Two-Faced Roles of Tumor-Associated Neutrophils in Cancer Development and Progression
Abstract
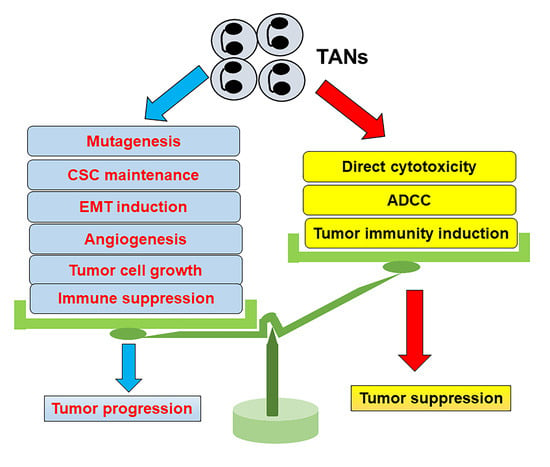
:1. Introduction
2. Differentiation and Plasticity of Neutrophils
3. TANs in Tumor Development and Growth
4. TANs in Tumor Immunity
5. TANs in Tumor Metastasis
6. Future Perspective on Anticancer Treatment Targeting TANs
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | antibody-dependent cell cytotoxicity |
| Ang | angiopoietin |
| AOM | azoxymethane |
| Arg | arginase |
| BCG | Mycobacterium bovis bacillus Calmette–Guérin |
| BMP | bone morphogenic protein |
| CSC | cancer stem cell |
| CTC | circulating tumor cell |
| CYLD | cylindromatosis lysine 63 deubiquitinase |
| DC | dendritic cell |
| DSS | dextran sodium sulfate |
| ECM | extracellular matrix |
| EMT | epithelial–mesenchymal transition |
| ER | endoplasmic reticulum |
| ESCC | esophageal squamous cell cancer |
| FGF | fibroblast growth factor |
| G–CSF | granulocyte colony-stimulating factor |
| GM–CSF | granulocyte macrophage colony-stimulating factor |
| GMP | granulocyte–monocyte progenitor |
| GVHD | graft-versus-host disease |
| HCC | hepatocellular carcinoma |
| HGF | hepatocyte growth factor |
| HSC | hematopoietic stem cell |
| IFN | interferon |
| IL | interleukin |
| LDN | low-density neutrophil |
| LOX-1 | lectin-type oxidized low-density lipoprotein receptor 1 |
| LPS | lipopolysaccharide |
| LT | leukotriene |
| M1dG | 3-(2-deoxy-β-d-erythro-pentofuranosyl)pyrimido [1,2-α] purin-10(3H)-one |
| MDSC | myeloid-derived suppressor cell |
| MHC | major histocompatibility antigen |
| miRNA | microRNA |
| MMP | matrix metalloproteinase |
| MPO | myeloperoxidase |
| NE | neutrophil elastase |
| NET | neutrophil extracellular trap |
| NK | natural killer |
| NLR | neutrophil–lymphocyte ratio |
| NSCLC | non-small cell lung cancer |
| OSM | oncostatin M |
| PDAC | pancreatic ductal adenocarcinoma |
| PDGF | platelet-derived growth factor |
| PI3K | phosphatidylinositol 3-kinase |
| PMN | polymorphonuclear leukocyte |
| Prok | prokineticin |
| R | receptor |
| ROS | reactive oxygen species |
| SCC | squamous cell carcinoma |
| TAN | tumor-associated neutrophil |
| TGF | transforming growth factor |
| TIMP | tissue inhibitor of matrix metalloproteinase |
| TNF | tumor necrosis factor |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
| Treg | regulatory T cell |
| TRMP2 | transient receptor potential cation channel, subfamily M, member 2 |
| VEGF | vascular endothelial growth factor |
References
- Ng, L.G.; Ostuni, R.; Hidalgo, A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.R.; Oppenheim, J.J. Poly’s lament: The neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol. Today 1992, 13, 169–172. [Google Scholar] [CrossRef]
- Tecchio, C.; Scapini, P.; Pizzolo, G.; Cassatella, M.A. On the cytokines produced by human neutrophils in tumors. Semin. Cancer Biol. 2013, 23, 159–170. [Google Scholar] [CrossRef]
- Tamassia, N.; Bianchetto-Aguilera, F.; Arruda-Silva, F.; Gardiman, E.; Gasperini, S.; Calzetti, F.; Cassatella, M.A. Cytokine production by human neutrophils: Revisiting the “dark side of the moon”. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12952. [Google Scholar] [CrossRef]
- Mollinedo, F. Neutrophil degranulation, plasticity, and cancer metastasis. Trends Immunol. 2019, 40, 228–242. [Google Scholar] [CrossRef]
- Pillay, J.; den Braber, I.; Vrisekoop, N.; Kwast, L.M.; de Boer, R.J.; Borghans, J.A.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2h2o reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef]
- Dancey, J.T.; Deubelbeiss, K.A.; Harker, L.A.; Finch, C.A. Neutrophil kinetics in man. J. Clin. Investig. 1976, 58, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. /Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Shen, M.; Hu, P.; Donskov, F.; Wang, G.; Liu, Q.; Du, J. Tumor-associated neutrophils as a new prognostic factor in cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e98259. [Google Scholar] [CrossRef] [Green Version]
- Rakaee, M.; Busund, L.T.; Paulsen, E.E.; Richardsen, E.; Al-Saad, S.; Andersen, S.; Donnem, T.; Bremnes, R.M.; Kilvaer, T.K. Prognostic effect of intratumoral neutrophils across histological subtypes of non-small cell lung cancer. Oncotarget 2016, 7, 72184–72196. [Google Scholar] [CrossRef] [Green Version]
- Berry, R.S.; Xiong, M.J.; Greenbaum, A.; Mortaji, P.; Nofchissey, R.A.; Schultz, F.; Martinez, C.; Luo, L.; Morris, K.T.; Hanson, J.A. High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage ii colorectal cancer. PLoS ONE 2017, 12, e0188799. [Google Scholar] [CrossRef] [Green Version]
- Cowland, J.B.; Borregaard, N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 2016, 273, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, L.W.; Safford, M.; Loken, M.R. Flow cytometric analysis of human bone marrow. Iii. Neutrophil maturation. Leukemia 1990, 4, 657–663. [Google Scholar] [PubMed]
- Velten, L.; Haas, S.F.; Raffel, S.; Blaszkiewicz, S.; Islam, S.; Hennig, B.P.; Hirche, C.; Lutz, C.; Buss, E.C.; Nowak, D.; et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat. Cell Biol. 2017, 19, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evrard, M.; Kwok, I.W.H.; Chong, S.Z.; Teng, K.W.W.; Becht, E.; Chen, J.; Sieow, J.L.; Penny, H.L.; Ching, G.C.; Devi, S.; et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity 2018, 48, 364–379.e368. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Ng, L.G. Granulopoiesis and neutrophil homeostasis: A metabolic, daily balancing act. Trends Immunol. 2019, 40, 598–612. [Google Scholar] [CrossRef]
- Paul, F.; Arkin, Y.A.; Giladi, A.; Jaitin, D.A.; Kenigsberg, E.; Keren-Shaul, H.; Winter, D.; Lara-Astiaso, D.; Gury, M.; Weiner, A.; et al. Transcriptional heterogeneity and lineage commitment in myeloid progenitors. Cell 2015, 163, 1663–1677. [Google Scholar] [CrossRef] [Green Version]
- Giladi, A.; Paul, F.; Herzog, Y.; Lubling, Y.; Weiner, A.; Yofe, I.; Jaitin, D.; Cabezas-Wallscheid, N.; Dress, R.; Ginhoux, F.; et al. Single-cell characterization of haematopoietic progenitors and their trajectories in homeostasis and perturbed haematopoiesis. Nat. Cell Biol. 2018, 20, 836–846. [Google Scholar] [CrossRef]
- De Filippo, K.; Rankin, S.M. Cxcr4, the master regulator of neutrophil trafficking in homeostasis and disease. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12949. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Awaji, M.; Saxena, S.; Varney, M.L.; Sharma, B.; Singh, R.K. Il-17–cxc chemokine receptor 2 axis facilitates breast cancer progression by up-regulating neutrophil recruitment. Am. J. Pathol. 2020, 190, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, H.; Yoshie, O. Functional roles of evolutionary conserved motifs and residues in vertebrate chemokine receptors. J. Leukoc. Biol. 2015, 97, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, A.; Chilvers, E.R.; Summers, C.; Koenderman, L. The neutrophil life cycle. Trends Immunol. 2019, 40, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Nourshargh, S.; Renshaw, S.A.; Imhof, B.A. Reverse migration of neutrophils: Where, when, how, and why? Trends Immunol. 2016, 37, 273–286. [Google Scholar] [CrossRef]
- Buckley, C.D.; Ross, E.A.; McGettrick, H.M.; Osborne, C.E.; Haworth, O.; Schmutz, C.; Stone, P.C.; Salmon, M.; Matharu, N.M.; Vohra, R.K.; et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J. Leukoc. Biol. 2006, 79, 303–311. [Google Scholar] [CrossRef]
- Rankin, E.B.; Giaccia, A.J. Hypoxic control of metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Casanova-Acebes, M.; Nicolás-Ávila, J.A.; Li, J.L.; García-Silva, S.; Balachander, A.; Rubio-Ponce, A.; Weiss, L.A.; Adrover, J.M.; Burrows, K.; A-González, N.; et al. Neutrophils instruct homeostatic and pathological states in naive tissues. J. Exp. Med. 2018, 215, 2778–2795. [Google Scholar] [CrossRef]
- Deniset, J.F.; Surewaard, B.G.; Lee, W.Y.; Kubes, P. Splenic ly6g(high) mature and ly6g(int) immature neutrophils contribute to eradication of s. Pneumoniae. J. Exp. Med. 2017, 214, 1333–1350. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by tgf-beta: “N1” versus “n2” tan. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, D.; Zhu, J. Dynamic balance between master transcription factors determines the fates and functions of cd4 t cell and innate lymphoid cell subsets. J. Exp. Med. 2017, 214, 1861–1876. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-associated macrophages: Recent insights and therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Kockritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type i ifns induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Mahiddine, K.; Blaisdell, A.; Ma, S.; Crequer-Grandhomme, A.; Lowell, C.A.; Erlebacher, A. Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J. Clin. Investig. 2020, 130, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [Green Version]
- Bergenfelz, C.; Leandersson, K. The generation and identity of human myeloid-derived suppressor cells. Front. Oncol. 2020, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Condamine, T.; Dominguez, G.A.; Youn, J.-I.; Kossenkov, A.V.; Mony, S.; Alicea-Torres, K.; Tcyganov, E.; Hashimoto, A.; Nefedova, Y.; Lin, C.; et al. Lectin-type oxidized ldl receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 2016, 1, aaf8943. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hu, Y.; Gu, F.; Liang, J.; Zeng, Y.; Hong, X.; Zhang, K.; Liu, L. Phenotypic and clinical characterization of low density neutrophils in patients with advanced lung adenocarcinoma. Oncotarget 2017, 8, 90969–90978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, B.E.; Tabaries, S.; Johnson, R.M.; Andrzejewski, S.; Senecal, J.; Lehuede, C.; Annis, M.G.; Ma, E.H.; Vols, S.; Ramsay, L.; et al. Immature low-density neutrophils exhibit metabolic flexibility that facilitates breast cancer liver metastasis. Cell Rep. 2019, 27, 3902–3915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassani, M.; Hellebrekers, P.; Chen, N.; van Aalst, C.; Bongers, S.; Hietbrink, F.; Koenderman, L.; Vrisekoop, N. On the origin of low-density neutrophils. J. Leukoc. Biol. 2020, 107, 809–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassatella, M.A.; Ostberg, N.K.; Tamassia, N.; Soehnlein, O. Biological roles of neutrophil-derived granule proteins and cytokines. Trends Immunol. 2019, 40, 648–664. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N.; Sorensen, O.E.; Theilgaard-Monch, K. Neutrophil granules: A library of innate immunity proteins. Trends Immunol. 2007, 28, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Gungor, N.; Knaapen, A.M.; Munnia, A.; Peluso, M.; Haenen, G.R.; Chiu, R.K.; Godschalk, R.W.; van Schooten, F.J. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis 2010, 25, 149–154. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Zhou, S.L.; Yin, D.; Hu, Z.Q.; Luo, C.B.; Zhou, Z.J.; Xin, H.Y.; Yang, X.R.; Shi, Y.H.; Wang, Z.; Huang, X.W.; et al. A positive feedback loop between cancer stem-like cells and tumor-associated neutrophils controls hepatocellular carcinoma progression. Hepatology 2019, 70, 1214–1230. [Google Scholar] [CrossRef]
- Mathis, B.J.; Lai, Y.; Qu, C.; Janicki, J.S.; Cui, T. Cyld-mediated signaling and diseases. Curr. Drug Targets 2015, 16, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Cumpian, A.M.; Caetano, M.S.; Ochoa, C.E.; De la Garza, M.M.; Lapid, D.J.; Mirabolfathinejad, S.G.; Dickey, B.F.; Zhou, Q.; Moghaddam, S.J. Promoting effect of neutrophils on lung tumorigenesis is mediated by cxcr2 and neutrophil elastase. Mol. Cancer 2013, 12, 154. [Google Scholar] [CrossRef] [Green Version]
- Houghton, A.M.; Rzymkiewicz, D.M.; Ji, H.; Gregory, A.D.; Egea, E.E.; Metz, H.E.; Stolz, D.B.; Land, S.R.; Marconcini, L.A.; Kliment, C.R.; et al. Neutrophil elastase-mediated degradation of irs-1 accelerates lung tumor growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [Green Version]
- Ardi, V.C.; Kupriyanova, T.A.; Deryugina, E.I.; Quigley, J.P. Human neutrophils uniquely release timp-free mmp-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 20262–20267. [Google Scholar] [CrossRef] [Green Version]
- Bekes, E.M.; Schweighofer, B.; Kupriyanova, T.A.; Zajac, E.; Ardi, V.C.; Quigley, J.P.; Deryugina, E.I. Tumor-recruited neutrophils and neutrophil timp-free mmp-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am. J. Pathol. 2011, 179, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, H.H.; Liang, S.; Henderson, A.J.; Dong, C. Regulation of interleukin-8 expression in melanoma-stimulated neutrophil inflammatory response. Exp. Cell Res. 2007, 313, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Hor, W.S.; Huang, W.L.; Lin, Y.S.; Yang, B.C. Cross-talk between tumor cells and neutrophils through the fas (apo-1, cd95)/fasl system: Human glioma cells enhance cell viability and stimulate cytokine production in neutrophils. J. Leukoc. Biol. 2003, 73, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, T.; Clarke, M.; Steele, C.W.; Samuel, M.S.; Neumann, J.; Jung, A.; Huels, D.; Olson, M.F.; Das, S.; Nibbs, R.J.B.; et al. Inhibition of cxcr2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J. Clin. Investig. 2012, 122, 3127–3144. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Ijichi, H.; Takahashi, R.; Miyabayashi, K.; Fujiwara, H.; Yamada, T.; Kato, H.; Nakatsuka, T.; Tanaka, Y.; Tateishi, K.; et al. Blocking cxcls–cxcr2 axis in tumor–stromal interactions contributes to survival in a mouse model of pancreatic ductal adenocarcinoma through reduced cell invasion/migration and a shift of immune-inflammatory microenvironment. Oncogenesis 2019, 8, 8. [Google Scholar] [CrossRef]
- Roth, A.D.; Delorenzi, M.; Tejpar, S.; Yan, P.; Klingbiel, D.; Fiocca, R.; d’Ario, G.; Cisar, L.; Labianca, R.; Cunningham, D.; et al. Integrated analysis of molecular and clinical prognostic factors in stage ii/iii colon cancer. J. Natl. Cancer Inst. 2012, 104, 1635–1646. [Google Scholar] [CrossRef] [Green Version]
- Voorneveld, P.W.; Jacobs, R.J.; Kodach, L.L.; Hardwick, J.C.H. A meta-analysis of smad4 immunohistochemistry as a prognostic marker in colorectal cancer. Transl. Oncol. 2015, 8, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Means, A.L.; Freeman, T.J.; Zhu, J.; Woodbury, L.G.; Marincola-Smith, P.; Wu, C.; Meyer, A.R.; Weaver, C.J.; Padmanabhan, C.; An, H.; et al. Epithelial smad4 deletion up-regulates inflammation and promotes inflammation-associated cancer. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 257–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Kawada, K.; Itatani, Y.; Inamoto, S.; Okamura, R.; Iwamoto, M.; Miyamoto, E.; Chen-Yoshikawa, T.F.; Hirai, H.; Hasegawa, S.; et al. Loss of smad4 promotes lung metastasis of colorectal cancer by accumulation of ccr1+ tumor-associated neutrophils through ccl15-ccr1 axis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 833–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, R.; Yamamoto, T.; Hirai, H.; Hanada, K.; Kiyasu, Y.; Nishikawa, G.; Mizuno, R.; Inamoto, S.; Itatani, Y.; Sakai, Y.; et al. Loss of smad4 promotes colorectal cancer progression by recruiting tumor-associated neutrophils via the cxcl1/8-cxcr2 axis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2887–2899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apte, R.S.; Chen, D.S.; Ferrara, N. Vegf in signaling and disease: Beyond discovery and development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudry, M.; Bregerie, O.; Andrieu, V.; El Benna, J.; Pocidalo, M.A.; Hakim, J. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood 1997, 90, 4153–4161. [Google Scholar] [CrossRef] [PubMed]
- Scapini, P.; Calzetti, F.; Cassatella, M.A. On the detection of neutrophil-derived vascular endothelial growth factor (vegf). J. Immunol. Methods 1999, 232, 121–129. [Google Scholar] [CrossRef]
- Ohki, Y.; Heissig, B.; Sato, Y.; Akiyama, H.; Zhu, Z.; Hicklin, D.J.; Shimada, K.; Ogawa, H.; Daida, H.; Hattori, K.; et al. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 2005–2007. [Google Scholar] [CrossRef]
- Scapini, P.; Morini, M.; Tecchio, C.; Minghelli, S.; Di Carlo, E.; Tanghetti, E.; Albini, A.; Lowell, C.; Berton, G.; Noonan, D.M.; et al. Cxcl1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-a. J. Immunol. 2004, 172, 5034–5040. [Google Scholar] [CrossRef] [Green Version]
- Jablonska, J.; Wu, C.F.; Andzinski, L.; Leschner, S.; Weiss, S. Cxcr2-mediated tumor-associated neutrophil recruitment is regulated by ifn-beta. Int. J. Cancer 2014, 134, 1346–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils responsive to endogenous ifn-beta regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Investig. 2010, 120, 1151–1164. [Google Scholar] [CrossRef]
- Neagoe, P.E.; Brkovic, A.; Hajjar, F.; Sirois, M.G. Expression and release of angiopoietin-1 from human neutrophils: Intracellular mechanisms. Growth Factors 2009, 27, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Steringer, J.P.; Nickel, W. The molecular mechanism underlying unconventional secretion of fibroblast growth factor 2 from tumour cells. Biol. Cell 2017, 109, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Weeks, A.N.; Lim, S.Y.; Yuzhalin, A.E.; Jones, K.; Markelc, B.; Kim, K.J.; Buzzelli, J.N.; Fokas, E.; Cao, Y.; Smart, S.; et al. Neutrophils promote hepatic metastasis growth through fibroblast growth factor 2-dependent angiogenesis in mice. Hepatology 2017, 65, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Negri, L.; Ferrara, N. The prokineticins: Neuromodulators and mediators of inflammation and myeloid cell-dependent angiogenesis. Physiol. Rev. 2018, 98, 1055–1082. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Qu, X.; Tan, M.; Meng, Y.G.; Ferrara, N. Characterization and regulation of bv8 in human blood cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 2675–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shojaei, F.; Wu, X.; Zhong, C.; Yu, L.; Liang, X.H.; Yao, J.; Blanchard, D.; Bais, C.; Peale, F.V.; van Bruggen, N.; et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 2007, 450, 825–831. [Google Scholar] [CrossRef]
- Shojaei, F.; Wu, X.; Qu, X.; Kowanetz, M.; Yu, L.; Tan, M.; Meng, Y.G.; Ferrara, N. G-csf-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-vegf therapy in mouse models. Proc. Natl. Acad. Sci. USA 2009, 106, 6742–6747. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the il-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Wijelath, E.S.; Carlsen, B.; Cole, T.; Chen, J.; Kothari, S.; Hammond, W.P. Oncostatin m induces basic fibroblast growth factor expression in endothelial cells and promotes endothelial cell proliferation, migration and spindle morphology. J. Cell Sci. 1997, 110 Pt 7, 871–879. [Google Scholar]
- Junk, D.J.; Bryson, B.L.; Smigiel, J.M.; Parameswaran, N.; Bartel, C.A.; Jackson, M.W. Oncostatin m promotes cancer cell plasticity through cooperative stat3-smad3 signaling. Oncogene 2017, 36, 4001–4013. [Google Scholar] [CrossRef] [Green Version]
- Queen, M.M.; Ryan, R.E.; Holzer, R.G.; Keller-Peck, C.R.; Jorcyk, C.L. Breast cancer cells stimulate neutrophils to produce oncostatin m: Potential implications for tumor progression. Cancer Res. 2005, 65, 8896–8904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonneau, M.; Frouin, E.; Huguier, V.; Jermidi, C.; Jegou, J.F.; Godet, J.; Barra, A.; Paris, I.; Levillain, P.; Cordier-Dirikoc, S.; et al. Oncostatin m is overexpressed in skin squamous-cell carcinoma and promotes tumor progression. Oncotarget 2018, 9, 36457–36473. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, Y.; Oda, T.; Kinoshita, T.; Nakahashi, C.; Hasebe, T.; Ohkohchi, N.; Ochiai, A. Overexpression of tgf-β by infiltrated granulocytes correlates with the expression of collagen mrna in pancreatic cancer. Br. J. Cancer 2004, 91, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Shen, M.; Zhang, P.; Zheng, C.; Pang, Z.; Zhu, L.; Du, J. Intratumoral neutrophil granulocytes contribute to epithelial-mesenchymal transition in lung adenocarcinoma cells. Tumour Biol. 2015, 36, 7789–7796. [Google Scholar] [CrossRef]
- Liu, X.; Sun, R.; Chen, J.; Liu, L.; Cui, X.; Shen, S.; Cui, G.; Ren, Z.; Yu, Z. Crosstalk mechanisms between hgf/c-met axis and ncrnas in malignancy. Front. Cell Dev. Biol. 2020, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Wislez, M.; Rabbe, N.; Marchal, J.; Milleron, B.; Crestani, B.; Mayaud, C.; Antoine, M.; Soler, P.; Cadranel, J. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: Role in tumor progression and death. Cancer Res. 2003, 63, 1405–1412. [Google Scholar]
- He, M.; Peng, A.; Huang, X.Z.; Shi, D.C.; Wang, J.C.; Zhao, Q.; Lin, H.; Kuang, D.M.; Ke, P.F.; Lao, X.M. Peritumoral stromal neutrophils are essential for c-met-elicited metastasis in human hepatocellular carcinoma. Oncoimmunology 2016, 5, e1219828. [Google Scholar] [CrossRef] [Green Version]
- Finisguerra, V.; Di Conza, G.; Di Matteo, M.; Serneels, J.; Costa, S.; Thompson, A.A.; Wauters, E.; Walmsley, S.; Prenen, H.; Granot, Z.; et al. Met is required for the recruitment of anti-tumoural neutrophils. Nature 2015, 522, 349–353. [Google Scholar] [CrossRef]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking tnf-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Investig. 2008, 118, 560–570. [Google Scholar]
- Shang, K.; Bai, Y.P.; Wang, C.; Wang, Z.; Gu, H.Y.; Du, X.; Zhou, X.Y.; Zheng, C.L.; Chi, Y.Y.; Mukaida, N.; et al. Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS ONE 2012, 7, e51848. [Google Scholar] [CrossRef] [Green Version]
- Triner, D.; Devenport, S.N.; Ramakrishnan, S.K.; Ma, X.; Frieler, R.A.; Greenson, J.K.; Inohara, N.; Nunez, G.; Colacino, J.A.; Mortensen, R.M.; et al. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Gastroenterology 2019, 156, 1467–1482. [Google Scholar] [CrossRef]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and nets in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef]
- Schedel, F.; Mayer-Hain, S.; Pappelbaum, K.I.; Metze, D.; Stock, M.; Goerge, T.; Loser, K.; Sunderkotter, C.; Luger, T.A.; Weishaupt, C. Evidence and impact of neutrophil extracellular traps in malignant melanoma. Pigment Cell Melanoma Res. 2020, 33, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demers, M.; Wong, S.L.; Martinod, K.; Gallant, M.; Cabral, J.E.; Wang, Y.; Wagner, D.D. Priming of neutrophils toward netosis promotes tumor growth. Oncoimmunology 2016, 5, e1134073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demers, M.; Wagner, D.D. Neutrophil extracellular traps: A new link to cancer-associated thrombosis and potential implications for tumor progression. Oncoimmunology 2013, 2, e22946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, L. The pro-tumor effect and the anti-tumor effect of neutrophils extracellular traps. Biosci. Trends 2020, 13, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013, 123, 3446–3458. [Google Scholar] [CrossRef]
- Najmeh, S.; Cools-Lartigue, J.; Rayes, R.F.; Gowing, S.; Vourtzoumis, P.; Bourdeau, F.; Giannias, B.; Berube, J.; Rousseau, S.; Ferri, L.E.; et al. Neutrophil extracellular traps sequester circulating tumor cells via beta1-integrin mediated interactions. Int. J. Cancer 2017, 140, 2321–2330. [Google Scholar] [CrossRef] [Green Version]
- van Kessel, K.P.; Verhoef, J. A view to a kill: Cytotoxic mechanisms of human polymorphonuclear leukocytes compared with monocytes and natural killer cells. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 1990, 58, 249–264. [Google Scholar] [CrossRef]
- Berkow, R.L.; Wang, D.; Larrick, J.W.; Dodson, R.W.; Howard, T.H. Enhancement of neutrophil superoxide production by preincubation with recombinant human tumor necrosis factor. J. Immunol. 1987, 139, 3783–3791. [Google Scholar]
- Takeshima, T.; Pop, L.M.; Laine, A.; Iyengar, P.; Vitetta, E.S.; Hannan, R. Key role for neutrophils in radiation-induced antitumor immune responses: Potentiation with g-csf. Proc. Natl. Acad. Sci. USA 2016, 113, 11300–11305. [Google Scholar] [CrossRef] [Green Version]
- Gershkovitz, M.; Caspi, Y.; Fainsod-Levi, T.; Katz, B.; Michaeli, J.; Khawaled, S.; Lev, S.; Polyansky, L.; Shaul, M.E.; Sionov, R.V.; et al. Trpm2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. 2018, 78, 2680–2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pericle, F.; Kirken, R.A.; Epling-Burnette, P.K.; Blanchard, D.K.; Djeu, J.Y. Direct killing of interleukin-2-transfected tumor cells by human neutrophils. Int. J. Cancer 1996, 66, 367–373. [Google Scholar] [CrossRef]
- Martin, A.; Seignez, C.; Racoeur, C.; Isambert, N.; Mabrouk, N.; Scagliarini, A.; Reveneau, S.; Arnould, L.; Bettaieb, A.; Jeannin, J.F.; et al. Tumor-derived granzyme b-expressing neutrophils acquire antitumor potential after lipid a treatment. Oncotarget 2018, 9, 28364–28378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushner, B.H.; Cheung, N.K. Absolute requirement of cd11/cd18 adhesion molecules, fcrii and the phosphatidylinositol-linked fcriii for monoclonal antibody-mediated neutrophil antihuman tumor cytotoxicity. Blood 1992, 79, 1484–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matlung, H.L.; Babes, L.; Zhao, X.W.; van Houdt, M.; Treffers, L.W.; van Rees, D.J.; Franke, K.; Schornagel, K.; Verkuijlen, P.; Janssen, H.; et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018, 23, 3946–3959.e3946. [Google Scholar] [CrossRef] [PubMed]
- Rosevear, H.M.; Lightfoot, A.J.; O’Donnell, M.A.; Griffith, T.S. The role of neutrophils and tnf-related apoptosis-inducing ligand (trail) in bacillus calmette-guerin (bcg) immunotherapy for urothelial carcinoma of the bladder. Cancer Metastasis Rev. 2009, 28, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Midorikawa, Y.; Yamashita, T.; Sendo, F. Modulation of the immune response to transplanted tumors in rats by selective depletion of neutrophils in vivo using a monoclonal antibody: Abrogation of specific transplantation resistance to chemical carcinogen-induced syngeneic tumors by selective depletion of neutrophils in vivo. Cancer Res. 1990, 50, 6243–6247. [Google Scholar]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020. [Google Scholar] [CrossRef]
- Beauvillain, C.; Delneste, Y.; Scotet, M.; Peres, A.; Gascan, H.; Guermonprez, P.; Barnaba, V.; Jeannin, P. Neutrophils efficiently cross-prime naive t cells in vivo. Blood 2007, 110, 2965–2973. [Google Scholar] [CrossRef]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Lore, K. Neutrophils acquire the capacity for antigen presentation to memory cd4(+) t cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushima, H.; Geng, S.; Lu, R.; Okamoto, T.; Yao, Y.; Mayuzumi, N.; Kotol, P.F.; Chojnacki, B.J.; Miyazaki, T.; Gallo, R.L.; et al. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood 2013, 121, 1677–1689. [Google Scholar] [CrossRef] [Green Version]
- Singhal, S.; Bhojnagarwala, P.S.; O’Brien, S.; Moon, E.K.; Garfall, A.L.; Rao, A.S.; Quatromoni, J.G.; Stephen, T.L.; Litzky, L.; Deshpande, C.; et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 2016, 30, 120–135. [Google Scholar] [CrossRef] [Green Version]
- Beauvillain, C.; Cunin, P.; Doni, A.; Scotet, M.; Jaillon, S.; Loiry, M.L.; Magistrelli, G.; Masternak, K.; Chevailler, A.; Delneste, Y.; et al. Ccr7 is involved in the migration of neutrophils to lymph nodes. Blood 2011, 117, 1196–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-associated neutrophils stimulate t cell responses in early-stage human lung cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, M.P.; Lombardi, L.; Stoppacciaro, A.; Melani, C.; Parenza, M.; Bottazzi, B.; Parmiani, G. Granulocyte colony-stimulating factor (g-csf) gene transduction in murine adenocarcinoma drives neutrophil-mediated tumor inhibition in vivo. Neutrophils discriminate between g-csf-producing and g-csf-nonproducing tumor cells. J. Immunol. 1992, 149, 113–119. [Google Scholar]
- Stoppacciaro, A.; Melani, C.; Parenza, M.; Mastracchio, A.; Bassi, C.; Baroni, C.; Parmiani, G.; Colombo, M.P. Regression of an established tumor genetically modified to release granulocyte colony-stimulating factor requires granulocyte-t cell cooperation and t cell-produced interferon gamma. J. Exp. Med. 1993, 178, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Pan, K.; Li, X.D.; She, K.L.; Zhao, J.J.; Wang, W.; Chen, J.G.; Chen, Y.B.; Yun, J.P.; Xia, J.C. The accumulation and prognosis value of tumor infiltrating il-17 producing cells in esophageal squamous cell carcinoma. PLoS ONE 2011, 6, e18219. [Google Scholar] [CrossRef]
- Chen, C.L.; Wang, Y.; Huang, C.Y.; Zhou, Z.Q.; Zhao, J.J.; Zhang, X.F.; Pan, Q.Z.; Wu, J.X.; Weng, D.S.; Tang, Y.; et al. Il-17 induces antitumor immunity by promoting beneficial neutrophil recruitment and activation in esophageal squamous cell carcinoma. Oncoimmunology 2017, 7, e1373234. [Google Scholar] [CrossRef]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. Cxcr1 and cxcr2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity 2020. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.; Jonkers, J.; et al. Il-17-producing gammadelta t cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- He, G.; Zhang, H.; Zhou, J.; Wang, B.; Chen, Y.; Kong, Y.; Xie, X.; Wang, X.; Fei, R.; Wei, L.; et al. Peritumoural neutrophils negatively regulate adaptive immunity via the pd-l1/pd-1 signalling pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase i production in the tumor microenvironment by mature myeloid cells inhibits t-cell receptor expression and antigen-specific t-cell responses. Cancer Res. 2004, 64, 5839–5849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotondo, R.; Barisione, G.; Mastracci, L.; Grossi, F.; Orengo, A.M.; Costa, R.; Truini, M.; Fabbi, M.; Ferrini, S.; Barbieri, O. Il-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int. J. Cancer 2009, 125, 887–893. [Google Scholar] [CrossRef] [PubMed]
- El-Hag, A.; Clark, R.A. Down-regulation of human natural killer activity against tumors by the neutrophil myeloperoxidase system and hydrogen peroxide. J. Immunol. 1984, 133, 3291–3297. [Google Scholar] [PubMed]
- Yang, T.H.; St John, L.S.; Garber, H.R.; Kerros, C.; Ruisaard, K.E.; Clise-Dwyer, K.; Alatrash, G.; Ma, Q.; Molldrem, J.J. Membrane-associated proteinase 3 on granulocytes and acute myeloid leukemia inhibits t cell proliferation. J. Immunol. 2018, 201, 1389–1399. [Google Scholar] [CrossRef]
- Mishalian, I.; Bayuh, R.; Eruslanov, E.; Michaeli, J.; Levy, L.; Zolotarov, L.; Singhal, S.; Albelda, S.M.; Granot, Z.; Fridlender, Z.G. Neutrophils recruit regulatory t-cells into tumors via secretion of ccl17--a new mechanism of impaired antitumor immunity. Int. J. Cancer 2014, 135, 1178–1186. [Google Scholar] [CrossRef]
- Zhou, S.L.; Zhou, Z.J.; Hu, Z.Q.; Huang, X.W.; Wang, Z.; Chen, E.B.; Fan, J.; Cao, Y.; Dai, Z.; Zhou, J. Tumor-associated neutrophils recruit macrophages and t-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology 2016, 150, 1646–1658. [Google Scholar] [CrossRef] [Green Version]
- Perobelli, S.M.; Mercadante, A.C.; Galvani, R.G.; Goncalves-Silva, T.; Alves, A.P.; Pereira-Neves, A.; Benchimol, M.; Nobrega, A.; Bonomo, A. G-csf-induced suppressor il-10+ neutrophils promote regulatory t cells that inhibit graft-versus-host disease in a long-lasting and specific way. J. Immunol. 2016, 197, 3725–3734. [Google Scholar] [CrossRef] [Green Version]
- LaGory, E.L.; Giaccia, A.J. The ever-expanding role of hif in tumour and stromal biology. Nat. Cell Biol. 2016, 18, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Heuvers, M.E.; Muskens, F.; Bezemer, K.; Lambers, M.; Dingemans, A.C.; Groen, H.J.M.; Smit, E.F.; Hoogsteden, H.C.; Hegmans, J.; Aerts, J. Arginase-1 mrna expression correlates with myeloid-derived suppressor cell levels in peripheral blood of nsclc patients. Lung Cancer 2013, 81, 468–474. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Shi, M.; Chen, Y.; Yu, D.; Zhao, C.; Gu, Y.; Yang, B.; Guo, S.; Ding, G.; et al. Cd13(hi) neutrophil-like myeloid-derived suppressor cells exert immune suppression through arginase 1 expression in pancreatic ductal adenocarcinoma. Oncoimmunology 2017, 6, e1258504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gielen, P.R.; Schulte, B.M.; Kers-Rebel, E.D.; Verrijp, K.; Bossman, S.A.; Ter Laan, M.; Wesseling, P.; Adema, G.J. Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients with glioblastoma highly express s100a8/9 and arginase and suppress t cell function. Neuro-Oncol. 2016, 18, 1253–1264. [Google Scholar]
- Najjar, Y.G.; Rayman, P.; Jia, X.; Pavicic, P.G., Jr.; Rini, B.I.; Tannenbaum, C.; Ko, J.; Haywood, S.; Cohen, P.; Hamilton, T.; et al. Myeloid-derived suppressor cell subset accumulation in renal cell carcinoma parenchyma is associated with intratumoral expression of il1beta, il8, cxcl5, and mip-1alpha. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 2346–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.; Overman, M.J.; Boutin, A.T.; Shang, X.; Zhao, D.; Dey, P.; Li, J.; Wang, G.; Lan, Z.; Li, J.; et al. Kras-irf2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 2019, 35, 559–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talmadge, J.E.; Fidler, I.J. Aacr centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biological principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [Green Version]
- Tabaries, S.; Ouellet, V.; Hsu, B.E.; Annis, M.G.; Rose, A.A.; Meunier, L.; Carmona, E.; Tam, C.E.; Mes-Masson, A.M.; Siegel, P.M. Granulocytic immune infiltrates are essential for the efficient formation of breast cancer liver metastases. Breast Cancer Res. Bcr 2015, 17, 45. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, J.; Yang, L.; Li, J.; Wu, W.; Huang, M.; Lin, L.; Su, S. Tumor-contacted neutrophils promote metastasis by a cd90-timp-1 juxtacrine-paracrine loop. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 1957–1969. [Google Scholar] [CrossRef]
- Li, S.; Cong, X.; Gao, H.; Lan, X.; Li, Z.; Wang, W.; Song, S.; Wang, Y.; Li, C.; Zhang, H.; et al. Tumor-associated neutrophils induce emt by il-17a to promote migration and invasion in gastric cancer cells. J. Exp. Clin. Cancer Res. Cr 2019, 38, 6. [Google Scholar] [CrossRef] [Green Version]
- Strilic, B.; Offermanns, S. Intravascular survival and extravasation of tumor cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Seubert, B.; Grunwald, B.; Kobuch, J.; Cui, H.; Schelter, F.; Schaten, S.; Siveke, J.T.; Lim, N.H.; Nagase, H.; Simonavicius, N.; et al. Tissue inhibitor of metalloproteinases (timp)-1 creates a premetastatic niche in the liver through sdf-1/cxcr4-dependent neutrophil recruitment in mice. Hepatology 2015, 61, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, Y.; Han, Y.; Zhang, Q.; Jiang, Z.; Zhang, X.; Huang, B.; Xu, X.; Zheng, J.; Cao, X. Tumor exosomal rnas promote lung pre-metastatic niche formation by activating alveolar epithelial tlr3 to recruit neutrophils. Cancer Cell 2016, 30, 243–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.F.; Andzinski, L.; Kasnitz, N.; Kroger, A.; Klawonn, F.; Lienenklaus, S.; Weiss, S.; Jablonska, J. The lack of type i interferon induces neutrophil-mediated pre-metastatic niche formation in the mouse lung. Int. J. Cancer 2015, 137, 837–847. [Google Scholar] [CrossRef]
- Andzinski, L.; Wu, C.F.; Lienenklaus, S.; Kroger, A.; Weiss, S.; Jablonska, J. Delayed apoptosis of tumor associated neutrophils in the absence of endogenous ifn-beta. Int. J. Cancer 2015, 136, 572–583. [Google Scholar]
- Yan, H.H.; Pickup, M.; Pang, Y.; Gorska, A.E.; Li, Z.; Chytil, A.; Geng, Y.; Gray, J.W.; Moses, H.L.; Yang, L. Gr-1+cd11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010, 70, 6139–6149. [Google Scholar] [CrossRef] [Green Version]
- Spicer, J.D.; McDonald, B.; Cools-Lartigue, J.J.; Chow, S.C.; Giannias, B.; Kubes, P.; Ferri, L.E. Neutrophils promote liver metastasis via mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012, 72, 3919–3927. [Google Scholar] [CrossRef] [Green Version]
- Wieland, E.; Rodriguez-Vita, J.; Liebler, S.S.; Mogler, C.; Moll, I.; Herberich, S.E.; Espinet, E.; Herpel, E.; Menuchin, A.; Chang-Claude, J.; et al. Endothelial notch1 activity facilitates metastasis. Cancer Cell 2017, 31, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Rayes, R.F.; Vourtzoumis, P.; Bou Rjeily, M.; Seth, R.; Bourdeau, F.; Giannias, B.; Berube, J.; Huang, Y.-H.; Rousseau, S.; Camilleri-Broet, S.; et al. Neutrophil extracellular trap–associated ceacam1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. J. Immunol. 2020, 204, 2285–2294. [Google Scholar] [CrossRef]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 2016, 8, 361ra138. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Ko, S.Y.; Mohamed, M.S.; Kenny, H.A.; Lengyel, E.; Naora, H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 2019, 216, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Wu, X.; Lee, J.; Tan, M.; Hagenbeek, T.; Qu, X.; Yu, L.; Ross, J.; Korsisaari, N.; Cao, T.; et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of ly6g+ly6c+ granulocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 21248–21255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.; Li, Q.; Ferrara, N. Metastatic growth instructed by neutrophil-derived transferrin. Proc. Natl. Acad. Sci. USA 2018, 115, 11060–11065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, S.; Baba, T.; Muranaka, H.; Tanabe, Y.; Takahashi, C.; Matsugo, S.; Mukaida, N. Involvement of prokineticin 2-expressing neutrophil infiltration in 5-fluorouracil-induced aggravation of breast cancer metastasis to lung. Mol. Cancer Ther. 2018, 17, 1515–1525. [Google Scholar] [CrossRef]
- Wculek, S.K.; Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 2015, 528, 413–417. [Google Scholar] [CrossRef]
- Saini, M.; Szczerba, B.M.; Aceto, N. Circulating tumor cell-neutrophil tango along the metastatic process. Cancer Res. 2019, 79, 6067–6073. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. Nf-kappab, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Tessitore, A.; Alesse, E.; Capalbo, C.; Zazzeroni, F. Cancer secretome and inflammation: The bright and the dark sides of nf-kappab. Semin. Cell Dev. Biol. 2018, 78, 51–61. [Google Scholar] [CrossRef]
- Jing, H.; Lee, S. Nf-kappab in cellular senescence and cancer treatment. Mol. Cells 2014, 37, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Nishida, J.; Momoi, Y.; Miyakuni, K.; Tamura, Y.; Takahashi, K.; Koinuma, D.; Miyazono, K.; Ehata, S. Epigenetic remodelling shapes inflammatory renal cancer and neutrophil-dependent metastasis. Nat. Cell Biol. 2020, 22, 465–475. [Google Scholar] [CrossRef]
- Granot, Z.; Henke, E.; Comen, E.A.; King, T.A.; Norton, L.; Benezra, R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 2011, 20, 300–314. [Google Scholar] [CrossRef] [Green Version]
- Teng, T.S.; Ji, A.L.; Ji, X.Y.; Li, Y.Z. Neutrophils and immunity: From bactericidal action to being conquered. J. Immunol. Res. 2017, 2017, 9671604. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.W.; Karim, S.A.; Leach, J.D.G.; Bailey, P.; Upstill-Goddard, R.; Rishi, L.; Foth, M.; Bryson, S.; McDaid, K.; Wilson, Z.; et al. Cxcr2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 2016, 29, 832–845. [Google Scholar] [CrossRef] [Green Version]
- Taromi, S.; Kayser, G.; Catusse, J.; von Elverfeldt, D.; Reichardt, W.; Braun, F.; Weber, W.A.; Zeiser, R.; Burger, M. Cxcr4 antagonists suppress small cell lung cancer progression. Oncotarget 2016, 7, 85185–85195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.; Heishi, T.; Incio, J.; Huang, Y.; Beech, E.Y.; Pinter, M.; Ho, W.W.; Kawaguchi, K.; Rahbari, N.N.; Chung, E.; et al. Targeting cxcr4-dependent immunosuppressive ly6c(low) monocytes improves antiangiogenic therapy in colorectal cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 10455–10460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, B.; Redfern, A.D.; Mouchemore, K.A.; Hamilton, J.A.; Anderson, R.L. The dark side of granulocyte-colony stimulating factor: A supportive therapy with potential to promote tumour progression. Clin. Exp. Metastasis 2018, 35, 255–267. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Lee, J.; Lee, S.; Lawler, S. Role of tumor-associated neutrophils in regulation of tumor growth in lung cancer development: A mathematical model. PLoS ONE 2019, 14, e0211041. [Google Scholar] [CrossRef]
- Eruslanov, E.B.; Singhal, S.; Albelda, S.M. Mouse versus human neutrophils in cancer: A major knowledge gap. Trends Cancer 2017, 3, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Zöllner, O.; Lenter, M.C.; Blanks, J.E.; Borges, E.; Steegmaier, M.; Zerwes, H.G.; Vestweber, D. L-selectin from human, but not from mouse neutrophils binds directly to e-selectin. J. Cell Biol. 1997, 136, 707–716. [Google Scholar] [CrossRef]
- Rausch, P.G.; Moore, T.G. Granule enzymes of polymorphonuclear neutrophils: A phylogenetic comparison. Blood 1975, 46, 913–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Mouse | Human | |
|---|---|---|
| Pre-neutrophil | Lin−c-kitintCD11b+CXCR4+ | Lin−CD66+CD15+CD33med CD49dmedCD101− |
| Immature neutrophil | Lin−c-kit−CD11b+Ly6G+ CXCR4−CXCR2− | Lin−CD66+CD15+CD33med CD49d−CD101medCD10−CD16med |
| Mature neutrophil | Lin−c-kit−CD11b+Ly6G+ CXCR4−CXCR2+ | Lin−CD66+CD15+CD33med CD49d−CD101medCD10+CD16high |
| Mouse | Human | |
|---|---|---|
| Total MDSC | Gr-1+CD11b+ | Not clearly determined |
| PMN–MDSC | CD11b+Ly6ClowLy6G+ | CD14−CD11b+CD15+CD66b+ |
| monocytic MDSC | CD11b+Ly6ChighLy6G− | CD14+CD11b+CD15−HLA-DRlow |
| early MDSC | Not clearly determined | Lin−HLA-DR−CD33+ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukaida, N.; Sasaki, S.-i.; Baba, T. Two-Faced Roles of Tumor-Associated Neutrophils in Cancer Development and Progression. Int. J. Mol. Sci. 2020, 21, 3457. https://doi.org/10.3390/ijms21103457
Mukaida N, Sasaki S-i, Baba T. Two-Faced Roles of Tumor-Associated Neutrophils in Cancer Development and Progression. International Journal of Molecular Sciences. 2020; 21(10):3457. https://doi.org/10.3390/ijms21103457
Chicago/Turabian StyleMukaida, Naofumi, So-ichiro Sasaki, and Tomohisa Baba. 2020. "Two-Faced Roles of Tumor-Associated Neutrophils in Cancer Development and Progression" International Journal of Molecular Sciences 21, no. 10: 3457. https://doi.org/10.3390/ijms21103457
APA StyleMukaida, N., Sasaki, S. -i., & Baba, T. (2020). Two-Faced Roles of Tumor-Associated Neutrophils in Cancer Development and Progression. International Journal of Molecular Sciences, 21(10), 3457. https://doi.org/10.3390/ijms21103457