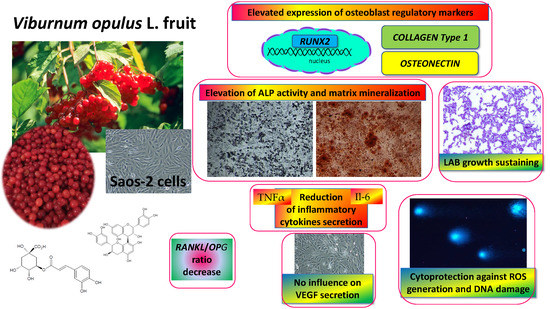
Viburnum opulus L. Juice Phenolic Compounds Influence Osteogenic Differentiation in Human Osteosarcoma Saos-2 Cells
Abstract
:1. Introduction
2. Results
2.1. Content of Phenolic Compounds in Fresh and Purified Juices of Viburnum opulus Fruit
2.2. V. opulus Influence on Cellular Metabolic Activity and Proliferation
2.3. V. opulus Influence on Alkaline Phosphatase Activity
2.4. V. opulus Influence on Matrix Mineralization
2.5. V. opulus Influence on Expression of Genes Associated with Osteogenesis
2.6. V. opulus Influence on Intracellular Reactive Oxygen Species Production and DNA Repair
2.7. V. opulus Influence on Pro-Inflammatory Markers: Il6, TNFα and VEGF Secretion
2.8. Antimicrobial Activity of V. opulus Against Lactic Acid Bacteria and Pathogens
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of V. opulus Samples, Identification and Quantitative Determination of Individual Phenolic Compounds by UPLC–PDA-Q/TOF-MS
4.3. Cell Culture
4.4. Cell Viability and Proliferation
4.5. Detection of Intracellular Reactive Oxygen Species Generation
4.6. DNA Damage and Repair
4.7. Alizarin Red Cells Staining
4.8. Estimation of Alkaline Phosphatase Activity
4.9. Gene Expresssion Analysis
4.10. Determination of Selected Proteins Levels
4.11. Antimicrobial Activity of V. opulus Juice
4.12. VEGF secretion
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| FJ | fresh juice |
| Il6 | interleukin 6 |
| NF-κB | nuclear factor kappa B |
| OPG | osteoprotegerin |
| RANKL | receptor activator of nuclear factor kappa-Β ligand |
| RUNX2 | Runt-related transcription factor 2 |
| ROS | reactive oxygen species |
| TNFα | tumor necrosis factor α |
| VEGF | vascular endothelial growth factor |
| PJ | purified juice |
References
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Nicolin, V.; De Tommasi, N.; Nori, S.L.; Costantinides, F.; Berton, F.; Di Lenarda, R. Modulatory effects of plant polyphenols on bone remodeling: A prospective view from the bench to bedside. Front. Endocrinol. 2019, 10, 494. [Google Scholar] [CrossRef]
- Mukudai, Y.; Kondo, S.; Koyama, T.; Li, C.; Banka, S.; Kogure, A.; Yazawa, K. Potential anti-osteoporotic effects of herbal extracts on osteoclasts, osteoblasts and chondrocytes in vitro. BMC Complement. Altern. Med. 2014, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Al Anouti, F.; Taha, Z.; Shamim, S.; Khalaf, K.; Al Kaabi, L.; Alsafar, H. An insight into the paradigms of osteoporosis: From genetics to biomechanics. Bone Rep. 2019, 11, 100216. [Google Scholar] [CrossRef]
- Komori, T. Regulation of proliferation, differentiation and functions of osteoblasts by runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-S.; Gu, Y.; Jiang, C.; Chen, L. Osteonectin regulates the extracellular matrix mineralization of osteoblasts through P38 signaling pathway. J. Cell. Physiol. 2020, 235, 2220–2231. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar]
- Shu, L.; Beier, E.; Sheu, T.; Zhang, H.; Zuscik, M.; Puzas, J.E.; Boyce, F.B.; Mooney, R.; Xing, L. High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif. Tissue Int. 2015, 96, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbo, F.; Brunetti, G.; Crupi, P.; Bortolotti, S.; Storlino, G.; Piacente, L.; Carocci, A.; Catalano, A.; Milani, G.; Colaianni, G.; et al. Effects of sweet cherry polyphenols on enhanced osteoclastogenesis associated with childhood obesity. Front. Immunol. 2019, 10, 1001. [Google Scholar] [CrossRef]
- Kyung, T.-W.; Lee, J.-E.; Van Phan, T.; Yu, R.; Choi, H.-S. Osteoclastogenesis by bone marrow-derived macrophages is enhanced in obese mice. J. Nutr. 2009, 139, 502–506. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Cao, L.; Xia, L.; Wu, Q.; Wang, J.; Wang, X.; Xu, L.; Zhou, Y.; Xu, Y.; Jiang, X. Evaluation of osteogenesis and angiogenesis of icariin in local controlled release and systemic delivery for calvarial defect in ovariectomized rats. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Library Global Report on Diabetes; WHO Press: Geneva, Switzerland, 2016; Volume 978, pp. 6–86. ISBN 978-92-4-156525-7. [Google Scholar]
- Chiva-Blanch, G.; Badimon, L. Effects of polyphenol intake on metabolic syndrome: Current evidences from human trials. Oxid. Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [Green Version]
- Bozzetto, L.; Annuzzi, G.; Pacini, G.; Costabile, G.; Vetrani, C.; Vitale, M.; Griffo, E.; Giacco, A.; De Natale, C.; Cocozza, S.; et al. Polyphenol-rich diets improve glucose metabolism in people at high cardiometabolic risk: A controlled randomised intervention trial. Diabetologia 2015, 58, 1551–1560. [Google Scholar] [CrossRef] [Green Version]
- Sequeira, I.R.; Poppitt, S.D. Unfolding novel mechanisms of polyphenol flavonoids for better glycaemic control: Targeting pancreatic islet amyloid polypeptide (IAPP). Nutrients 2017, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Popova, A.; Alexieva, I.; Krastanov, A.; Lante, A. Polyphenols as Suitable Control for Obesity and Diabetes. Open Biotechnol. J. 2018, 12, 219–228. [Google Scholar] [CrossRef]
- Torre, E. Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary polyphenols and gene expression in molecular pathways associated with type 2 diabetes mellitus: A review. Int. J. Mol. Sci. 2020, 21, 140. [Google Scholar] [CrossRef] [Green Version]
- Austermann, K.; Baecker, N.; Stehle, P.; Heer, M. Putative effects of nutritive polyphenols on bone metabolism in vivo—Evidence from human studies. Nutrients 2019, 11, 871. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Lee, S.K.; Chun, O.K. Soy isoflavones and osteoporotic bone loss: A review with an emphasis on modulation of bone remodeling. J. Med. Food 2016, 19, 1–14. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Budryn, G.; Grzelczyk, J.; Perez-Sanchez, H.; Żyżelewicz, D. Evaluation of Isoflavones as bone resorption inhibitors upon interactions with receptor activator of nuclear factor-κb ligand (RANKL). Molecules 2020, 25, 206. [Google Scholar] [CrossRef] [Green Version]
- Torre, E.; Iviglia, G.; Cassinelli, C.; Morra, M.; Russo, N. Polyphenols from grape pomace induce osteogenic differentiation in mesenchymal stem cells. Int. J. Mol. Med. 2020, 45, 1721–1734. [Google Scholar] [CrossRef] [Green Version]
- Zakłos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus fruit phenolic compounds as cytoprotective agents able to decrease free fatty acids and glucose uptake by Caco-2 cells. Antioxidants 2019, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Zakłos-Szyda, M.; Majewska, I.; Redzynia, M.; Koziołkiewicz, M. Antidiabetic effect of polyphenolic extracts from selected edible plants as α-amylase, α-glucosidase and PTP1B inhibitors, and β pancreatic cells cytoprotective agents—A comparative study. Curr. Top. Med. Chem. 2015, 15, 2431–2444. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pawlik, N. The influence of Viburnum opulus polyphenolic compounds on metabolic activity and migration of HeLa and MCF cells. Acta Innov. 2019, 33, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Act, J.O.P.; Ruosi, C.; Querques, F.; Granata, F.; Colella, G.; Liccardo, S.; Lombardo, B.; Pastore, L. Cellular and animal models for the identification of osteoporosis determinants increasing vertebral compression fractures risk. Osteoporos. Phys. Act. 2015, 3, 2. [Google Scholar]
- Česoniene, L.; Daubaras, R.; Vencloviene, J.; Viškelis, P. Biochemical and agro-biological diversity of Viburnum opulus genotypes. Cent. Eur. J. Biol. 2010, 5, 864–871. [Google Scholar] [CrossRef]
- Zakłos, M.; Pietrzyk, N.; Szustak, M.; Podsędek, A. Viburnum opulus L. juice phenolics inhibit mouse 3T3-L1 cells adipogenesis and pancreatic lipase activity. Nutrients 2020, 12, 2003. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Kowalska-Baron, A.; Pietrzyk, N.; Drzazga, A. Evaluation of Viburnum opulus L. fruit phenolics cytoprotective potential on insulinoma MIN6 cells relevant for diabetes mellitus and obesity. Antioxidants 2020, 9, 433. [Google Scholar] [CrossRef]
- Min, J.; Yuan, Z.; Zhang, Q.; Lin, S.; Wang, K.; Luo, J. Analysis of anti-osteoporosis function of chlorogenic acid by gene microarray profiling in ovariectomy rat model. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Perova, I.B.; Zhogova, A.A.; Cherkashin, A.V.; Éller, K.I.; Ramenskaya, G.V. Biologically active substances from european guelder berry fruits. Pharm. Chem. J. 2014, 48, 332–339. [Google Scholar] [CrossRef]
- Ghiacci, G.; Lumetti, S.; Mori, D.; Macaluso, G.M.; Sala, R. Stanozolol promotes osteogenic gene expression and apposition of bone mineral in vitro Abstract. J. Appl. Oral Serv. 2019, 27, e20180014. [Google Scholar]
- Prideaux, M.; Wijenayaka, A.R.; Kumarasinghe, D.D.; Ormsby, R.T.; Evdokiou, A.; Findlay, D.M.; Atkins, G.J. SaOS2 osteosarcoma cells as an in vitro model for studying the transition of human osteoblasts to osteocytes. Calcif. Tissue Int. 2014, 95, 183–193. [Google Scholar] [CrossRef]
- Graef, J.L.; Rendina-ruedy, E.; Crockett, E.K.; Ouyang, P.; Jarrod, B.; Cichewicz, R.H.; Lucas, E.A.; Smith, B.J. Select polyphenolic fractions from dried plum enhance osteoblast activity through BMP-2 signaling. J. Nutr. Biochem. 2019, 55, 59–67. [Google Scholar] [CrossRef]
- Bin, H.S.; Jeong, J.H.; Choi, U.K. Chlorogenic acid promotes osteoblastogenesis in human adipose tissue-derived mesenchymal stem cells. Food Sci. Biotechnol. 2013, 22, 107–112. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S.; Song, S.; Kim, W.H.; Song, E.; Lee, J.; Lee, S.; Han, D.; Lee, J. Protective effects of melon extracts on bone strength, mineralization, and metabolism in rats with ovariectomy-induced osteoporosis. Antioxidants 2019, 8, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.P.; Lin, S.J.; Wan, W.B.; Zuo, H.L.; Yao, F.F.; Ruan, H.B.; Xu, J.; Song, W.; Zhou, Y.C.; Wen, S.Y.; et al. Chlorogenic acid prevents osteoporosis by Shp2/PI3K/Akt pathway in ovariectomized rats. PLoS ONE 2016, 11, e0166751. [Google Scholar] [CrossRef]
- Byun, M.R.; Sung, M.K.; Kim, A.R.; Lee, C.H.; Jang, E.J.; Jeong, M.G.; Noh, M.; Hwang, E.S.; Hong, J.H. (−)-Epicatechin gallate (ECG) stimulates osteoblast differentiation via runt-related transcription factor 2 (RUNX2) and transcriptional coactivator with PDZ-binding motif (TAZ)-mediated transcriptional activation. J. Biol. Chem. 2014, 289, 9926–9935. [Google Scholar] [CrossRef] [Green Version]
- Kovar, H.; Bierbaumer, L.; Radic-sarikas, B. The YAP/TAZ pathway in osteogenesis and bone sarcoma pathogenesis. Cells 2020, 9, 972. [Google Scholar] [CrossRef] [Green Version]
- El Ouarrat, D.; Isaac, R.; Lee, Y.S.; Oh, D.Y.; Wollam, J.; Lackey, D.; Riopel, M.; Bandyopadhyay, G.; Seo, J.B.; Sampath-Kumar, R.; et al. TAZ is a negative regulator of PPARγ activity in adipocytes and TAZ deletion improves insulin sensitivity and glucose tolerance. Cell Metab. 2020, 31, 162–173.e5. [Google Scholar] [CrossRef]
- Lin, S.Y.; Kang, L.; Wang, C.Z.; Huang, H.H.; Cheng, T.L.; Huang, H.T.; Lee, M.J.; Lin, Y.S.; Ho, M.L.; Wang, G.J.; et al. (−)-Epigallocatechin-3-gallate (EGCG) enhances osteogenic differentiation of human bone marrow mesenchymal stem cells. Molecules 2018, 23, 3221. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Research 2013, 1, 11–26. [Google Scholar]
- Shin, J.; Kim, J.E.; Pak, K.J.; Kang, J.I.; Kim, T.S.; Lee, S.Y.; Yeo, I.H.; Park, J.H.Y.; Kim, J.H.; Kang, N.J.; et al. A combination of soybean and Haematococcus extract alleviates ultraviolet B-induced photoaging. Int. J. Mol. Sci. 2017, 18, 682. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.T.; Kang, L.; Wang, C.Z.; Huang, P.J.; Huang, H.T.; Lin, S.Y.; Chou, S.H.; Lu, C.C.; Shen, P.C.; Lin, Y.S.; et al. (−)-Epigallocatechin-3-gallate decreases osteoclastogenesis via modulation of RANKL and osteoprotegrin. Molecules 2019, 24, 156. [Google Scholar] [CrossRef] [Green Version]
- Kwak, S.C.; Lee, C.; Kim, J.Y.; Oh, H.M.; So, H.S.; Lee, M.S.; Rho, M.C.; Oh, J. Chlorogenic acid inhibits osteoclast differentiation and bone resorption by down-regulation of receptor activator of nuclear factor kappa-b ligand-induced nuclear factor of activated t cells c1 expression. Biol. Pharm. Bull. 2013, 36, 1779–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sungkamanee, S.; Wattanathorn, J.; Muchimapura, S.; Thukham-Mee, W. Antiosteoporotic effect of combined extract of morus alba and polygonum odoratum. Oxid. Med. Cell. Longev. 2014, 2014, 579305. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Nie, Y.; Cao, D.P.; Xue, Y.Y.; Wang, J.S.; Zhao, L.; Rahman, K.; Zhang, Q.Y.; Qin, L.P. Potential antiosteoporotic agents from plants: A comprehensive review. Evid.-Based Complement. Altern. Med. 2012, 2012, 364604. [Google Scholar] [CrossRef]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-κB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, L.; Zhao, Z.; Shi, J.; Wang, X.; Huang, J. Grape seed proanthocyanidins inhibit H2O2-induced osteoblastic MC3T3-E1 cell apoptosis via ameliorating H2O2-induced mitochondrial dysfunction. J. Toxicol. Sci. 2014, 39, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Yeh, Y.; Shyu, H. The Preventive effect of biochanin a on bone loss in ovariectomized rats: Involvement in regulation of growth and activity of osteoblasts and osteoclasts. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar]
- Nash, L.A.; Ward, W.E. Comparison of black, green and rooibos tea on osteoblast activity. Food Funct. 2016, 7, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Shapses Sue, A.; Claudia, P.; Wang, Y. Obesity is a concern for bone health with aging. Nutr. Res. 2017, 39, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Park, J.J.; Hwang, S.J.; Park, J.H.; Lee, H.J. Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1α/AKT pathway. Cell. Oncol. 2015, 38, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ana, C.; Mark, W.; Christina, M.; Paul, N.; Kroon, P.A. Molecular structure-function relationship of dietary polyphenols for inhibiting VEGF-induced VEGFR-2 activity. Mol. Nutr. Food Res. 2015, 59, 2119–2131. [Google Scholar]
- Tsakiroglou, P.; Weber, J.; Ashworth, S.; Del Bo, C.; Klimis-Zacas, D. Phenolic and anthocyanin fractions from wild blueberries (V. angustifolium) differentially modulate endothelial cell migration partially through RHOA and RAC1. J. Cell. Biochem. 2019, 120, 11056–11067. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Leong, D.J.; He, Z.; Xu, L.; Liu, L.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Procyanidins mitigate osteoarthritis pathogenesis by, at least in part, suppressing vascular endothelial growth factor signaling. Int. J. Mol. Sci. 2016, 17, 2065. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; dePaula Lima, J. Consumption of chlorogenic acids through coffee and health implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic Acids from Green Coffee Extract are Highly Bioavailable in Humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in humans. Compr. Rev. Food Sci. Food Saf. 2020, 1–54. [Google Scholar] [CrossRef]
- Gwiazdowska, D.; Juś, K.; Jasnowska-Małecka, J.; Kluczyńska, K. The impact of polyphenols on Bifidobacterium growth. Acta Biochim. Pol. 2015, 62, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hu, K.L.J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fed mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef]
- Yan, J.; Takakura, A.; Zandi-Nejad, K.; Charles, J.F. Mechanisms of gut microbiota-mediated bone remodeling. Gut Microbes 2018, 9, 84–92. [Google Scholar] [CrossRef]
- Gilman, J.; Cashman, K.D. The effect of probiotic bacteria on transepithelial calcium transport and calcium uptake in human intestinal-like caco-2 cells. Curr. Issues Intest. Microbiol. 2006, 7, 1–6. [Google Scholar]
- Gatej, S.M.; Marino, V.; Bright, R.; Fitzsimmons, T.R.; Gully, N.; Zilm, P.; Gibson, R.J.; Edwards, S.; Bartold, P.M. Probiotic Lactobacillus rhamnosus GG prevents alveolar bone loss in a mouse model of experimental periodontitis. J. Clin. Periodontol. 2018, 45, 204–212. [Google Scholar] [CrossRef]
- Xu, X.; Jia, X.; Mo, L.; Liu, C.; Zheng, L.; Yuan, Q.; Zhou, X. Intestinal microbiota: A potential target for the treatment of postmenopausal osteoporosis. Bone Res. 2017, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Cronin, O.; Keohane, D.M.; Cormac, E.M.; Nugent, H.; Nugent, M.; Molloy, C.; O’toole, P.W.; Shanahan, F.; Molloy, M.G.; et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatol. 2019, 58, 2295–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Rao, S.; Cheng, Y.; Zhuo, X.; Deng, C.; Xu, N.; Zhang, H.; Yang, L. Microbial osteoporosis: The interplay between the gut microbiota and bones via host metabolism and immunity. Microbiologyopen 2019, 8, e00810. [Google Scholar] [CrossRef] [Green Version]
- Nowak, A.; Śliżewska, K.; Otlewska, A. Antigenotoxic activity of lactic acid bacteria, prebiotics, and products of their fermentation against selected mutagens. Regul. Toxicol. Pharmacol. 2015, 73, 938–946. [Google Scholar] [CrossRef]
- Muthusami, S.; Senthilkumar, K.; Vignesh, C.; Ilangovan, R.; Stanley, J.; Selvamurugan, N.; Srinivasan, N. Effects of Cissus quadrangularis on the Proliferation, differentiation and matrix mineralization of human osteoblast like SaOS-2 Cells. J. Cell. Biochem. 2011, 1045, 1035–1045. [Google Scholar] [CrossRef]












| Phenolic Compound | Content (mg/g) | ||
|---|---|---|---|
| Fresh Juice (FJ) | Purified Juice (PJ) | ||
| FLAVANOLS | (+)-Catechin | 0.657 ± 0.006 | 40.729 ± 0.596 |
| (−)-Epicatechin | 0.135 ± 0.002 | 8.002 ± 0.116 | |
| (Epi)catechin derivative Ia | 0.103 ± 0.001 | 6.998 ± 0.221 | |
| (Epi)catechin derivative IIa | 0.080 ± 0.001 | 6.006 ± 0.165 | |
| Gallocatechin gallatea | 0.031 ± 0.000 | 1.876 ± 0.085 | |
| Procyanidin dimer B1 | 0.759 ± 0.003 | 47.596 ± 0.148 | |
| Procyanidin dimer B2 | 0.199 ± 0.002 | 11.540 ± 0.148 | |
| Procyanidin dimerb | 0.024 ± 0.001 | 1.602 ± 0.258 | |
| B-type procyanidin dimer derivative Ib | 0.016 ± 0.000 | 2.071 ± 0.097 | |
| B-type procyanidin dimer derivative IIb | 0.035 ± 0.000 | 2.293 ± 0.094 | |
| Procyanidin trimer C1 | 0.033 ± 0.001 | 3.212 ± 0.351 | |
| Procyanidin trimer Ic | 0.112 ± 0.001 | 6.866 ± 0.342 | |
| Procyanidin trimer IIc | 0.030 ± 0.006 | 2.634 ± 0.270 | |
| Procyanidin trimer IIIc | 0.032 ± 0.000 | 1.796 ± 0.053 | |
| HYDROXYCINNAMIC ACIDS | Chlorogenic acid | 8.039 ± 0.145 | 645.492 ± 1.984 |
| Cryptochlorogenic acid | 0.004 ± 0.000 | 0.484 ± 0.023 | |
| Neochlorogenic acid | 0.007 ± 0.001 | 0,215 ± 0.019 | |
| Caffeoylquinic acidd | 0.745 ± 0.001 | 44.344 ± 0.176 | |
| Caffeoylquinic acid derivative Id | 0.015 ± 0.000 | 1.289 ± 0.058 | |
| Caffeoylquinic acid derivative IId | 0.024 ± 0.002 | 1.051 ± 0.008 | |
| Caffeoylquinic acid derivative IIId | 0.017 ± 0.001 | 1.220 ± 0.020 | |
| Caffeoylquinic acid derivative IVd | 0.034 ± 0.000 | 3.306 ± 0.014 | |
| Caffeoylquinic acid derivative Vd | 0.034 ± 0.000 | 3.268 ± 0.010 | |
| Feruloylquinic acid Id | n.d. | 5.722 ± 0.021 | |
| Feruloylquinic acid IId | n.d. | 0.528 ± 0.005 | |
| FLAVONOLS | Quercetin-3-vicianosidee | 0.020 ± 0.000 | 1.266 ± 0.007 |
| Quercetin-3-galactosidee | n.d. | 0.149 ± 0.011 | |
| Quercetin-3-rutinoside | 0.016 ± 0.000 | 0.921 ± 0.007 | |
| Quercetin-3—rhamnosidee | 0.007 ± 0.000 | 0.491 ± 0.002 | |
| ANTHO-CYANINS | Cyanidin-3-sambubioside | 0.093 ± 0.000 | 7.010 ± 0.003 |
| Cyanidin-3-glucoside | 0.139 ± 0.000 | 13.583 ± 0.799 | |
| Cyanidin-3-rutinoside | 0.068 ± 0.001 | 5.246 ± 0.016 | |
| Strain | Inhibition Zone [mm] | |
|---|---|---|
| FJ | PJ | |
| S. aureus ATTC 25923 | 2.0 | 7.0 |
| S. aureus ATTC 6538 | 12.3 | 9.0 |
| L. monocytogenes ATCC 19115 | 0 | 8.7 |
| Ent. faecalis ATCC 29212 | 6.0 | 10.0 |
| E. coli ATCC 10536 | 0 | 0 |
| E. coli ATCC 8739 | 0 | 0 |
| P. aeruginosa ATCC 15442 | 0 | 0 |
| P. aeruginosa ATCC 24755 | 0 | 0 |
| E. cloacae ATCC 13047 | 0 | 0 |
| S. typhimurium ATCC 14028 | 0 | 0 |
| S. enteritidis ATCC 13076 | 0 | 0 |
| C. albicans ATCC 10231 | 0 | 0 |
| Lb. rhamnosus GG | 0 | 0 |
| Lb. plantarum ŁOCK 0981 | 0 | 0 |
| Lb. brevis ŁOCK 0983 | 0 | 0 |
| Lb. paracasei ŁOCK 0985 | 0 | 0 |
| Lb. delbrueckii ŁOCK 0987 | 0 | 0 |
| Lb. plantarum ŁOCK 0989 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakłos-Szyda, M.; Nowak, A.; Pietrzyk, N.; Podsędek, A. Viburnum opulus L. Juice Phenolic Compounds Influence Osteogenic Differentiation in Human Osteosarcoma Saos-2 Cells. Int. J. Mol. Sci. 2020, 21, 4909. https://doi.org/10.3390/ijms21144909
Zakłos-Szyda M, Nowak A, Pietrzyk N, Podsędek A. Viburnum opulus L. Juice Phenolic Compounds Influence Osteogenic Differentiation in Human Osteosarcoma Saos-2 Cells. International Journal of Molecular Sciences. 2020; 21(14):4909. https://doi.org/10.3390/ijms21144909
Chicago/Turabian StyleZakłos-Szyda, Małgorzata, Adriana Nowak, Nina Pietrzyk, and Anna Podsędek. 2020. "Viburnum opulus L. Juice Phenolic Compounds Influence Osteogenic Differentiation in Human Osteosarcoma Saos-2 Cells" International Journal of Molecular Sciences 21, no. 14: 4909. https://doi.org/10.3390/ijms21144909
APA StyleZakłos-Szyda, M., Nowak, A., Pietrzyk, N., & Podsędek, A. (2020). Viburnum opulus L. Juice Phenolic Compounds Influence Osteogenic Differentiation in Human Osteosarcoma Saos-2 Cells. International Journal of Molecular Sciences, 21(14), 4909. https://doi.org/10.3390/ijms21144909