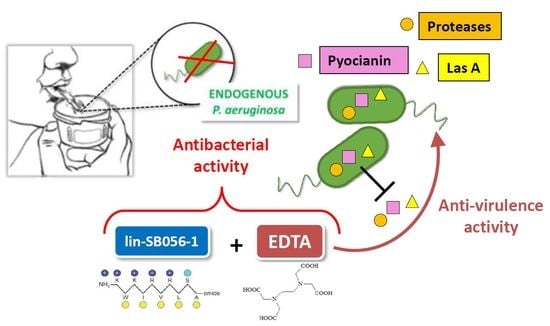
Targeting Pseudomonas aeruginosa in the Sputum of Primary Ciliary Dyskinesia Patients with a Combinatorial Strategy Having Antibacterial and Anti-Virulence Potential
Abstract
:1. Introduction
2. Results
2.1. Killing Activity of lin-SB056-1 in Combination with Ethylenediaminetetraacetic Acid (EDTA) against Endogenous P. aeruginosa
2.2. Effects of EDTA and lin-SB056-1 on Virulence Factors’ Production by P. aeruginosa PaM1 and PaM5
3. Discussion
4. Materials and Methods
4.1. Sputum Collection and Treatment
4.2. Peptide and EDTA Solutions
4.3. Susceptibility Testing
4.4. Bactericidal Assay in Patients’ Sputum
4.5. Assays for Evaluation of Virulence Factors in Culture Supernatants
4.6. Biofilm Inhibition Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| AMPs | Antimicrobial peptides |
| ANOVA | Analysis of variance |
| CF | Cystic fibrosis |
| CFU | Colony forming units |
| CV | Crystal violet |
| EDTA | Ethylenediaminetetraacetic acid |
| LB | Luria-Bertani broth |
| LPS | Lipopolysaccharide |
| MIC | Minimum Inhibitory Concentration |
| OD | Optical density |
| PCD | Primary Ciliary Dyskinesia |
| SPB | Sodium Phosphate Buffer |
| TSB | Tryptone Soy Broth |
References
- Wijers, C.D.M.; Chmiel, J.F.; Gaston, B.M. Bacterial infections in patients with primary ciliary dyskinesia: Comparison with cystic fibrosis. Chron. Respir. Dis. 2017, 14, 392–406. [Google Scholar] [CrossRef]
- Cohen-Cymberknoh, M.; Weigert, N.; Gileles-Hillel, A.; Breuer, O.; Simanovsky, N.; Boon, M.; De Boeck, K.; Barbato, A.; Snijders, D.; Collura, M.; et al. Clinical impact of Pseudomonas aeruginosa colonization in patients with primary ciliary dyskinesia. Respir. Med. 2017, 131, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, S.; Holgersen, M.G.; Nielsen, K.G. Variation in treatment strategies for the eradication of Pseudomonas aeruginosa in primary ciliary dyskinesia across European centers. Chron. Respir. Dis. 2019, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, I.; Jeukens, J.; Freschi, L.; Alcalá-Franco, B.; Facchini, M.; Boyle, B.; Molinaro, A.; Kukavica-Ibrulj, I.; Tümmler, B.; Levesque, R.C.; et al. Comparative genomics and biological characterization of sequential Pseudomonas aeruginosa isolates from persistent airways infection. BMC Genom. 2015, 16, 1105–1118. [Google Scholar] [CrossRef] [Green Version]
- Alanin Mikkel, C. Bacteriology and treatment of infections in the upper and lower airways in patients with primary ciliary dyskinesia: Addressing the paranasal sinuses. Dan. Med. J. 2017, 64, 5361–5378. [Google Scholar]
- Haney, E.F.; Mansour, S.C.; Hancock, R.E. Antimicrobial Peptides: An introduction. Methods Mol. Biol. 2017, 1548, 3–22. [Google Scholar] [CrossRef]
- Grassi, L.; Di Luca, M.; Maisetta, G.; Rinaldi, A.C.; Esin, S.; Trampuz, A.; Batoni, G. Generation of persister cells of Pseudomonas aeruginosa and Staphylococcus aureus by chemical treatment and evaluation of their susceptibility to membrane-targeting agents. Front. Microbiol. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef]
- Maisetta, G.; Di Luca, M.; Esin, S.; Florio, W.; Brancatisano, F.L.; Bottai, D.; Campa, M.; Batoni, G. Evaluation of the inhibitory effects of human serum components on bactericidal activity of human beta defensin 3. Peptides 2008, 29, 1–6. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S.; Campa, M. Human beta-defensin-3: A promising antimicrobial peptide. Mini Rev. Med. Chem. 2006, 6, 1063–1073. [Google Scholar] [CrossRef]
- Sommer, L.M.; Alanin, M.C.; Marvig, R.L.; Nielsen, K.G.; Høiby, N.; von Buchwald, C.; Molin, S.; Johansen, H.K. Bacterial evolution in PCD and CF patients follows the same mutational steps. Sci. Rep. 2016, 28, 28732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, V.; Smyth, A. Cystic fibrosis microbiology: Advances in antimicrobial therapy. J. Cyst. Fibros. 2015, 14, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, E.; Vighetto, C.; Di Martino, P.; Larreta Garde, V.; Seyer, D. Synergistic antibiofilm efficacy of various commercial antiseptics, enzymes and EDTA: A study of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Int. J. Antimicrob. Agents 2016, 48, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Salisbury, A.M. The Efficacy of tetrasodium EDTA on biofilms. Adv. Exp. Med. Biol. 2018, 1057, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, M.E. Rationale for the successful management of EDTA chelation therapy in human burden by toxic metals. Biomed. Res. Int. 2016, 2016, 8274504. [Google Scholar] [CrossRef] [Green Version]
- Maisetta, G.; Grassi, L.; Esin, S.; Serra, I.; Scorciapino, M.A.; Rinaldi, A.C.; Batoni, G. The semi-synthetic peptide lin-SB056-1 in combination with EDTA exerts strong antimicrobial and antibiofilm activity against Pseudomonas aeruginosa in conditions mimicking cystic fibrosis sputum. Int. J. Mol. Sci. 2017, 16, 1994. [Google Scholar] [CrossRef]
- Bush, A.; Payne, D.; Pike, S.; Jenkins, G.; Henke, M.O.; Rubin, B.K. Mucus properties in children with primary ciliary dyskinesia: Comparison with cystic fibrosis. Chest 2006, 129, 118–123. [Google Scholar] [CrossRef]
- Kim, W.D. Lung mucus: A clinician’s view. Eur. Respir. J. 1997, 10, 1914–1917. [Google Scholar] [CrossRef]
- Olson, J.C.; Ohman, D.E. Efficient production and processing of elastase and LasA by Pseudomonas aeruginosa require zinc and calcium ions. J. Bacteriol. 1992, 174, 4140–4147. [Google Scholar] [CrossRef] [Green Version]
- Guragain, M.; King, M.M.; Williamson, K.S.; Pérez-Osorio, A.C.; Akiyama, T.; Khanam, S.; Patrauchan, M.A.; Franklin, M.J. The Pseudomonas aeruginosa PAO1 two-component regulator CarSR regulates calcium homeostasis and calcium-induced virulence factor production through its regulatory targets CarO and CarP. J. Bacteriol. 2016, 198, 951–963. [Google Scholar] [CrossRef] [Green Version]
- Rada, B.; Leto, T.L. Pyocyanin effects on respiratory epithelium: Relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. 2013, 21, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tingpej, P.; Smith, L.; Rose, B.; Zhu, H.; Conibear, T.; Al Nassafi, K.; Manos, J.; Elkins, M.; Bye, P.; Willcox, M.; et al. Phenotypic characterization of clonal and nonclonal Pseudomonas aeruginosa strains isolated from lungs of adults with cystic fibrosis. J. Clin. Microbiol. 2007, 45, 1697–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, P.W.; Pier, G.B.; Preston, M.J.; Goldberger, O.; Fitzgerald, M.L.; Bernfield, M. Syndecan-1 shedding is enhanced by LasA, a secreted virulence factor of Pseudomonas aeruginosa. J. Biol. Chem. 2000, 275, 3057–3064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.J.; Hampton, G.E.; Garcia, G.P.C.; Maher, M.J.; Perugini, M.A.; Ackerley, D.F.; Lamont, I.L. Integrated activities of two alternative sigma factors coordinate iron acquisition and uptake by Pseudomonas aeruginosa. Mol. Microbiol. 2017, 106, 891–904. [Google Scholar] [CrossRef] [Green Version]
- Hodges, N.A.; Gordon, C.A. Protection of Pseudomonas aeruginosa against ciprofloxacin and beta-lactams by homologous alginate. Antimicrob. Agents Chemother. 1991, 35, 2450–2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leid, J.G.; Willson, C.J.; Shirtliff, M.E.; Hassett, D.J.; Parsek, M.R.; Jeffers, A.K. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J. Immunol. 2005, 175, 7512–7518. [Google Scholar] [CrossRef] [Green Version]
- Grassi, L.; Batoni, G.; Ostyn, L.; Rigole, P.; Van den Bossche, S.; Rinaldi, A.C.; Maisetta, G.; Esin, S.; Coenye, T.; Crabbé, A. The antimicrobial peptide lin-SB056-1 and its dendrimeric derivative prevent Pseudomonas aeruginosa biofilm formation in physiologically relevant models of chronic infections. Front. Microbiol. 2019, 10, 198. [Google Scholar] [CrossRef] [Green Version]
- Müller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.V.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.G.; Schneider, T.; et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. USA 2016, 113, 7077–7086. [Google Scholar] [CrossRef] [Green Version]
- Sajjan, U.S.; Tran, L.T.; Sole, N.; Rovaldi, C.; Akiyama, A.; Friden, P.M.; Forstner, J.F.; Rothstein, D.M. P-113D, an antimicrobial peptide active against Pseudomonas aeruginosa, retains activity in the presence of sputum from cystic fibrosis patients. Antimicrob. Agents Chemother. 2001, 45, 3437–3444. [Google Scholar] [CrossRef] [Green Version]
- Hachem, R.; Bahna, P.; Hanna, H.; Stephens, L.C.; Raad, I. EDTA as an adjunct antifungal agent for invasive pulmonary aspergillosis in a rodent model. Antimicrob. Agents Chemother. 2006, 50, 1823–1827. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lin, Y.; Lu, Q.; Li, F.; Yu, J.; Wang, Z.; He, Y.; Song, C. In vitro and in vivo activity of EDTA and antibacterial agents against the biofilm of mucoid Pseudomonas aeruginosa. Infection 2017, 45, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sarkisova, S.; Patrauchan, M.A.; Berglund, D.; Nivens, D.E.; Franklin, M.J. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J. Bacteriol. 2005, 187, 4327–4337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Orazio, M.; Mastropasqua, M.C.; Cerasi, M.; Pacello, F.; Consalvo, A.; Chirullo, B.; Mortensen, B.; Skaar, E.P.; Ciavardelli, D.; Pasquali, P.; et al. The capability of Pseudomonas aeruginosa to recruit zinc under conditions of limited metal availability is affected by inactivation of the ZnuABC transporter. Metallomics 2015, 7, 1023–1035. [Google Scholar] [CrossRef] [Green Version]
- Marquart, M.E.; Dajcs, J.J.; Caballero, A.R.; Thibodeaux, B.A.; O’Callaghan, R.J. Calcium and magnesium enhance the production of Pseudomonas aeruginosa protease IV, a corneal virulence factor. Med. Microbiol. Immunol. 2005, 194, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Laux, D.C.; Corson, J.M.; Givskov, M.; Hentzer, M.; Møller, A.; Wosencroft, K.A.; Olson, J.C.; Krogfelt, K.A.; Goldberg, J.B.; Cohen, P.S. Lysophosphatidic acid inhibition of the accumulation of Pseudomonas aeruginosa PAO1 alginate, pyoverdin, elastase and LasA. Microbiology 2002, 148, 1709–1723. [Google Scholar] [CrossRef] [Green Version]
- Körstgens, V.; Flemming, H.C.; Wingender, J.; Borchard, W. Influence of calcium ions on the mechanical properties of a model biofilm of mucoid Pseudomonas aeruginosa. Water Sci. Technol. 2001, 43, 49–57. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). EUCAST definitive document E. Def 1.2, May 2000: Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Essar, D.W.; Eberly, L.; Hadero, A.; Crawford, IP. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990, 172, 884–900. [Google Scholar] [CrossRef] [Green Version]
- El-Mowafy, S.A.; Abd, E.; Galil, K.H.; El-Messery, S.M.; Shaaban, M.I. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb. Pathog. 2014, 74, 25–32. [Google Scholar] [CrossRef]
- Kessler, E.; Safrin, M.; Olson, J.C.; Ohman, D.E. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J. Biol. Chem. 1993, 268, 7503–7508. [Google Scholar] [CrossRef]
- Cox, C.D.; Adams, P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect. Immun. 1985, 48, 130–138. [Google Scholar]
- Heidari, A.; Noshiranzadeh, N.; Haghi, F.; Bikas, R. Inhibition of quorum sensing related virulence factors of Pseudomonas aeruginosa by pyridoxal lactohydrazone. Microb. Pathogen. 2017, 112, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complement. Altern. Med. 2019, 19, 82–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maisetta, G.; Grassi, L.; Esin, S.; Kaya, E.; Morelli, A.; Puppi, D.; Piras, M.; Chiellini, F.; Pifferi, M.; Batoni, G. Targeting Pseudomonas aeruginosa in the Sputum of Primary Ciliary Dyskinesia Patients with a Combinatorial Strategy Having Antibacterial and Anti-Virulence Potential. Int. J. Mol. Sci. 2020, 21, 69. https://doi.org/10.3390/ijms21010069
Maisetta G, Grassi L, Esin S, Kaya E, Morelli A, Puppi D, Piras M, Chiellini F, Pifferi M, Batoni G. Targeting Pseudomonas aeruginosa in the Sputum of Primary Ciliary Dyskinesia Patients with a Combinatorial Strategy Having Antibacterial and Anti-Virulence Potential. International Journal of Molecular Sciences. 2020; 21(1):69. https://doi.org/10.3390/ijms21010069
Chicago/Turabian StyleMaisetta, Giuseppantonio, Lucia Grassi, Semih Esin, Esingül Kaya, Andrea Morelli, Dario Puppi, Martina Piras, Federica Chiellini, Massimo Pifferi, and Giovanna Batoni. 2020. "Targeting Pseudomonas aeruginosa in the Sputum of Primary Ciliary Dyskinesia Patients with a Combinatorial Strategy Having Antibacterial and Anti-Virulence Potential" International Journal of Molecular Sciences 21, no. 1: 69. https://doi.org/10.3390/ijms21010069
APA StyleMaisetta, G., Grassi, L., Esin, S., Kaya, E., Morelli, A., Puppi, D., Piras, M., Chiellini, F., Pifferi, M., & Batoni, G. (2020). Targeting Pseudomonas aeruginosa in the Sputum of Primary Ciliary Dyskinesia Patients with a Combinatorial Strategy Having Antibacterial and Anti-Virulence Potential. International Journal of Molecular Sciences, 21(1), 69. https://doi.org/10.3390/ijms21010069