Connexin-Mediated Signaling at the Immunological Synapse
Abstract
:1. Introduction
2. Cx43-Mediated Signaling at the DC-T Cell IS
3. Cx43 Channels also Impact DC-Mediated T Cell Activation by Amplifying Antigen Cross-Presentation Pathways
4. Cx43-Mediated Signaling at the CIS
5. Cx43 Interactome Reveals Multiple Proteins Associated with the IS
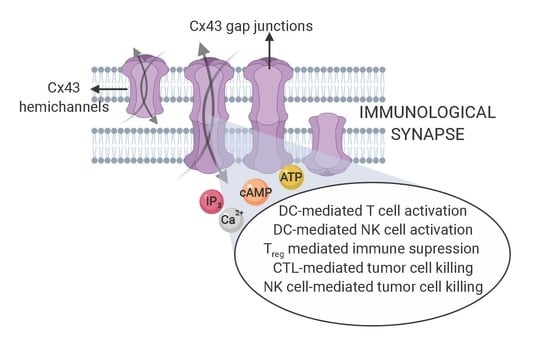
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Grakoui, A.; Bromley, S.K.; Sumen, C.; Davis, M.M.; Shaw, A.S.; Allen, P.M.; Dustin, M.L. The immunological synapse: A molecular machine controlling T cell activation. Science 1999, 285, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Brilot, F.; Strowig, T.; Roberts, S.M.; Arrey, F.; Münz, C. NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Ralpha. J. Clin. Investig. 2007, 117, 3316–3329. [Google Scholar] [CrossRef]
- Liu, D.; Bryceson, Y.T.; Meckel, T.; Vasiliver-Shamis, G.; Dustin, M.L.; Long, E.O. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity 2009, 31, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Goodridge, H.S.; Reyes, C.N.; Becker, C.A.; Katsumoto, T.R.; Ma, J.; Wolf, A.J.; Bose, N.; Chan, A.S.; Magee, A.S.; Danielson, M.E.; et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 2011, 472, 471–475. [Google Scholar] [CrossRef]
- Carroll-Portillo, A.; Spendier, K.; Pfeiffer, J.; Griffiths, G.; Li, H.; Lidke, K.A.; Oliver, J.M.; Lidke, D.S.; Thomas, J.L.; Wilson, B.S.; et al. Formation of a mast cell synapse: Fc epsilon RI membrane dynamics upon binding mobile or immobilized ligands on surfaces. J. Immunol. 2010, 184, 1328–1338. [Google Scholar] [CrossRef] [Green Version]
- Yuseff, M.I.; Reversat, A.; Lankar, D.; Diaz, J.; Fanget, I.; Pierobon, P.; Randrian, V.; Larochette, N.; Vascotto, F.; Desdouets, C.; et al. Polarized secretion of lysosomes at the B cell synapse couples antigen extraction to processing and presentation. Immunity 2011, 35, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.G.; Arvey, A.; Jin, W.; Rudensky, A.Y. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 2014, 15, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Tato, C.M.; Davis, M.M. How the immune system talks to itself: The varied role of synapses. Immunol. Rev. 2013, 251, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Monks, C.R.; Freiberg, B.A.; Kupfer, H.; Sciaky, N.; Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998, 395, 82–86. [Google Scholar] [CrossRef]
- Freiberg, B.A.; Kupfer, H.; Maslanik, W.; Delli, J.; Kappler, J.; Zaller, D.M.; Kupfer, A. Staging and resetting T cell activation in SMACs. Nat. Immunol. 2002, 3, 911–917. [Google Scholar] [CrossRef]
- Dustin, M.L.; Long, E.O. Cytotoxic immunological synapses. Immunol. Rev. 2010, 235, 24–34. [Google Scholar] [CrossRef]
- Basu, R.; Huse, M. Mechanical Communication at the Immunological Synapse. Trends. Cell. Biol. 2017, 27, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Naranjo, A.; Bouma, G.; Pereda, C.; Ramírez, M.; Webb, K.F.; Tittarelli, A.; López, M.N.; Kalergis, A.M.; Thrasher, A.J.; Becker, D.L.; et al. Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J. Immunol. 2011, 187, 3121–3132. [Google Scholar] [CrossRef]
- Segretain, D.; Falk, M.M. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim. Biophys. Acta 2004, 1662, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Sáez, P.J.; Shoji, K.F.; Aguirre, A.; Sáez, J.C. Regulation of hemichannels and gap junction channels by cytokines in antigen-presenting cells. Mediat. Inflamm. 2014, 2014, 742734. [Google Scholar] [CrossRef] [Green Version]
- Neijssen, J.; Pang, B.; Neefjes, J. Gap junction-mediated intercellular communication in the immune system. Prog. Biophys. Mol. Biol. 2007, 94, 207–218. [Google Scholar] [CrossRef]
- Gleisner, M.A.; Navarrete, M.; Hofmann, F.; Salazar-Onfray, F.; Tittarelli, A. Mind the Gaps in Tumor Immunity: Impact of Connexin-Mediated Intercellular Connections. Front. Immunol. 2017, 8, 1067. [Google Scholar] [CrossRef] [Green Version]
- Valdebenito, S.; Barreto, A.; Eugenin, E.A. The role of connexin and pannexin containing channels in the innate and acquired immune response. Biochim. Biophys. Acta Biomembr. 2018, 1860, 154–165. [Google Scholar] [CrossRef]
- Oviedo-Orta, E.; Hoy, T.; Evans, W.H. Intercellular communication in the immune system: Differential expression of connexin40 and 43, and perturbation of gap junction channel functions in peripheral blood and tonsil human lymphocyte subpopulations. Immunology 2000, 99, 578–590. [Google Scholar] [CrossRef]
- Fonseca, P.C.; Nihei, O.K.; Urban-Maldonado, M.; Abreu, S.; de Carvalho, A.C.; Spray, D.C.; Savino, W.; Alves, L.A. Characterization of connexin 30.3 and 43 in thymocytes. Immunol. Lett. 2004, 94, 65–75. [Google Scholar] [CrossRef]
- Schajnovitz, A.; Itkin, T.; D’Uva, G.; Kalinkovich, A.; Golan, K.; Ludin, A.; Cohen, D.; Shulman, Z.; Avigdor, A.; Nagler, A.; et al. CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat. Immunol. 2011, 12, 391–398. [Google Scholar] [CrossRef]
- Hills, C.; Price, G.W.; Wall, M.J.; Kaufmann, T.J.; Chi-Wai Tang, S.; Yiu, W.H.; Squires, P.E. Transforming Growth Factor Beta 1 Drives a Switch in Connexin Mediated Cell-to-Cell Communication in Tubular Cells of the Diabetic Kidney. Cell Physiol. Biochem. 2018, 45, 2369–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oviedo-Orta, E.; Evans, W.H. Gap junctions and connexins: Potential contributors to the immunological synapse. J. Leukoc. Biol. 2002, 72, 636–642. [Google Scholar] [PubMed]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Yatim, N.; Cullen, S.; Albert, M.L. Dying cells actively regulate adaptive immune responses. Nat. Rev. Immunol. 2017, 17, 262–275. [Google Scholar] [CrossRef]
- Junger, W.G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Kummerow, C.; Junker, C.; Kruse, K.; Rieger, H.; Quintana, A.; Hoth, M. The immunological synapse controls local and global calcium signals in T lymphocytes. Immunol. Rev. 2009, 231, 132–147. [Google Scholar] [CrossRef]
- Trebak, M.; Kinet, J.P. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef]
- Baranova, A.; Ivanov, D.; Petrash, N.; Pestova, A.; Skoblov, M.; Kelmanson, I.; Shagin, D.; Nazarenko, S.; Geraymovych, E.; Litvin, O.; et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 2004, 83, 706–716. [Google Scholar] [CrossRef]
- Penuela, S.; Bhalla, R.; Gong, X.Q.; Cowan, K.N.; Celetti, S.J.; Cowan, B.J.; Bai, D.; Shao, Q.; Laird, D.W. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 2007, 120, 3772–3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenk, U.; Westendorf, A.M.; Radaelli, E.; Casati, A.; Ferro, M.; Fumagalli, M.; Verderio, C.; Buer, J.; Scanziani, E.; Grassi, F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal. 2008, 1, ra6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woehrle, T.; Yip, L.; Elkhal, A.; Sumi, Y.; Chen, Y.; Yao, Y.; Insel, P.A.; Junger, W.G. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 2010, 116, 3475–3484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sáez, P.J.; Vargas, P.; Shoji, K.F.; Harcha, P.A.; Lennon-Duménil, A.M.; Sáez, J.C. ATP promotes the fast migration of dendritic cells through the activity of pannexin 1 channels and P2X7 receptors. Sci. Signal. 2017, 10, eaah7107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manohar, M.; Hirsh, M.I.; Chen, Y.; Woehrle, T.; Karande, A.A.; Junger, W.G. ATP release and autocrine signaling through P2X4 receptors regulate γδ T cell activation. J. Leukoc. Biol. 2012, 92, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, A.; Tsukimoto, M.; Harada, H.; Moriyama, Y.; Kojima, S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J. Biol. Chem. 2010, 285, 17406–17416. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Naranjo, A.; Sáez, P.J.; Johansson, C.C.; Ramírez, M.; Mandakovic, D.; Pereda, C.; López, M.N.; Kiessling, R.; Sáez, J.C.; Salazar-Onfray, F. Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. J. Immunol. 2007, 178, 6949–6957. [Google Scholar] [CrossRef] [Green Version]
- Oviedo-Orta, E.; Perreau, M.; Evans, W.H.; Potolicchio, I. Control of the proliferation of activated CD4+ T cells by connexins. J. Leukoc. Biol. 2010, 88, 79–86. [Google Scholar] [CrossRef]
- Ni, X.; Wang, A.; Zhang, L.; Shan, L.Y.; Zhang, H.C.; Li, L.; Si, J.Q.; Luo, J.; Li, X.Z.; Ma, K.T. Up-regulation of gap junction in peripheral blood T lymphocytes contributes to the inflammatory response in essential hypertension. PLoS ONE 2017, 12, e0184773. [Google Scholar] [CrossRef] [Green Version]
- Elgueta, R.; Tobar, J.A.; Shoji, K.F.; De Calisto, J.; Kalergis, A.M.; Bono, M.R.; Rosemblatt, M.; Saez, J.C. Gap junctions at the dendritic cell-T cell interface are key elements for antigen-dependent T cell activation. J. Immunol. 2009, 183, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kurlander, R.J. Comparison of anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for expanding human T cells: Differing impact on CD8 T cell phenotype and responsiveness to restimulation. J. Transl. Med. 2010, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Leybaert, L.; Sanderson, M.J. Intercellular Ca(2+) waves: Mechanisms and function. Physiol. Rev. 2012, 92, 1359–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazhin, A.V.; Kahnert, S.; Kimpfler, S.; Schadendorf, D.; Umansky, V. Distinct metabolism of cyclic adenosine monophosphate in regulatory and helper CD4+ T cells. Mol. Immunol. 2010, 47, 678–684. [Google Scholar] [CrossRef]
- Bopp, T.; Becker, C.; Klein, M.; Klein-Heßling, S.; Palmetshofer, A.; Serfling, E.; Heib, V.; Becker, M.; Kubach, J.; Schmitt, S.; et al. Cyclic adenosine monophosphate is a key component of regulatory T cell–mediated suppression. J. Exp. Med. 2007, 204, 1303–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehbi, V.L.; Taskén, K. Molecular mechanisms for cAMP-mediated immunoregulation in T cells-Role of anchored protein kinase A signaling units. Front. Immunol. 2016, 7, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadokoro, C.E.; Shakhar, G.; Shen, S.; Ding, Y.; Lino, A.C.; Maraver, A.; Lafaille, J.J.; Dustin, M.L. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med. 2006, 203, 505–511. [Google Scholar] [CrossRef]
- Weber, M.; Lupp, C.; Stein, P.; Kreft, A.; Bopp, T.; Wehler, T.C.; Schmitt, E.; Schild, H.; Radsak, M.P. Mechanisms of cyclic nucleotide phosphodiesterases in modulating T cell responses in murine graft-versus-host disease. PLoS ONE 2013, 8, e58110. [Google Scholar] [CrossRef]
- Kuczma, M.; Wang, C.Y.; Ignatowicz, L.; Gourdie, R.; Kraj, P. Altered connexin 43 expression underlies age-dependent decrease of regulatory T cell suppressor function in nonobese diabetic mice. J. Immunol. 2015, 194, 5261–5271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhett, J.M.; Jourdan, J.; Gourdie, R.G. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol. Biol. Cell. 2011, 22, 1516–1528. [Google Scholar] [CrossRef]
- Luckey, U.; Schmidt, T.; Pfender, N.; Romer, M.; Lorenz, N.; Martin, S.F.; Bopp, T.; Schmitt, E.; Nikolaev, A.; Yogev, N.; et al. Crosstalk of regulatory T cells and tolerogenic dendritic cells prevents contact allergy in subjects with low zone tolerance. J. Allergy Clin. Immunol. 2012, 130, 781–797. [Google Scholar] [CrossRef]
- Ring, S.; Karakhanova, S.; Johnson, T.; Enk, A.H.; Mahnke, K. Gap junctions between regulatory T cells and dendritic cells prevent sensitization of CD8(+) T cells. J. Allergy Clin. Immunol. 2010, 125, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, M.E.; Rueda, C.M.; Rusie, L.K.; Chougnet, C.A. Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood 2011, 117, 5372–5380. [Google Scholar] [CrossRef] [PubMed]
- Matsue, H.; Yao, J.; Matsue, K.; Nagasaka, A.; Sugiyama, H.; Aoki, R.; Kitamura, M.; Shimada, S. Gap junction-mediated intercellular communication between dendritic cells (DCs) is required for effective activation of DCs. J. Immunol. 2006, 176, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruez, R.; Dubrot, J.; Zoso, A.; Bacchetta, M.; Molica, F.; Hugues, S.; Kwak, B.R.; Chanson, M. Dendritic Cell Migration Toward CCL21 Gradient Requires Functional Cx43. Front. Physiol. 2018, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Neijssen, J.; Herberts, C.; Drijfhout, J.W.; Reits, E.; Janssen, L.; Neefjes, J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature 2005, 434, 83–88. [Google Scholar] [CrossRef]
- Benlalam, H.; Jalil, A.; Hasmim, M.; Pang, B.; Tamouza, R.; Mitterrand, M.; Godet, Y.; Lamerant, N.; Robert, C.; Avril, M.F.; et al. Gap junction communication between autologous endothelial and tumor cells induce cross-recognition and elimination by specific CTL. J. Immunol. 2009, 182, 2654–2664. [Google Scholar] [CrossRef] [Green Version]
- Saccheri, F.; Pozzi, C.; Avogadri, F.; Barozzi, S.; Faretta, M.; Fusi, P.; Rescigno, M. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci. Transl. Med. 2010, 2, 44ra57. [Google Scholar] [CrossRef]
- Huang, M.N.; Nicholson, L.T.; Batich, K.A.; Swartz, A.M.; Kopin, D.; Wellford, S.; Prabhakar, V.K.; Woroniecka, K.; Nair, S.K.; Fecci, P.E.; et al. Antigen-loaded monocyte administration induces potent therapeutic antitumor T cell responses. J. Clin. Investig. 2020, 130, 774–788. [Google Scholar] [CrossRef]
- Pang, B.; Neijssen, J.; Qiao, X.; Janssen, L.; Janssen, H.; Lippuner, C.; Neefjes, J. Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J. Immunol. 2009, 183, 1083–1090. [Google Scholar] [CrossRef] [Green Version]
- Mazzini, E.; Massimiliano, L.; Penna, G.; Rescigno, M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity 2014, 40, 248–261. [Google Scholar] [CrossRef] [Green Version]
- Tittarelli, A.; Mendoza-Naranjo, A.; Farías, M.; Guerrero, I.; Ihara, F.; Wennerberg, E.; Riquelme, S.; Gleisner, A.; Kalergis, A.; Lundqvist, A.; et al. Gap junction intercellular communications regulate NK cell activation and modulate NK cytotoxic capacity. J. Immunol. 2014, 192, 1313–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keefe, D.; Shi, L.; Feske, S.; Massol, R.; Navarro, F.; Kirchhausen, T.; Lieberman, J. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity 2005, 23, 249–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery, J.; Keefe, D.; Saarian, S.; Martinvalet, D.; Walch, M.; Boucrot, E.; Kirchhausen, T.; Lieberman, J. Perforin activates clathrin- and dynamin-dependent endocytosis, which is required for plasma membrane repair and delivery of granzyme B for granzyme-mediated apoptosis. Blood 2010, 115, 1582–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery, J.; Keefe, D.; Boulant, S.; Boucrot, E.; Walch, M.; Martinvalet, D.; Goping, I.S.; Bleackley, R.C.; Kirchhausen, T.; Lieberman, J. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat. Immunol. 2011, 12, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Tittarelli, A.; Janji, B.; Van Moer, K.; Noman, M.Z.; Chouaib, S. The selective degradation of synaptic connexin 43 protein by hypoxia-induced autophagy impairs natural killer cell-mediated tumor cell killing. J. Biol. Chem. 2015, 290, 23670–23679. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, F.; Navarrete, M.; Álvarez, J.; Guerrero, I.; Gleisner, M.A.; Tittarelli, A.; Salazar-Onfray, F. Cx43-Gap Junctions Accumulate at the Cytotoxic Immunological Synapse Enabling Cytotoxic T Lymphocyte Melanoma Cell Killing. Int. J. Mol. Sci. 2019, 20, 4509. [Google Scholar] [CrossRef] [Green Version]
- Davenport, A.J.; Cross, R.S.; Watson, K.A.; Liao, Y.; Shi, W.; Prince, H.M.; Beavis, P.A.; Trapani, J.A.; Kershaw, M.H.; Ritchie, D.S.; et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl. Acad. Sci. USA 2018, 115, E2068–E2076. [Google Scholar] [CrossRef] [Green Version]
- Leithe, E.; Mesnil, M.; Aasen, T. The connexin 43 C-terminus: A tail of many tales. Biochim. Biophys. Acta Biomembr. 2018, 1860, 48–64. [Google Scholar] [CrossRef]
- Machtaler, S.; Dang-Lawson, M.; Choi, K.; Jang, C.; Naus, C.C.; Matsuuchi, L. The gap junction protein Cx43 regulates B-lymphocyte spreading and adhesion. J. Cell Sci. 2011, 124, 2611–2621. [Google Scholar] [CrossRef] [Green Version]
- Martín-Cófreces, N.B.; Sánchez-Madrid, F. Sailing to and Docking at the Immune Synapse: Role of Tubulin Dynamics and Molecular Motors. Front. Immunol. 2018, 9, 1174. [Google Scholar] [CrossRef]
- Singh, D.; Lampe, P.D. Identification of connexin-43 interacting proteins. Cell. Commun. Adhes. 2003, 10, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tello-Lafoz, M.; Martínez-Martínez, G.; Rodríguez-Rodríguez, C.; Albar, J.P.; Huse, M.; Gharbi, S.; Merida, I. Sorting nexin 27 interactome in T-lymphocytes identifies zona occludens-2 dynamic redistribution at the immune synapse. Traffic 2017, 18, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Suzuki, R.; Furuno, T.; Teshima, R.; Nakanishi, M. N-cadherin plays a role in the synapse-like structures between mast cells and neurites. Biol. Pharm. Bull. 2004, 27, 1891–1894. [Google Scholar] [CrossRef] [Green Version]
- Combs, J.; Kim, S.J.; Tan, S.; Ligon, L.A.; Holzbaur, E.L.; Kuhn, J.; Poenie, M. Recruitment of dynein to the Jurkat immunological synapse. Proc. Natl. Acad. Sci. USA 2006, 103, 14883–14888. [Google Scholar] [CrossRef] [Green Version]
- Heissmeyer, V.; Macián, F.; Im, S.H.; Varma, R.; Feske, S.; Venuprasad, K.; Gu, H.; Liu, Y.C.; Dustin, M.L.; Rao, A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 2004, 5, 255–265. [Google Scholar] [CrossRef]
- González-Granado, J.M.; Silvestre-Roig, C.; Rocha-Perugini, V.; Trigueros-Motos, L.; Cibrián, D.; Morlino, G.; Blanco-Berrocal, M.; Osorio, F.G.; Freije, J.; López-Otín, C.; et al. Nuclear envelope lamin-A couples actin dynamics with immunological synapse architecture and T cell activation. Sci. Signal. 2014, 7, ra37. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Molldrem, J.J.; Ma, Q. LFA-1 regulates CD8+ T cell activation via T cell receptor-mediated and LFA-1-mediated Erk1/2 signal pathways. J. Biol. Chem. 2009, 284, 21001–21010. [Google Scholar] [CrossRef] [Green Version]
- Zyss, D.; Ebrahimi, H.; Gergely, F. Casein kinase I delta controls centrosome positioning during T cell activation. J. Cell Biol. 2011, 195, 781–797. [Google Scholar] [CrossRef]
- Gomez, T.S.; Hamann, M.J.; McCarney, S.; Savoy, D.N.; Lubking, C.M.; Heldebrant, M.P.; Labno, C.M.; McKean, D.J.; McNiven, M.A.; Burkhardt, J.K.; et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat. Immunol. 2005, 6, 261–270. [Google Scholar] [CrossRef]
- Lasserre, R.; Alcover, A. Microtubule dynamics and signal transduction at the immunological synapse: New partners and new connections. EMBO J. 2012, 31, 4100–4102. [Google Scholar] [CrossRef] [Green Version]
- Calabia-Linares, C.; Robles-Valero, J.; de la Fuente, H.; Perez-Martinez, M.; Martín-Cofreces, N.; Alfonso-Pérez, M.; Gutierrez-Vázquez, C.; Mittelbrunn, M.; Ibiza, S.; Urbano-Olmos, F.R.; et al. Endosomal clathrin drives actin accumulation at the immunological synapse. J. Cell Sci. 2011, 124, 820–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onnis, A.; Baldari, C.T. Orchestration of Immunological Synapse Assembly by Vesicular Trafficking. Front. Cell Dev. Biol. 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dustin, M.L. Coordination of T cell activation and migration through formation of the immunological synapse. Ann. N. Y. Acad. Sci. 2003, 987, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Nath, S.; Christian, L.; Tan, S.Y.; Ki, S.; Ehrlich, L.I.; Poenie, M. Dynein Separately Partners with NDE1 and Dynactin to Orchestrate T Cell Focused Secretion. J. Immunol. 2016, 197, 2090–2101. [Google Scholar] [CrossRef]




| Protein | Annotation | Score | Reference |
|---|---|---|---|
| TJP1 | Tight junction protein-1 (zonula occludens-1; ZO-1). | 0.994 | |
| CDH2 | Cadherin 2, type 1, N-cadherin. Neuroimmune synapses with mast cells that involve N-cadherin expression on the mast cells may be important in allergy. | 0.99 | [73] |
| CTNNB1 | Catenin (cadherin-associated protein), beta 1. Key downstream component of the canonical Wnt signaling pathway. Serves as anchor protein at actin-rich adherent junctions and at the IS. | 0.964 | [74] |
| JUP | Junction plakoglobin. Catenin (cadherin-associated protein), gamma. | 0.947 | |
| NEDD4 | E3 ubiquitin-protein ligase. Involved in the proteolytic degradation of IS signaling proteins (PLC-γ1 and PKC-θ), from T cell-APC contact membrane in T cell anergy. | 0.947 | [75] |
| CTNND1 | Catenin (cadherin-associated protein), delta 1. | 0.927 | |
| MAPK3 | Mitogen-activated protein kinase 3 (ERK1). Serine/threonine kinase which acts as an essential component of the MAP kinase signal transduction pathway. Mediates the signaling of lamin-A-dependent F-actin-mediated IS formation and T cell activation. Mediates LFA-1-dependent TCR activation in CD8+ T cells. | 0.938 | [76,77] |
| CTNNA1 | Catenin (cadherin-associated protein), alpha 1. | 0.934 | |
| MAPK1 | Mitogen-activated protein kinase 1 (ERK2). Serine/threonine kinase which acts as an essential component of the MAP kinase signal transduction pathway. Mediates the signaling of lamin-A-dependent F-actin-mediated IS formation and T cell activation. | 0.985 | [76,77] |
| LEF1 | Lymphoid enhancer-binding factor 1. | 0.922 | |
| CSNK1D | Casein kinase 1, delta (CKIδ). Phosphorylates Cx43/GJA1, MAP1A, SNAPIN, MAPT/TAU, TOP2A, DCK, HIF1A, EIF6, p53/TP53, DVL2, DVL3, ESR1, AIB1/NCOA3, DNMT1, PKD2, YAP1, PER1 and PER2. Controls centrosome recruitment to the IS during T cell activation. | 0.922 | [78] |
| RIC1 | RAB6A-GEF complex partner protein 1. Required for phosphorylation and localization of Cx43. | 0.922 | |
| DAB2 | Disabled homolog 2; Adapter protein that functions as clathrin-associated sorting protein required for clathrin-mediated endocytosis of selected cargo proteins. | 0.92 | |
| DNM2 | Dynamin-2. Regulates T cell activation by controlling actin polymerization at the IS. | 0.919 | [79] |
| MAPRE1 | Microtubule-associated protein, RP/EB family, member 1 (EB1). Binds to the microtubules plus-end and regulates dynamics of microtubule cytoskeleton. CKIδ/EB1 contributes to the increase in microtubule growth speeds in polarized T cells and to centrosome recruitment to the IS during T cell activation. Mediates the organization of an IS fully functional to transduce activation signals. | 0.916 | [78,80] |
| CLTC | Clathrin heavy chain 1. Clathrin is recruited to the IS and drives actin cytoskeleton accumulation. Promotes endocytosis of cytotoxic granules in target cells during in CIS. | 0.91 | [81,82] |
| SRC | v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (avian). Non-receptor protein tyrosine kinase which is activated following engagement of many different classes of cellular receptors including immune response receptors and integrins. Src is activated early during IS formation. | 0.909 | [83] |
| PKP2 | Plakophilin 2. | 0.908 | |
| MYO6 | Unconventional myosin-VI. | 0.906 | |
| DNM1 | Dynamin-1. | 0.905 | |
| DCTN1 | Dynactin 1. Mediates the accumulation of CTLA4 and granzyme B-containing intracellular vesicles at the IS and CTL-mediated lysis. Dynein/dynactin contribute to the internal forces that control organelle positioning and function at the T cell-APC contact area. | 0.905 | [70,84] |
| CLTCL1 | Clathrin heavy chain 2. Clathrin is recruited to the IS and drives actin cytoskeleton accumulation. Promotes endocytosis of cytotoxic granules in target cells during in CIS. | 0.902 | [81,82] |
| CLTB | Clathrin light chain B. Clathrin is recruited to the IS and drives actin cytoskeleton accumulation. Promotes endocytosis of cytotoxic granules in target cells during in CIS. | 0.902 | [81,82] |
| CLTA | Clathrin light chain A. Clathrin is recruited to the IS and drives actin cytoskeleton accumulation. Promotes endocytosis of cytotoxic granules in target cells during in CIS. | 0.902 | [81,82] |
| AP2M1 | AP-2 complex subunit mu. Involved in clathrin-dependent endocytosis in which cargo proteins are incorporated into vesicles surrounded by clathrin, which are destined for fusion with the early endosome. Participates in TCR recycling at the IS. | 0.9 | [82] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tittarelli, A.; Navarrete, M.; Gleisner, M.A.; Gebicke-Haerter, P.; Salazar-Onfray, F. Connexin-Mediated Signaling at the Immunological Synapse. Int. J. Mol. Sci. 2020, 21, 3736. https://doi.org/10.3390/ijms21103736
Tittarelli A, Navarrete M, Gleisner MA, Gebicke-Haerter P, Salazar-Onfray F. Connexin-Mediated Signaling at the Immunological Synapse. International Journal of Molecular Sciences. 2020; 21(10):3736. https://doi.org/10.3390/ijms21103736
Chicago/Turabian StyleTittarelli, Andrés, Mariela Navarrete, María Alejandra Gleisner, Peter Gebicke-Haerter, and Flavio Salazar-Onfray. 2020. "Connexin-Mediated Signaling at the Immunological Synapse" International Journal of Molecular Sciences 21, no. 10: 3736. https://doi.org/10.3390/ijms21103736
APA StyleTittarelli, A., Navarrete, M., Gleisner, M. A., Gebicke-Haerter, P., & Salazar-Onfray, F. (2020). Connexin-Mediated Signaling at the Immunological Synapse. International Journal of Molecular Sciences, 21(10), 3736. https://doi.org/10.3390/ijms21103736