Butylparaben Is Toxic to Porcine Oocyte Maturation and Subsequent Embryonic Development Following In Vitro Fertilization
Abstract
:1. Introduction
2. Results
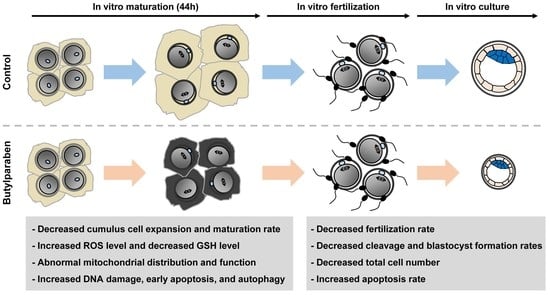
2.1. BP Treatment Impairs Meiotic Progression in Porcine Oocytes
2.2. BP Treatment during IVM Reduces the Developmental Competence of Porcine IVF Embryos
2.3. BP Treatment Negatively Affects Intracellular Levels of ROS and GSH in Porcine Oocytes
2.4. BP Treatment Interferes with Mitochondrial Organization and Function in Porcine Oocytes
2.5. BP Treatment Triggers DNA Damage, Early Apoptosis, and Autophagy in Porcine Oocytes
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Oocyte Collection and IVM
4.3. Treatment of Butylparaben
4.4. Assessment of Cumulus Cell Expansion
4.5. Assessment of Nuclear Maturation of Oocytes
4.6. IVF of Oocytes
4.7. Terminal Deoxynucleotidyl Transferase-Mediated dUTP-Digoxygenin Nick End-Labeling (TUNEL) Assay
4.8. Measurement of Intracellular ROS and GSH Levels
4.9. Assessment of Mitochondrial Distribution and Membrane Potential
4.10. Annexin-V Staining
4.11. Immunocytochemistry
4.12. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BP | butylparaben |
| ROS | reactive oxygen species |
| GSH | glutathione |
| IVM | in vitro maturation |
| IVF | in vitro fertilization |
| COCs | Cumulus-oocyte complexes |
| MII | metaphase II |
| ICM | inner cell mass |
| TE | trophectoderm |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated dUTP-digoxygenin nick end-labeling |
| LC3 | microtubule-associated protein light chain 3 |
| PMSG | pregnant mare serum gonadotropin |
| hCG | human chorionic gonadotropin |
| DMSO | dimethylsulfoxide |
| DPBS | Dulbecco’s phosphate-buffered saline |
| BSA | bovine serum albumin |
| mTBM | modified Tris-buffered medium |
| PVA | polyvinyl alcohol |
| RT | room temperature |
| SEM | standard error of the mean |
| ANOVA | analysis of variance |
References
- Cashman, A.L.; Warshaw, E.M. Parabens: A review of epidemiology, structure, allergenicity, and hormonal properties. Dermat. Contact Atopic Occup. Drug 2005, 16, 57–66. [Google Scholar]
- Ma, Y.; Marquis, R. Irreversible paraben inhibition of glycolysis by Streptococcus mutans GS-5. Lett. Appl. Microbiol. 1996, 23, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Freese, E.; Sheu, C.W.; Galliers, E. Function of Lipophilic Acids as Antimicrobial Food Additives. Nature 1973, 241, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Andersen, F.A. Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int. J. Toxicol. 2008, 27 (Suppl. 4), 1–82. [Google Scholar]
- Caon, T.; Costa, A.C.O.; De Oliveira, M.; Micke, G.A.; Simões, C.M.O. Evaluation of the transdermal permeation of different paraben combinations through a pig ear skin model. Int. J. Pharm. 2010, 391, 1–6. [Google Scholar] [CrossRef]
- Nagar, Y.; Thakur, R.S.; Parveen, T.; Patel, D.K.; Ram, K.R.; Satish, A. Toxicity assessment of parabens in Caenorhabditis elegans. Chemosphere 2020, 246, 125730. [Google Scholar] [CrossRef]
- Barr, L.; Metaxas, G.; Harbach, C.A.J.; Savoy, L.A.; Darbre, P. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J. Appl. Toxicol. 2012, 32, 219–232. [Google Scholar] [CrossRef]
- Hines, E.; Mendola, P.; Von Ehrenstein, O.S.; Ye, X.; Calafat, A.M.; Fenton, S.E. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod. Toxicol. 2014, 54, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Meeker, J.D.; Yang, T.C.; Ye, X.; Calafat, A.M.; Hauser, R. Urinary Concentrations of Parabens and Serum Hormone Levels, Semen Quality Parameters, and Sperm DNA Damage. Environ. Health Perspect. 2010, 119, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Sun, L.; Hu, Y.; Jiao, J.; Hu, J. Inverse antagonist activities of parabens on human oestrogen-related receptor gamma (ERRgamma): In vitro and in silico studies. Toxicol. Appl. Pharmacol. 2013, 270, 16–22. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107 (Suppl. 6), 907–938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, S.; Qiao, P.; Dong, L.; Yu, M.; Wang, C.; Zhang, M.; Zhang, L.; Li, Y.; Tang, N.; et al. n-butylparaben induces male reproductive disorders via regulation of estradiol and estrogen receptors. J. Appl. Toxicol. 2016, 36, 1223–1234. [Google Scholar] [CrossRef]
- Oishi, S. Effects of butylparaben on the male reproductive system in rats. Toxicol. Ind. Health 2001, 17, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hoberman, A.; Schreur, D.K.; Leazer, T.; Daston, G.P.; Carthew, P.; Re, T.; Loretz, L.; Mann, P. Lack of effect of butylparaben and methylparaben on the reproductive system in male rats. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2008, 83, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Lemini, C.; Hernández, A.; Jaimez, R.; Franco, Y.; E Avila, M.; Castell, A. Morphometric analysis of mice uteri treated with the preservatives methyl, ethyl, propyl, and butylparaben. Toxicol. Ind. Health 2004, 20, 123–132. [Google Scholar] [CrossRef]
- Vo, T.T.; Yoo, Y.-M.; Choi, K.-C.; Jeung, E.-B. Potential estrogenic effect(s) of parabens at the prepubertal stage of a postnatal female rat model. Reprod. Toxicol. 2010, 29, 306–316. [Google Scholar] [CrossRef]
- Ahn, H.-J.; An, B.-S.; Jung, E.-M.; Yang, H.; Choi, K.-C.; Jeung, E.-B. Parabens inhibit the early phase of folliculogenesis and steroidogenesis in the ovaries of neonatal rats. Mol. Reprod. Dev. 2012, 79, 626–636. [Google Scholar] [CrossRef]
- Martignoni, M.; Groothuis, G.M.M.; De Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef]
- Schatten, H.; Sun, Q.-Y. Centrosome dynamics during mammalian oocyte maturation with a focus on meiotic spindle formation. Mol. Reprod. Dev. 2011, 78, 757–768. [Google Scholar] [CrossRef]
- Brevini, T.A.L.; Vassena, R.; Francisci, C.; Gandolfi, F. Role of Adenosine Triphosphate, Active Mitochondria, and Microtubules in the Acquisition of Developmental Competence of Parthenogenetically Activated Pig Oocytes1. Boil. Reprod. 2005, 72, 1218–1223. [Google Scholar] [CrossRef] [Green Version]
- Calafat, A.M.; Ye, X.; Wong, L.-Y.; Bishop, A.M.; Needham, L.L. Urinary Concentrations of Four Parabens in the U.S. Population: NHANES 2005–2006. Environ. Health Perspect. 2010, 118, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Craig, Z.; Ziv-Gal, A. Pretty Good or Pretty Bad? The Ovary and Chemicals in Personal Care Products. Toxicol. Sci. 2017, 162, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, F.; Jiao, X.; Chen, F.; Zhang, X.; Duan, Z.; Ding, Z.; Wu, D.; Wang, Y.; Zhang, S.; Miao, Y.; et al. Isobutylparaben Negatively Affects Porcine Oocyte Maturation Through Increasing Oxidative Stress and Cytoskeletal Abnormalities. Environ. Mol. Mutagen. 2020, 61, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Russell, P.T.; Larsen, W.J. Functional significance of cumulus expansion in the mouse: Roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol. Reprod. Dev. 1993, 34, 87–93. [Google Scholar] [CrossRef]
- Tanghe, S.; Van Soom, A.; Nauwynck, H.; Coryn, M.; De Kruif, A. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol. Reprod. Dev. 2002, 61, 414–424. [Google Scholar] [CrossRef]
- Palta, P.; Chauhan, M.S. Laboratory production of buffalo (Bubalus bubalis) embryos. Reprod. Fertil. Dev. 1998, 10, 379–392. [Google Scholar] [CrossRef]
- De Matos, D.; Furnus, C.C. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development: Effect of β-mercaptoethanol, cysteine and cystine. Theriogenology 2000, 53, 761–771. [Google Scholar] [CrossRef]
- Hashimoto, S.; Minami, N.; Takakura, R.; Yamada, M.; Imai, H.; Kashima, N. Low oxygen tension during in vitro maturation is beneficial for supporting the subsequent development of bovine cumulus-oocyte complexes. Mol. Reprod. Dev. 2000, 57, 353–360. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M. Role of glutathione in the maturation and fertilization of pig oocytes in vitro. Mol. Reprod. Dev. 1993, 35, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.H.; Verma, R.J. Butyl p-hydroxybenzoic acid induces oxidative stress in mice liver—An in vivo study. Acta Pol. Pharm. Drug Res. 2011, 68, 875–879. [Google Scholar]
- Yang, C.; Lim, W.; Bazer, F.W.; Song, G. Butyl paraben promotes apoptosis in human trophoblast cells through increased oxidative stress-induced endoplasmic reticulum stress. Environ. Toxicol. 2018, 33, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jee, B.C. Effects of Butylparaben Supplementation on In Vitro Development of Mouse Preantral Follicle. Reprod. Sci. 2020, 27, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-H.; Liang, S.; Kim, S.-H.; Cui, X.-S.; Kim, N.-H. Fe(III) Is Essential for Porcine Embryonic Development via Mitochondrial Function Maintenance. PLoS ONE 2015, 10, e0130791. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.K.; Lane, S.I.R.; Merriman, J.A.; Jones, K. DNA damage induces a meiotic arrest in mouse oocytes mediated by the spindle assembly checkpoint. Nat. Commun. 2015, 6, 8553. [Google Scholar] [CrossRef]
- Kuo, L.J.; Yang, L.-X. Gamma-H2AX—A novel biomarker for DNA double-strand breaks. In Vivo 2008, 22, 305–309. [Google Scholar]
- Riad, M.A.; Abd-Rabo, M.M.; El Aziz, S.A.A.; El Behairy, A.M.; Badawy, M.M. Reproductive toxic impact of subchronic treatment with combined butylparaben and triclosan in weanling male rats. J. Biochem. Mol. Toxicol. 2018, 32, e22037. [Google Scholar] [CrossRef]
- Chaube, S.K.; Shrivastav, T.G.; Tiwari, M.; Prasad, S.; Tripathi, A.; Pandey, A. Neem (Azadirachta indica L.) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. SpringerPlus 2014, 3, 464. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Wang, Q.-C.; Chen, K.-L.; Zhu, C.-C.; Liu, J.; Sun, S.-C. Acrylamide toxic effects on mouse oocyte quality and fertility in vivo. Sci. Rep. 2015, 5, 11562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.-S.; Kim, J.-S.; Kim, Y.-H.; Sim, B.-W.; Yoon, S.-B.; Cha, J.-J.; Choi, S.-A.; Yang, H.-J.; Mun, S.-E.; Park, Y.-H.; et al. Induction of autophagy during in vitro maturation improves the nuclear and cytoplasmic maturation of porcine oocytes. Reprod. Fertil. Dev. 2014, 26, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Park, J.-H.; Kim, E.-Y.; Ko, J.-J.; Park, K.-S.; Lee, K.-A. The role of Rad51 in safeguarding mitochondrial activity during the meiotic cell cycle in mammalian oocytes. Sci. Rep. 2016, 6, 34110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderhyden, B.C.; Caron, P.J.; Buccione, R.; Eppig, J.J. Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev. Boil. 1990, 140, 307–317. [Google Scholar] [CrossRef]
- Lee, S.; Jin, J.; Taweechaipaisankul, A.; Kim, G.A.; Ahn, C.; Lee, B.C. Melatonin influences the sonic hedgehog signaling pathway in porcine cumulus oocyte complexes. J. Pineal Res. 2017, 63, e12424. [Google Scholar] [CrossRef]
- Lee, S.; Jin, J.; Taweechaipaisankul, A.; Kim, G.A.; Ahn, C.; Lee, B.C. Sonic hedgehog signaling mediates resveratrol to improve maturation of pig oocytes in vitro and subsequent preimplantation embryo development. J. Cell. Physiol. 2018, 233, 5023–5033. [Google Scholar] [CrossRef]
- Jeong, P.-S.; Yoon, S.-B.; Lee, M.-H.; Son, H.-C.; Lee, H.-Y.; Lee, S.; Koo, B.-S.; Jeong, K.-J.; Lee, J.-H.; Jin, Y.B.; et al. Embryo aggregation regulates in vitro stress conditions to promote developmental competence in pigs. PeerJ 2019, 7, e8143. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, P.-S.; Lee, S.; Park, S.-H.; Kim, M.J.; Kang, H.-G.; Nanjidsuren, T.; Son, H.-C.; Song, B.-S.; Koo, D.-B.; Sim, B.-W.; et al. Butylparaben Is Toxic to Porcine Oocyte Maturation and Subsequent Embryonic Development Following In Vitro Fertilization. Int. J. Mol. Sci. 2020, 21, 3692. https://doi.org/10.3390/ijms21103692
Jeong P-S, Lee S, Park S-H, Kim MJ, Kang H-G, Nanjidsuren T, Son H-C, Song B-S, Koo D-B, Sim B-W, et al. Butylparaben Is Toxic to Porcine Oocyte Maturation and Subsequent Embryonic Development Following In Vitro Fertilization. International Journal of Molecular Sciences. 2020; 21(10):3692. https://doi.org/10.3390/ijms21103692
Chicago/Turabian StyleJeong, Pil-Soo, Sanghoon Lee, Soo-Hyun Park, Min Ju Kim, Hyo-Gu Kang, Tsevelmaa Nanjidsuren, Hee-Chang Son, Bong-Seok Song, Deog-Bon Koo, Bo-Woong Sim, and et al. 2020. "Butylparaben Is Toxic to Porcine Oocyte Maturation and Subsequent Embryonic Development Following In Vitro Fertilization" International Journal of Molecular Sciences 21, no. 10: 3692. https://doi.org/10.3390/ijms21103692
APA StyleJeong, P. -S., Lee, S., Park, S. -H., Kim, M. J., Kang, H. -G., Nanjidsuren, T., Son, H. -C., Song, B. -S., Koo, D. -B., Sim, B. -W., & Kim, S. -U. (2020). Butylparaben Is Toxic to Porcine Oocyte Maturation and Subsequent Embryonic Development Following In Vitro Fertilization. International Journal of Molecular Sciences, 21(10), 3692. https://doi.org/10.3390/ijms21103692