Nicotinamide Phosphoribosyltransferase (Nampt)/Nicotinamide Adenine Dinucleotide (NAD) Axis Suppresses Atrial Fibrillation by Modulating the Calcium Handling Pathway
Abstract
:1. Introduction
2. Results
2.1. HFD Reduces Nampt Expression in the Atrial Tissues
2.2. NKO and HFD Do Not Affect Cardiac Function and Morphology
2.3. HFD Increases Body Weight (BW) and Fat Volume, but Not Affected by NKO
2.4. NKO and HFD Increase AF Susceptibility and Duration
2.5. NKO and HFD Accelerate Diastolic Calcium Leaks Under Isoproterenol (Iso) Stimulation in Cardiomyocytes
2.6. NKO and HFD Promote CaMKII Oxidation and RyR2 Phosphorylation
2.7. NAD Precursor NR Increases Nampt Amount and Decreases AF Duration
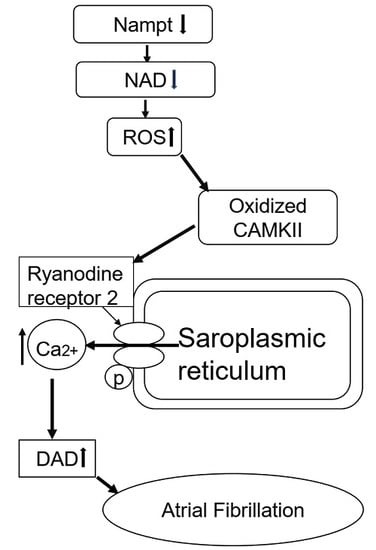
3. Discussion
4. Materials and Methods
4.1. Mouse Model
4.2. Blood Pressure Measurement
4.3. Echocardiography
4.4. Body Fat Composition Analysis
4.5. Electrophysiological Study and AF Induction
4.6. Calcium Imaging
4.7. RNA Expression Analysis
4.8. Histology and Immunohistochemistry
4.9. Western Blotting Analysis
4.10. NAD Detection Assay
4.11. NR Treatment
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of Atrial Fibrillation on the Risk of Death: The Framingham Heart Study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agostino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation 2003, 107, 2920–2925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.-H.; McAnulty, J.H., Jr.; Zheng, Z.-J.; et al. Worldwide Epidemiology of Atrial Fibrillation. A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Sumeray, M.; Steiner, M.; Sutton, P.; Treasure, T. Age and obesity as risk factors in perioperative atrial fibrillation. Lancet 1988, 20, 448. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A. Independent Risk Factors for Atrial Fibrillation in a Population-Based Cohort: The Framingham Heart Study. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Parise, H.; Levy, D.; D’Agostino, R.B., Sr.; Wolf, P.A.; Vasan, R.S.; Benjamin, E.J. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004, 292, 2471–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef]
- Lubbers, E.R.; Price, M.V.; Mohler, P.J. Arrhythmogenic substrates for atrial fibrillation in obesity. Front. Physiol. 2018, 9, 1482. [Google Scholar] [CrossRef]
- Neef, S.; Dybkova, N.; Sossalla, S.; Ort, K.R.; Fluschnik, N.; Neumann, K.; Seipelt, R.; Schöndube, F.A.; Hasenfuss, G.; Maier, L.S. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ. Res. 2010, 106, 1134–1144. [Google Scholar] [CrossRef] [Green Version]
- Purohit, A.; Rokita, A.G.; Guan, X.; Chen, B.; Koval, O.M.; Voigt, N.; Neef, S.; Sowa, T.; Gao, Z.; Luczak, E.D.; et al. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 2013, 128, 1748–1757. [Google Scholar] [CrossRef] [Green Version]
- Revollo, J.R.; Korner, A.; Mills, K.F.; Satoh, A.; Wang, T.; Garten, A.; Dasgupta, B.; Sasaki, Y.; Wolberger, C.; Townsend, R.R.; et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007, 6, 363–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Zhou, R.; Xia, B.; Dang, W.; Yang, Z.; Chen, X. NAMPT maintains mitochondria content via NRF2-PPARα/AMPKα pathway to promote cell survival under oxidative stress. Cell Signal. 2020, 66, 109496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tang, W.; Chen, X.; Wang, S.; Wang, X.; Xu, H.; Li, L. The NAMPT/E2F2/SIRT1 axis promotes proliferation and inhibits p53-dependent apoptosis in human melanoma cells. Biochem. Biophys. Res. Commun. 2017, 493, 77–84. [Google Scholar] [CrossRef]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011, 14, 528–538. [Google Scholar] [CrossRef] [Green Version]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-kB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef]
- Stromsdorfer, K.L.; Yamaguchi, S.; Yoon, M.J.; Moseley, A.C.; Franczyk, M.P.; Kelly, S.C.; Qi, N.; Imai, S.; Yoshino, J. NAMPT-mediated NAD(+) biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep. 2016, 16, 1851–1860. [Google Scholar] [CrossRef] [Green Version]
- Diguet, N.; Trammell, S.A.J.; Tannous, C.; Deloux, R.; Piquereau, J.; Mougenout, N.; Gouge, A.; Gressette, M.; Manoury, B.; Blanc, J.; et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 2018, 137, 2256–2273. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Byun, J.; Zhai, P.; Ikeda, Y.; Oka, S.; Sadoshima, J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE 2014, 9, e98972. [Google Scholar] [CrossRef] [Green Version]
- Byun, J.; Oka, S.I.; Imai, N.; Huang, C.Y.; Ralda, G.; Zhai, P.; Ikeda, Y.; Ikeda, S.; Sadoshima, J. Both gain and loss of Nampt function promote pressure overload-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H711–H725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Yang, X.; Hua, X.; Liu, J.; Fan, M.B.; Li, G.Q.; Song, J.; Xu, T.Y.; Li, Z.Y.; Guan, Y.F.; et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic liver disease in ageing. Br. J. Pharmacol. 2016, 173, 2352–2368. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, K.N.; Peics, J.; Ma, T.; Karavaeva, I.; Dall, M.; Chubanava, S.; Basse, A.L.; Dmytriyeva, O.; Treebak, J.T.; Gerhart-Hines, Z. NAMPT-mediated NAD+ biosynthesis is indispensable for adipose tissue plasticity and development obesity. Mol. Metab. 2018, 11, 178–188. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial remodeling and atrial fibrillation: Recent advances and translational perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef] [Green Version]
- Luczak, E.D.; Anderson, M.E. CaMKII oxidative activation and the pathogenesis of cardiac disease. J. Mol. Cell Cardiol. 2014, 0, 112–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedeschi, P.M.; Bansal, N.; Kerrigan, J.E.; Abali, E.E.; Scotto, K.W.; Bertino, J.R. NAD+ kinase as a therapeutic target in cancer. Clin. Cancer Res. 2016, 22, 5189–5195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.M.; Park, C.W.; Kim, S.W.; Nam, Y.J.; Yu, J.H.; Shin, J.H.; Yun, C.H.; Im, S.H.; Kim, K.T.; Sung, Y.C.; et al. Nampt suppresses glucose deprivation-induced oxidative stress by increasing NADPH levels in breast cancer. Oncogene 2016, 35, 3544–3554. [Google Scholar] [CrossRef]
- Sociali, G.; Grozio, A.; Caffa, I.; Schuster, S.; Becherini, P.; Damonte, P.; Sturla, L.; Fresia, C.; Passalacqua, M.; Mazzola, F.; et al. SIRT6 deacetylase activity regulates NAMPT activity and NAD(P)H pools in cancer cells. FASEB J. 2019, 33, 3704–3717. [Google Scholar] [CrossRef]
- Trammell, S.A.; Weidemann, B.J.; Chadda, A.; Yorek, M.S.; Holmes, A.; Coppey, L.J.; Obrosov, A.; Kardon, R.H.; Yorek, M.A.; Brenner, C. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci. Rep. 2017, 6, 26933. [Google Scholar] [CrossRef] [Green Version]
- Dudley, S.C., Jr.; Hoch, N.E.; McCann, L.A.; Honeycutt, C.; Diamandopoulos, L.; Fukai, T.; Harrison, D.G.; Dikalov, S.I.; Langberg, J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: Role of the NADPH and xanthine oxidases. Circulation 2005, 112, 1266–1273. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Guzik, T.J.; Zhang, Y.H.; Zhang, M.H.; Kattach, H.; Ratnatunga, C.; Pillai, R.; Channon, K.M.; Casadei, B. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ. Res. 2005, 97, 629–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldman, M.; Nudelman, V.; Shainberg, A.; Abraham, N.G.; Kornwoski, R.; Aravot, M.; Arad, M.; Hochhauser, E. PARP-1 inhibition protects the diabetic heart through activation of SIRT1-PGC-1α axis. Exp. Cell Res. 2018, 373, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Cao, Q.; Wen, K.; Xiao, Y.F.; Chen, T.T.; Guan, X.H.; Liu, Y.; Zou, L.; Qian, Y.S.; Deng, K.Y.; et al. CD38 deficiency alleviates D-galactose-induced myocardial cell senescence through NAD+/Sirt1 signaling pathway. Front. Physiol. 2019, 10, 1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.P.; Oka, S.; Shao, D.; Hariharan, N.; Sadoshima, J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ. Res. 2009, 105, 481–491. [Google Scholar] [CrossRef] [Green Version]
- Harada, M.; Van Wagoner, D.R.; Nattel, S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 2015, 79, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Wu, H.C.; Liao, C.C.; Chou, Y.C.; Pan, S.F.; Chiu, C.M. Secretion of one adipokine Nampt/Visfatin suppresses the inflammatory stress-induced NF-κB activity and affects Nampt-dependent cell viability in Huh-7 cells. Mediat. Inflamm. 2015, 2015, 392471. [Google Scholar] [CrossRef]
- Fletcher, R.S.; Ratajczak, J.; Doig, C.L.; Oakey, L.A.; Callingham, R.; Da Silva Xavier, G.; Garten, A.; Elhassan, Y.S.; Redpath, P.; Migaud, M.E.; et al. Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol. Metab. 2017, 6, 819–832. [Google Scholar] [CrossRef]
- Chowdhry, S.; Zanca, C.; Rajkumar, U.; Koga, T.; Diao, Y.; Raviram, R.; Liu, F.; Turner, K.; Yang, H.; Brunk, E.; et al. NAD metabolic dependency in cancer is shaped by gene amplification and enhancer remodelling. Nature 2019, 597, 570–575. [Google Scholar] [CrossRef]
- Qin, R.; Murakoshi, N.; Xu, D.; Tajiri, K.; Feng, D.; Stujanna, E.N.; Yonebayashi, S.; Nakagawa, Y.; Shimano, H.; Nogami, A.; et al. Exercise training reduces ventricular arrhythmias through restoring calcium handling and sympathetic tone in myocardial infarction mice. Physiol. Rep. 2019, 7, e13972. [Google Scholar] [CrossRef] [Green Version]
- Canto, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.Y.; Luan, J.J.; YFan Olatunji, O.J.; Song, J.; Zuo, J. α-Mangostin reduced the viability of A594 cells in vitro by provoking ROS production through downregulation of NAMPT/NAD. Cell Stress Chaperones 2020, 25, 163–172. [Google Scholar] [CrossRef] [PubMed]
- de Guia, R.M.; Hassing, A.S.; Skov, L.J.; Ratner, C.; Plucinska, K.; Madsen, S.; Diep, T.A.; Dela Cruz, G.V.; Trammell, S.A.J.; Sustarsic, E.G.; et al. Fasting- and ghrelin-induced food intake is regulated by NAMPT in the hypothalamus. Acta Physiol. (Oxf.) 2020, 228, e13437. [Google Scholar] [PubMed]
- Mukherjee, S.; Chellappa, K.; Moffitt, A.; Ndungu, J.; Dellinger, R.W.; Davis, J.G.; Agarwal, B.; Baur, J.A. Nicotinamide adenine dinucleotide biosynthesis promotes liver regeneration. Hepatology 2017, 65, 616–630. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.B.; Huang, Y.; Moreno-Vinasco, L.; Sammani, S.; Moitra, J.; Barnard, J.W.; Ma, S.F.; Mirzapoiazova, T.; Evenoski, C.; Reeves, R.R.; et al. Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2008, 178, 605–617. [Google Scholar] [CrossRef] [Green Version]
- Stujanna, E.N.; Murakoshi, N.; Tajiri, K.; Xu, D.; Kimura, T.; Qin, R.; Feng, D.; Yonebayashi, S.; Ogura, Y.; Yamagami, F.; et al. Rev-erb agonist improves adverse cardiac remodeling and survival in myocardial infarction through an anti-inflammatory mechanism. PLoS ONE 2017, 12, e0189330. [Google Scholar] [CrossRef] [Green Version]
- Lubura, M.; Hesse, D.; Neumann, N.; Scherneck, S.; Wiedmer, P.; Schurmann, A. Non-invasive quantification of white and brown adipose tissue and liver fat content by computed tomography in mice. PLoS ONE 2012, 7, e37026. [Google Scholar] [CrossRef]
- Xu, D.; Murakoshi, N.; Igarashi, M.; Hirayama, A.; Ito, Y.; Seo, Y.; Tada, H.; Aonuma, K. PPAR-γ activator pioglitazone prevents age-related atrial fibrillation susceptibility by improving antioxidant capacity and reducing apoptosis in a rat model. J. Cardiovasc. Electrophysiol. 2012, 23, 209–217. [Google Scholar] [CrossRef]
- Xu, D.; Murakoshi, N.; Tada, H.; Igarashi, M.; Sekiguchi, Y.; Aonuma, K. Age-related increase in atrial fibrillation induced by transvenous catheter-based atrial burst pacing: An In-Vivo rat model of inducible atrial fibrillation. J. Cardiovasc. Electrophysiol. 2010, 21, 88–93. [Google Scholar] [CrossRef]
- Akers-Johnson, M.; Li, P.Y.; Holmes, A.P.; O’Brien, S.M.; Pavlovic, D.; Foo, R.S. A simplified, Langendorff-free method for concomitant isolation of viable cardiac myocytes and nonmyocytes from the adult mouse heart. Circ. Res. 2016, 119, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Song, L.S.; Shirokova, N.; Gonzalez, A.; Lakatta, E.G.; Rios, E.; Stern, M.D. Amplitude distribution of calcium sparks in confocal images: Theory and studies with an automatic detection method. Biophys. J. 1999, 76, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelins: Minimum information for publication of quantitative real-time PCR experiment. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Echocardiographic Parameters | WT | NKO | ||
|---|---|---|---|---|
| ND | HFD | ND | HFD | |
| LVDd (mm) | 3.89 ± 0.49 | 3.71 ± 0.51 | 3.96 ± 0.47 | 3.71 ± 0.35 |
| LVDs (mm) | 2.50 ± 0.21 | 2.51 ±0.18 | 2.49 ± 0.16 | 2.53 ± 0.18 |
| FS (%) | 32.48 ± 9.53 | 31.66 ± 7.06 | 31.59 ± 6.40 | 29.82 ± 5.92 |
| EF (%) | 59.66 ± 12.73 | 60.10 ± 10.45 | 59.92 ± 9.34 | 57.41 ± 8.82 |
| LAD (mm) | 1.09 ± 0.06 | 1.10 ± 0.15 | 1.11 ± 0.02 | 1.12 ± 0.07 |
| LVSV (μL) | 50.28 ± 15.33 | 51.10 ± 13.19 | 58.58 ± 21.84 | 57.28 ± 12.96 |
| Basic Informations | WT | NKO | ||
|---|---|---|---|---|
| ND | HFD | ND | HFD | |
| SBP (mmHg) | 130.4 ± 11.1 | 130.6 ± 8.2 | 129.6 ± 14.5 | 137.2 ± 12.7 * |
| DBP (mmHg) | 105.4 ± 9.5 | 97.6 ± 7.7 | 101.8 ± 11.5 | 109.8 ± 9.5 * |
| MBP (mmHg) | 113.6 ± 9,6 | 107 ± 6.7 | 110.6 ± 11.9 | 118.4 ± 6.1 * |
| BW (g) | 26.84 ± 0.62 | 34.04 ± 3.30 | 26.48 ± 1.07 | 35.36 ± 1.55 * |
| HW/BW (mg/g) | 4.61 ± 0.28 | 3.54 ± 1.55 * | 4.37 ± 0.20 | 3.50 ± 0.32 * |
| LV/BW (mg/g) | 3.21 ± 0.16 | 2.96 ± 0.78 * | 3.12 ± 0.17 | 3.02 ± 0.24 * |
| AV/BW (mg/g) | 0.32 ±0.05 | 0.29 ± 0.10 | 0.30 ±0.06 | 0.30 ± 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, D.; Xu, D.; Murakoshi, N.; Tajiri, K.; Qin, R.; Yonebayashi, S.; Okabe, Y.; Li, S.; Yuan, Z.; Aonuma, K.; et al. Nicotinamide Phosphoribosyltransferase (Nampt)/Nicotinamide Adenine Dinucleotide (NAD) Axis Suppresses Atrial Fibrillation by Modulating the Calcium Handling Pathway. Int. J. Mol. Sci. 2020, 21, 4655. https://doi.org/10.3390/ijms21134655
Feng D, Xu D, Murakoshi N, Tajiri K, Qin R, Yonebayashi S, Okabe Y, Li S, Yuan Z, Aonuma K, et al. Nicotinamide Phosphoribosyltransferase (Nampt)/Nicotinamide Adenine Dinucleotide (NAD) Axis Suppresses Atrial Fibrillation by Modulating the Calcium Handling Pathway. International Journal of Molecular Sciences. 2020; 21(13):4655. https://doi.org/10.3390/ijms21134655
Chicago/Turabian StyleFeng, Duo, DongZhu Xu, Nobuyuki Murakoshi, Kazuko Tajiri, Rujie Qin, Saori Yonebayashi, Yuta Okabe, Siqi Li, Zixun Yuan, Kazutaka Aonuma, and et al. 2020. "Nicotinamide Phosphoribosyltransferase (Nampt)/Nicotinamide Adenine Dinucleotide (NAD) Axis Suppresses Atrial Fibrillation by Modulating the Calcium Handling Pathway" International Journal of Molecular Sciences 21, no. 13: 4655. https://doi.org/10.3390/ijms21134655
APA StyleFeng, D., Xu, D., Murakoshi, N., Tajiri, K., Qin, R., Yonebayashi, S., Okabe, Y., Li, S., Yuan, Z., Aonuma, K., & Ieda, M. (2020). Nicotinamide Phosphoribosyltransferase (Nampt)/Nicotinamide Adenine Dinucleotide (NAD) Axis Suppresses Atrial Fibrillation by Modulating the Calcium Handling Pathway. International Journal of Molecular Sciences, 21(13), 4655. https://doi.org/10.3390/ijms21134655