and beta-Cell Dysfunction. Oxid. Med. Cell. Longev. 2015, 2015, 181643. [Google Scholar] [CrossRef] [Green Version]
- Khodabandehloo, H.; Gorgani-Firuzjaee, S.; Panahi, G.; Meshkani, R. Molecular and cellular mechanisms linking inflammation to insulin resistance and beta-cell dysfunction. Transl. Res. 2016, 167, 228–256. [Google Scholar] [CrossRef]
- Saxena, R.; Elbers, C.C.; Guo, Y.; Peter, I.; Gaunt, T.R.; Mega, J.L.; Lanktree, M.B.; Tare, A.; Castillo, B.A.; Li, Y.R.; et al. Large-Scale Gene-Centric Meta-Analysis across 39 Studies Identifies Type 2 Diabetes Loci. Am. J. Hum. Genet. 2012, 90, 410–425. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, P.; Khanna, D.; Balakumar, P. Catechin averts experimental diabetes mellitus-induced vascular endothelial structural and functional abnormalities. Cardiovasc. Toxicol. 2014, 14, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. Aqueous-Methanol Extracts of Orange-Fleshed Sweet Potato (Ipomoeabatatas) Ameliorate Oxidative Stress and Modulate Type 2 Diabetes Associated Genes in Insulin Resistant C2C12 Cells. Molecules 2018, 23, 2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Kampmann, U.; Christensen, B.; Nielsen, T.S.; Pedersen, S.B.; Orskov, L.; Lund, S.; Moller, N.; Jessen, N. GLUT4 and UBC9 protein expression is reduced in muscle from type 2 diabetic patients with severe insulin resistance. PLoS ONE 2011, 6, e27854. [Google Scholar] [CrossRef]
- Anderson, C.M.; Hu, J.; Barnes, R.M.; Heidt, A.B.; Cornelissen, I.; Black, B.L. Myocyte enhancer factor 2C function in skeletal muscle is required for normal growth and glucose metabolism in mice. Skelet. Muscle 2015, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Schenk, S.; McCurdy, C.E.; Philp, A.; Chen, M.Z.; Holliday, M.J.; Bandyopadhyay, G.K.; Osborn, O.; Baar, K.; Olefsky, J.M. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J. Clin. Investig. 2011, 121, 4281–4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [Green Version]
- Modi, S.; Yaluri, N.; Kokkola, T.; Laakso, M. Plant-derived compounds strigolactone GR24 and pinosylvin activate SIRT1 and enhance glucose uptake in rat skeletal muscle cells. Sci. Rep. 2017, 7, 17606. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.N.; Wang, C.J.; Yang, Y.S.; Lin, C.L.; Peng, C.H. Hibiscus sabdariffa polyphenols prevent palmitate-induced renal epithelial mesenchymal transition by alleviating dipeptidyl peptidase-4-mediated insulin resistance. Food Funct. 2016, 7, 475–482. [Google Scholar] [CrossRef]
- Kim, D.; Han, G.D. Ameliorating Effects of Fermented Rice Bran Extract on Oxidative Stress Induced by High Glucose and Hydrogen Peroxide in 3T3-L1 Adipocytes. Plant Foods Hum. Nutr. 2011, 66, 285. [Google Scholar] [CrossRef]
- Boue, S.M.; Daigle, K.W.; Chen, M.-H.; Cao, H.; Heiman, M.L. Antidiabetic Potential of Purple and Red Rice (Oryza sativa L.) Bran Extracts. J. Agric. Food Chem. 2016, 64, 5345–5353. [Google Scholar] [CrossRef]
- Johnson, R.; Dludla, P.V.; Muller, C.J.; Huisamen, B.; Essop, M.F.; Louw, J. The Transcription Profile Unveils the Cardioprotective Effect of Aspalathin against Lipid Toxicity in an In Vitro H9c2 Model. Molecules 2017, 22, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooi, J.; Adamu, H.A.; Imam, M.U.; Ithnin, H.; Ismail, M. Polyphenol-rich ethyl acetate fraction isolated from Molineria latifolia ameliorates insulin resistance in experimental diabetic rats via IRS1/AKT activation. Biomed. Pharmacother. 2018, 98, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Sun, W.; Fan, Y.; Guo, X.; Xu, G.; Xu, T.; Hou, Y.; Zhao, B.; Feng, X.; Liu, T. Effect of mulberry leaf (Folium Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling pathway in type 2 diabetes mellitus rats. Pharm. Biol. 2016, 54, 2685–2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucci, S.A.; Boyland, E.J.; Halford, J.C. The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: A review of current and emerging therapeutic agents. Diabetes Metab. Syndr. Obes. Targets Ther. 2010, 3, 125. [Google Scholar] [CrossRef] [Green Version]
- Seymour, E.M.; Lewis, S.K.; Urcuyo-Llanes, D.E.; Tanone, I.I.; Kirakosyan, A.; Kaufman, P.B.; Bolling, S.F. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J. Med. Food 2009, 12, 935–942. [Google Scholar] [CrossRef]
- Iwaki, M.; Matsuda, M.; Maeda, N.; Funahashi, T.; Matsuzawa, Y.; Makishima, M.; Shimomura, I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 2003, 52, 1655–1663. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Bao, J. Polyphenols in whole rice grain: Genetic diversity and health benefits. Food Chem. 2015, 180, 86–97. [Google Scholar] [CrossRef]
- Hiemori, M.; Koh, E.; Mitchell, A.E. Influence of Cooking on Anthocyanins in Black Rice (Oryza sativa L. japonica var. SBR). J. Agric. Food Chem. 2009, 57, 1908–1914. [Google Scholar] [CrossRef]
- Friedmsan, M. Rice brans, rice bran oils, and rice hulls: Composition, food and industrial uses, and bioactivities in humans, animals, and cells. J. Agric. Food Chem. 2013, 61, 10626–10641. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Rico, C.W.; Lee, S.C.; Kang, M.Y. Modulatory effect of rice bran and phytic acid on glucose metabolism in high fat-fed C57BL/6N mice. J. Clin. Biochem. Nutr. 2010, 47, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, E.H.; Sung, R.K.; Hwang, I.K.; Tae, Y.H. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J. Agric. Food Chem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; Blay, M.; Blade, M.; Salvado, M.; Arola, L.; Ardevol, A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology 2004, 145, 4985–4990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhabasa-Lhoret, R.; Chiasson, J. International Textbook of Diabetes Mellitus; Wiley-Blackwell: Hoboken, NJ, USA, 2004; Volume 3, pp. 901–914. [Google Scholar]
- Muller, C.J.; Joubert, E.; de Beer, D.; Sanderson, M.; Malherbe, C.J.; Fey, S.J.; Louw, J. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomed. Int. J. Phytother. Phytopharmacol. 2012, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ishak, N.A.; Ismail, M.; Hamid, M.; Ahmad, Z.; Abd Ghafar, S.A. Antidiabetic and hypolipidemic activities of curculigo latifolia fruit: Root extract in high fat fed diet and low dose STZ induced diabetic rats. J. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rask-Madsen, C.; Kahn, C.R. Tissue–specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2052–2059. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.D.; Islam, M.S. Effects of white mulberry (Morus alba) leaf tea investigated in a type 2 diabetes model of rats. Acta Pol. Pharm. 2015, 72, 153–160. [Google Scholar]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Kikuchi, A.; Takamura, T. Where does liver fat go? A possible molecular link between fatty liver and diabetes. J. Diabetes Investig. 2017, 8, 152–154. [Google Scholar] [CrossRef] [Green Version]
- Snel, M.; Jonker, J.T.; Schoones, J.; Lamb, H.; De Roos, A.; Pijl, H.; Jazet, I.M. Ectopic Fat and Insulin Resistance: Pathophysiology and Effect of Diet and Lifestyle Interventions. Int. J. Endocrinol. 2012, 2012, 18. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Gill, J.M.R. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014, 12, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, P.A.; Yokoyama, W. Cinnamon intake lowers fasting blood glucose: Meta-analysis. J. Med. Food 2011, 14, 884–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.M.; Kuhn, P.; Poulev, A.; Rojo, L.E.; Lila, M.A.; Raskin, I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012, 135, 2994–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J.-Y.; Hu, L.-H.; Li, J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Hsu, C.-Y.; Chen, C.-Y.; Liu, H.-K. Fructus Corni suppresses hepatic gluconeogenesis related gene transcription, enhances glucose responsiveness of pancreatic beta-cells, and prevents toxin induced beta-cell death. J. Ethnopharmacol. 2008, 117, 483–490. [Google Scholar] [CrossRef]
- Collins, Q.F.; Liu, H.-Y.; Pi, J.; Liu, Z.; Quon, M.J.; Cao, W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J. Biol. Chem. 2007, 282, 30143–30149. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-T.; Ha, J.; Park, I.-J.; Lee, S.-K.; Baik, H.W.; Kim, Y.M.; Park, O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef]
- Robinson, K.A.; Stewart, C.A.; Pye, Q.N.; Nguyen, X.; Kenney, L.; Salzman, S.; Floyd, R.A.; Hensley, K. Redox-sensitive protein phosphatase activity regulates the phosphorylation state of p38 protein kinase in primary astrocyte culture. J. Neurosci. Res. 1999, 55, 724–732. [Google Scholar] [CrossRef]
- Adamu, H.A.; Imam, M.U.; Ooi, D.-J.; Esa, N.M.; Rosli, R.; Ismail, M. In utero exposure to germinated brown rice and its oryzanol-rich extract attenuated high fat diet-induced insulin resistance in F1 generation of rats. BMC Complement. Altern. Med. 2017, 17, 67. [Google Scholar] [CrossRef] [Green Version]
- Imam, M.U.; Azmi, N.H.; Bhanger, M.I.; Ismail, N.; Ismail, M. Antidiabetic Properties of Germinated Brown Rice: A Systematic Review. J. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.U.; Ismail, M. Nutrigenomic effects of germinated brown rice and its bioactives on hepatic gluconeogenic genes in type 2 diabetic rats and HEPG2 cells. Mol. Nutr. Food Res. 2013, 57, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Kim, H.J.; Lee, J.S.; Lee, M.K.; Kim, H.O.; Park, E.J.; Kim, H.K.; Jeong, T.S.; Choi, M.S. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin. Nutr. 2003, 22, 561–568. [Google Scholar] [CrossRef]
- Jung, U.J.; Lee, M.K.; Park, Y.B.; Kang, M.A.; Choi, M.S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int. J. Biochem. Cell Biol. 2006, 38, 1134–1145. [Google Scholar] [CrossRef]
- Do, G.-M.; Jung, U.J.; Park, H.-J.; Kwon, E.-Y.; Jeon, S.-M.; McGregor, R.A.; Choi, M.-S. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol. Nutr. Food Res. 2012, 56, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Zulkawi, N.; Ng, K.H.; Zamberi, R.; Yeap, S.K.; Jaganath, I.B.; Satharasinghe, D.; Yong, C.Y.; Jamaluddin, A.B.; Tan, S.W.; Ho, W.Y. The in vivo hepato-recovery effects of the polyphenol-rich fermented food Xeniji™ on ethanol-induced liver damage. RSC Adv. 2017, 7, 38287–38299. [Google Scholar] [CrossRef] [Green Version]
- Holt, M.P.; Cheng, L.; Ju, C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J. Leukoc. Biol. 2008, 84, 1410–1421. [Google Scholar] [CrossRef]
- Teresa Vanessa, F.; Annamaria, P.; Pengou, Z.; Franco, F. Hyperglycemia-induced Oxidative Stress and its Role in Diabetes Mellitus Related Cardiovascular Diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef]
- Les, F.; Carpéné, C.; Arbonés-Mainar, J.M.; Decaunes, P.; Valero, M.S.; López, V. Pomegranate juice and its main polyphenols exhibit direct effects on amine oxidases from human adipose tissue and inhibit lipid metabolism in adipocytes. J. Funct. Foods 2017, 33, 323–331. [Google Scholar] [CrossRef]
- Banihani, S.; Swedan, S.; Alguraan, Z. Pomegranate and type 2 diabetes. Nutr. Res. 2013, 33, 341–348. [Google Scholar] [CrossRef]
- Ekhlasi, G.; Shidfar, F.; Agah, S.; Merat, S.; Hosseini, A.F. Effects of Pomegranate and Orange Juice on Antioxidant Status in Non-Alcoholic Fatty Liver Disease Patients: A Randomized Clinical Trial. Int. J. Vitam. Nutr. Res. 2015, 85, 292–298. [Google Scholar] [CrossRef]
- Xu, K.Z.-Y.; Zhu, C.; Kim, M.S.; Yamahara, J.; Li, Y. Pomegranate flower ameliorates fatty liver in an animal model of type 2 diabetes and obesity. J. Ethnopharmacol. 2009, 123, 280–287. [Google Scholar] [CrossRef]
- Hontecillas, R.; O’Shea, M.; Einerhand, A.; Diguardo, M.; Bassaganya-Riera, J. Activation of PPAR γ and α by Punicic Acid Ameliorates Glucose Tolerance and Suppresses Obesity-Related Inflammation. J. Am. Coll. Nutr. 2009, 28, 184–195. [Google Scholar] [CrossRef]
- Furuhashi, M.; Saitoh, S.; Shimamoto, K.; Miura, T. Fatty Acid-Binding Protein 4 (FABP4): Pathophysiological Insights and Potent Clinical Biomarker of Metabolic and Cardiovascular Diseases. Clin. Med. Insights Cardiol. 2015, 8, 23–33. [Google Scholar] [CrossRef]
- Anusree, S.S.; Priyanka, A.; Nisha, V.M.; Das, A.A.; Raghu, K.G. An in vitro study reveals the nutraceutical potential of punicic acid relevant to diabetes via enhanced GLUT4 expression and adiponectin secretion. Food Funct. 2014, 5, 2590–2601. [Google Scholar] [CrossRef]
- Les, F.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Pomegranate polyphenols and urolithin A inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J. Ethnopharmacol. 2018, 220, 67–74. [Google Scholar] [CrossRef]
- Woo, M.-S.; Choi, H.-S.; Seo, M.-J.; Jeon, H.-J.; Lee, B.-Y. Ellagic Acid Suppresses Lipid Accumulation by Suppressing Early Adipogenic Events and Cell Cycle Arrest. Phytother. Res. 2015, 29, 398–406. [Google Scholar] [CrossRef]
- Laskin, D.L.; Sunil, V.R.; Gardner, C.R.; Laskin, J.D. Macrophages and tissue injury: Agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 2011, 51, 267–288. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Shi, Y.; Ren, Y.; Wu, H.; Yao, F.; Wei, J.; Wu, M.; Hou, Y.; Duan, H. Anthocyanins inhibit high-glucose-induced cholesterol accumulation and inflammation by activating LXRalpha pathway in HK-2 cells. Drug Des. Devel. Ther. 2015, 9, 5099–5113. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Wu, H.; Zhang, H.; Li, F.; Chen, S.; Hou, B.; Shi, Y.; Zhao, L.; Duan, H. Anthocyanins inhibit high glucose-induced renal tubular cell apoptosis caused by oxidative stress in db/db mice. Int. J. Mol. Med. 2018, 41, 1608–1618. [Google Scholar] [CrossRef]
- Miranda-Diaz, A.G.; Pazarin-Villasenor, L.; Yanowsky-Escatell, F.G.; Andrade-Sierra, J. Oxidative Stress in Diabetic Nephropathy with Early Chronic Kidney Disease. J. Diabetes Res. 2016, 2016, 7047238. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.-M.; Jialal, I.; Devaraj, S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 2011, 22, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Cordero-Herrera, I.; Chen, X.; Ramos, S.; Devaraj, S. (-)-Epicatechin attenuates high-glucose-induced inflammation by epigenetic modulation in human monocytes. Eur. J. Nutr. 2017, 56, 1369–1373. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 2007, 4, 8. [Google Scholar] [CrossRef] [Green Version]


| Polyphenols | Plasma Conc. (Cmax) | Half-Life (T1/2) | Quantities | Food Source | Ref. |
|---|---|---|---|---|---|
| Quercetin | 0.3–0.75 µmol/L | 0.6 h | 80–100 mg | Onion | [39] |
| EGCG and EC | 0.1–0.7 μmol/L | 1 h | 90–150 mg | Green tea | [40] |
| Epichatechin | 0.25–0.7 μmol/L | 2 h | 70–165 mg | Cocoa | [41] |
| Catechin | 0.09 μmol/L | 1 h | 35 mg | Red wine | [42] |
| Hesperetin | 1.3–2.2 μmol/L | 5–7 h | 130–220 mg | Orange | [43] |
| Naringenin | 6 μmol/L | 5–7 h | 200 mg | Grapefruit | [44] |
| Anthocyanins | 97.4 nmol/L | 1.11 h | 110–200 mg | Elderberry extracts | [45] |
| Lignan | 30 nmol/L | 9–24 h | 25 mg | Linseed | [46] |
| Isoflavones | 1.4–4 μmol/L | 6–8 h | 50 mg | Soy | [47] |
| Polyphenols/Conc. | Genes Affected | Function | Pathways | Cells/Tissue Type | Ref. |
|---|---|---|---|---|---|
| In vitro models | |||||
| Resveratrol (25 µm) | ↑ SIRT1, ↑ Glut2, ↑ GK, ↑ Pdx-1, ↑ Hnf-1α, ↑ Tfam | ↑ Insulin biogenesis | Mitochondrial | Cells-INS-1E | [9] |
| Resveratrol (0.1 µM) and curcumin (1 PMOL/l) | ↑ cAMP ↓ PDE ↓ Pde3b, ↓ Pde8a, ↓ Pde10a | ↑ insulin secretion | cAMP, Insulin secretion | Cells-β-Min6, HP62 | [72] |
| Cocoa catchechins (25 µg/mL) | ↑ Hmox1, ↑ Nqo1, ↑ Nrf1, ↑ GABPA, | ↑ Mitochondrial electron complexes | Electron transport chain | Cells-INS-1 832/13 | [76] |
| Tyrosol (25,50 µM/mL) | ↓ GRP78, ↓ PERK, ↓ eIFα, ↓ CHOP↓ XBP-1, ↓ p-JNK | ↓ Apoptosis, ↑ β-cells survival | JNK | Cells-NIT-1, | [77] |
| Jojoba seed extracts (150 µg/mL) | ↑ Nfr2, ↓ p22phox, ↓ Casp-3, ↑ SOD & CAT | ↓ ROS/OS | Mitochondrial | Cells-RINm5f | [78] |
| γ-Oryzanol (0.2 or 2.0 µg/mL) | ↓ Dnajb9, ↓ Xbp1s, ↓ Chop, ↓ Casp3, ↓ CAD | ↑ β-cell function, ↓ ER stress | ER Stress | Cells-MIN6 | [79] |
| Epigallocatechin gallate (1–10 µM) | ↑ Pdx-1, ↑ FOXO1 ↑ pAkt | ↑ Β-cell function, ↑ insulin secretion | pAkt/Pdx-1 | Cells-RIN-m5F | [80] |
| Centratherum anthelminticum seeds (6.25–50 µg/mL) | ↑ Glut2, | ↑ β-cell function | Insulin secretion | Cells-β-TC6, | [81] |
| In vivo model | |||||
| γ-Oryzanol (320 µg/g BW) | ↓ Dnajb9, ↓ Xbp1s, ↓ Chop, ↓ Casp3, ↓ CAD | ↑ β-cell function, ↓ ER stress | ER Stress | Pancreaticisliets-C57BL/6J mice, | [79] |
| Polyphenols/Conc. | Gene Affected | Function | Pathways | Cells/Tissue Type | Ref. |
|---|---|---|---|---|---|
| In vitro models | |||||
| OSPT (500 µg/mL) and OSPL (100 µg/mL) | ↑ Glut4, ↑ Nrf1, ↑ Mef2a, ↓ Acc2. | ↓ Hyperinsulinemia, ↓ Lipid peroxidation | Insulin sensitivity | Cells-C2C12 | [94] |
| Strigolactone GR24 and pinosylvin (60–100 µM) | ↑ SIRT1, ↑ Glut4 ↑ FOXO1 ↑ IRS-1 ↑ Akt2, | ↑ Insulin sensitivity, ↑ Glucose uptake | AKt2 | Cells-L6 myoblasts | [100] |
| Hibiscus sabdariffa (Various dose) | ↑ IRS-1, ↑ PI3K, ↓ DPP4, ↓ GLP-1R | ↑ Insulin sensitivity, ↓ Starch breakdown | Insulin receptor activation (PI3K) | Cells-HK-2 | [101] |
| C3G and PCA (10–100 µmol) | ↑ PPARγ, ↑ Glut4, ↑ Adiponectin | ↑ Glucose uptake | PPARγ | Cells-3T3-L1 | [10] |
| Rice bran extracts (10 μg/mL and 50 μg) | ↑ PPARγ, ↑ Adiponectin ↓ TNF-α | ↑ Insulin sensitivity | PPARγ/adipogenesis | Cells-3T3-L1 | [102] |
| Pigmented rice bran extracts (50 µg/mL) | ↑ INSR, ↑ PI3K, ↑ Glut4, ↓ DDP-4 | ↑ Insulin sensitivity, ↓ Starch breakdown | Akt2/PI3K | Cells-3T3-L1 | [103] |
| Aspalathin (1 µM) | ↑ Glut4, ↑ UCP2, ↓ CPT1, ↑ Bcl-1 | ↑ Cell viability, ↑ Insulin sensitivity, ↑ Glucose uptake | pAMPK | Cells-H9c2 | [104] |
| In vivo models | |||||
| Polyphenol-rich ethyl acetate fraction (200 mg/kg BW) | ↑ Insr, ↑ IRS1, ↑ IRS2 ↑ Akt2, ↑ Glut4 | ↑ Insulin sensitivity | IRS1/AKT | Skeletal muscle-Sprague-Dawley rats | [105] |
| Folium Mori Extract (2 g/kg BW) | ↑ IRS-1, ↑ PI3Kp85α, ↑ Glut-4 | ↑ Glucose uptake | IRS-1/PI3K/Glut-4 signalling | Skeletal muscle- Sprague-Dawley rats | [106] |
| Polyphenols | Genes Affected | Function | Pathways | Cells/Tissue Type | Ref. |
|---|---|---|---|---|---|
| In vitro models | |||||
| Cinnamon extract (1–25 µg/mL) | ↓ PEPCK, ↓ G6Pase | ↓ Hepatic glucose output | PEPCK | Cells-H4IIE | [126] |
| Fructus Corni (50 mg/mL) | ↓ PEPCK | ↓ Hepatic Glucose out put | Gluconeogenesis | Cells-H4IIE | [128] |
| EGCG (5–25 µM) | ↓ PEPCK, ↓ G6Pase | ↓ Hepatic glucose out put | PI3K | Cells-H4IIE | [57] |
| EGCG (≤1–10 µM) | ↓ PEPCK, ↓ G6Pase | ↓ Hepatic glucose output | AMPK/CaMKK | Cells-H4IIE | [130] |
| Germinated black rice (50 ppm) | ↓ Pck1, ↓ Fbp1 | ↓ Hepatic glucose output | Gluconeogenesis | Cells-HepG2 | [134] |
| In vivo models | |||||
| Germinated black rice (50–100 ppm) | ↓ Pck1, ↓ Fbp1 | ↓ Hepatic glucose output | Gluconeogenesis | Liver-Sprague-Dawley rats | [134] |
| Hesperidin and Naringin (0.2 g/kg BW) | ↓ G6Pase, ↓ PEPCK | ↓ Hepatic glucose output | Gluconeogenesis | Liver-C57BL/KsJ-db/db mice | [136] |
| Resveratrol (0.02% w/w) | ↓ PECK, ↓ G6P, ↑ GK, ↓ SREBP-1c | ↑ Hepatic glucose uptake | PEPCK/AMPK | Liver-C57BL/KsJ-db/db mice | [137] |
| Fermented food paste (0.1–1.0 kg/BW) | ↑ G6PD, ↑ GCK, ↑ PFK, ↑ 6PGD | ↑ Glycogen synthesis, ↑ Hepatic insulin sensitivity, ↓ Hepatic glucose output | Glycolysis | Liver-Balb/c mice | [138] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review. Int. J. Mol. Sci. 2020, 21, 140. https://doi.org/10.3390/ijms21010140
Kang GG, Francis N, Hill R, Waters D, Blanchard C, Santhakumar AB. Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review. International Journal of Molecular Sciences. 2020; 21(1):140. https://doi.org/10.3390/ijms21010140
Chicago/Turabian StyleKang, Gideon Gatluak, Nidhish Francis, Rodney Hill, Daniel Waters, Christopher Blanchard, and Abishek Bommannan Santhakumar. 2020. "Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review" International Journal of Molecular Sciences 21, no. 1: 140. https://doi.org/10.3390/ijms21010140
APA StyleKang, G. G., Francis, N., Hill, R., Waters, D., Blanchard, C., & Santhakumar, A. B. (2020). Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review. International Journal of Molecular Sciences, 21(1), 140. https://doi.org/10.3390/ijms21010140