Mangosteen Pericarp and Its Bioactive Xanthones: Potential Therapeutic Value in Alzheimer’s Disease, Parkinson’s Disease, and Depression with Pharmacokinetic and Safety Profiles
Abstract
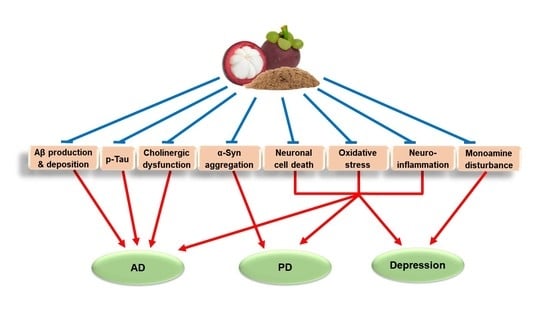
:1. Introduction
2. Major Pathologies and Therapeutic Strategies of AD, PD, and Depression
3. Pharmacological Effects of MP-Derived Agents in AD Models
3.1. Pharmacological Effects of MPE and MP Diet in AD Models
3.2. Pharmacological Effects of Xanthones Isolated from MP in AD Models
4. Pharmacological Effects of MP-Derived Agents in PD Models
5. Pharmacological Effects of MP-Derived Agents in Depression Models
6. Pharmacokinetic (PK) Profiles of MP-Derived Agents
6.1. In Vitro and In Vivo PK Profiles of MP-Derived Agents
6.2. PK Profiles of MP-Derived Agents in Humans
6.3. Proposed Solutions to Improve the PK Profiles of MP-Derived Agents
7. Safety Profiles of MP-Derived Agents
7.1. Safety Profiles of MP-Derived Agents in Animals
7.2. Safety Profiles of MP-Derived Agents in Humans
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Höglund, K.; Salter, H. Molecular biomarkers of neurodegeneration. Expert Rev. Mol. Diagn. 2013, 13, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020. [Google Scholar] [CrossRef]
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przedborski, S. The two-century journey of Parkinson disease research. Nat. Rev. Neurosci. 2017, 18, 251. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; Collado-Mateo, D. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Lu, Y.; Tang, C.; Liow, C.S.; Ng, W.W.; Ho, C.S.; Ho, R.C. A regressional analysis of maladaptive rumination, illness perception and negative emotional outcomes in Asian patients suffering from depressive disorder. Asian J. Psychiatry 2014, 12, 69–76. [Google Scholar] [CrossRef]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Di Paolo, M.; Papi, L.; Gori, F.; Turillazzi, E. Natural Products in Neurodegenerative Diseases: A Great Promise but an Ethical Challenge. Int. J. Mol. Sci. 2019, 20, 5170. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, S.M.; Daglia, M.; Braidy, N.; Nabavi, S.F. Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutr. Neurosci. 2017, 20, 180–194. [Google Scholar] [CrossRef]
- Pathak, L.; Agrawal, Y.; Dhir, A. Natural polyphenols in the management of major depression. Expert Opin. Investig. Drugs 2013, 22, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Kinghorn, A.D. Structural Characterization, Biological Effects, and Synthetic Studies on Xanthones from Mangosteen (Garcinia mangostana), a Popular Botanical Dietary Supplement. Mini Rev. Org. Chem. 2008, 5, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Pedraza-Chaverri, J.; Cardenas-Rodriguez, N.; Orozco-Ibarra, M.; Perez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef] [Green Version]
- Sriyanti, I.; Edikresnha, D.; Rahma, A.; Munir, M.M.; Rachmawati, H.; Khairurrijal, K. Mangosteen pericarp extract embedded in electrospun PVP nanofiber mats: Physicochemical properties and release mechanism of alpha-mangostin. Int. J. Nanomed. 2018, 13, 4927–4941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wang, S.; Ouyang, Y.; Tu, Y.; Liu, A.; Tian, Y.; He, M.; Pi, R. Garcinone D, a natural xanthone promotes C17.2 neural stem cell proliferation: Possible involvement of STAT3/Cyclin D1 pathway and Nrf2/HO-1 pathway. Neurosci. Lett. 2016, 626, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y.; Wang, S.N.; Yang, X.H.; Lan, W.J.; Chen, Z.W.; Chen, J.K.; Xie, J.H.; Han, Y.F.; Pi, R.B.; Yang, X.B. Gartanin Protects Neurons against Glutamate-Induced Cell Death in HT22 Cells: Independence of Nrf-2 but Involvement of HO-1 and AMPK. Neurochem. Res. 2016, 41, 2267–2277. [Google Scholar] [CrossRef]
- Tangpong, J.; Miriyala, S.; Noel, T.; Sinthupibulyakit, C.; Jungsuwadee, P.; St. Clair, D.K. Doxorubicin-induced central nervous system toxicity and protection by xanthone derivative of Garcinia mangostana. Neuroscience 2011, 175, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Nava Catorce, M.; Acero, G.; Pedraza-Chaverri, J.; Fragoso, G.; Govezensky, T.; Gevorkian, G. Alpha-mangostin attenuates brain inflammation induced by peripheral lipopolysaccharide administration in C57BL/6J mice. J. Neuroimmunol. 2016, 297, 20–27. [Google Scholar] [CrossRef]
- Phyu, M.P.; Tangpong, J. Neuroprotective effects of xanthone derivative of Garcinia mangostana against lead-induced acetylcholinesterase dysfunction and cognitive impairment. Food Chem. Toxicol. 2014, 70, 151–156. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Reyes-Fermin, L.M.; Nolasco-Amaya, E.G.; Orozco-Ibarra, M.; Medina-Campos, O.N.; Gonzalez-Cuahutencos, O.; Rivero-Cruz, I.; Mata, R. ROS scavenging capacity and neuroprotective effect of alpha-mangostin against 3-nitropropionic acid in cerebellar granule neurons. Exp. Toxicol. Pathol. 2009, 61, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Fermin, L.M.; Gonzalez-Reyes, S.; Tarco-Alvarez, N.G.; Hernandez-Nava, M.; Orozco-Ibarra, M.; Pedraza-Chaverri, J. Neuroprotective effect of alpha-mangostin and curcumin against iodoacetate-induced cell death. Nutr. Neurosci. 2012, 15, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Suthammarak, W.; Numpraphrut, P.; Charoensakdi, R.; Neungton, N.; Tunrungruangtavee, V.; Jaisupa, N.; Charoensak, S.; Moongkarndi, P.; Muangpaisan, W. Antioxidant-enhancing property of the polar fraction of mangosteen pericarp extract and evaluation of its safety in humans. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.H.; Zhang, K.J.; Gu, Q.L.; Bi, X.L.; Wang, J.X. Pharmacology of mangostins and their derivatives: A comprehensive review. Chin. J. Nat. Med. 2017, 15, 81–93. [Google Scholar] [CrossRef]
- Ashton, M.M.; Dean, O.M.; Walker, A.J.; Bortolasci, C.C.; Ng, C.H.; Hopwood, M.; Harvey, B.H.; Moller, M.; McGrath, J.J.; Marx, W. The therapeutic potential of mangosteen pericarp as an adjunctive therapy for bipolar disorder and schizophrenia. Front. Psychiatry 2019, 10, 115. [Google Scholar] [CrossRef] [Green Version]
- Menendez-Gonzalez, M.; Padilla-Zambrano, H.S.; Alvarez, G.; Capetillo-Zarate, E.; Tomas-Zapico, C.; Costa, A. Targeting Beta-Amyloid at the CSF: A New Therapeutic Strategy in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 100. [Google Scholar] [CrossRef]
- Lee, L.L.; Ha, H.; Chang, Y.T.; DeLisa, M.P. Discovery of amyloid-beta aggregation inhibitors using an engineered assay for intracellular protein folding and solubility. Protein Sci. 2009, 18, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Salomone, S.; Caraci, F.; Leggio, G.M.; Fedotova, J.; Drago, F. New pharmacological strategies for treatment of Alzheimer’s disease: Focus on disease modifying drugs. Br. J. Clin. Pharmacol. 2012, 73, 504–517. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J. The amyloid hypothesis for Alzheimer’s disease: A critical reappraisal. J. Neurochem. 2009, 110, 1129–1134. [Google Scholar] [CrossRef]
- Chakravarthy, M.; Chen, S.; Dodd, P.R.; Veedu, R.N. Nucleic acid-based theranostics for tackling alzheimer’s disease. Theranostics 2017, 7, 3933. [Google Scholar] [CrossRef] [PubMed]
- Pooler, A.M.; Polydoro, M.; Wegmann, S.; Nicholls, S.B.; Spires-Jones, T.L.; Hyman, B.T. Propagation of tau pathology in Alzheimer’s disease: Identification of novel therapeutic targets. Alzheimers Res. Ther. 2013, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Kumar, A.; Keegan, R.M.; Deshmukh, R. Recent advances in the neurobiology and neuropharmacology of Alzheimer’s disease. Biomed. Pharmacother. 2018, 98, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Cheng, B.; Davis, D.; Bryant, K.; Lieberburg, I.; Rydel, R.E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 1992, 12, 376–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couratier, P.; Lesort, M.; Sindou, P.; Esclaire, F.; Yardin, C.; Hugon, J. Modifications of neuronal phosphorylated τ immunoreactivity induced by NMDA toxicity. Mol. Chem. Neuropathol. 1996, 27, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–186. [Google Scholar] [CrossRef]
- Giacobini, E. Cholinergic function and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2003, 18, S1–S5. [Google Scholar] [CrossRef]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Dickson, D.W. Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2, a009258. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.R. Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease; Springer Science & Business Media: Basel, Switzerland, 2012; Volume 65. [Google Scholar]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef]
- Cheng, H.C.; Ulane, C.M.; Burke, R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Mahlknecht, P. Pharmacologic Treatment of Motor Symptoms Associated with Parkinson Disease. Neurol. Clin. 2020, 38, 255–267. [Google Scholar] [CrossRef]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J.; Lucki, I.; Drevets, W.C. 5-HT1A receptor function in major depressive disorder. Prog. Neurobiol. 2009, 88, 17–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mintun, M.A.; Sheline, Y.I.; Moerlein, S.M.; Vlassenko, A.G.; Huang, Y.; Snyder, A.Z. Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: In vivo measurement with [18F]altanserin positron emission tomography. Biol. Psychiatry 2004, 55, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ruhe, H.G.; Mason, N.S.; Schene, A.H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol. Psychiatry 2007, 12, 331–359. [Google Scholar] [CrossRef] [Green Version]
- Sanacora, G.; Gueorguieva, R.; Epperson, C.N.; Wu, Y.T.; Appel, M.; Rothman, D.L.; Krystal, J.H.; Mason, G.F. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry 2004, 61, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Gelenberg, A.; Freeman, M.; Markowitz, J.; Rosenbaum, J.; Thase, M.; Trivedi, M.; Van Rhoads, R.J.A.J.P. American Psychiatric Association practice guidelines for the treatment of patients with major depressive disorder. Am. J. Psychiatry 2010, 167, 9–118. [Google Scholar]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking neuroinflammation and neurodegeneration in Parkinson’s disease. J. Immunol. Res. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Tan, E.K.; Chao, Y.X.; West, A.; Chan, L.L.; Poewe, W.; Jankovic, J. Parkinson disease and the immune system—Associations, mechanisms and therapeutics. Nat. Rev. Neurol. 2020, 16, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Caraci, F.; Cuello, A.C.; Caruso, G.; Nisticò, R.; Corbo, M.; Baldacci, F.; Toschi, N.; Garaci, F.; Chiesa, P.A.; et al. A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer’s Disease. Front. Immunol. 2020, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Anisman, H.; Merali, Z. Cytokines, stress and depressive illness: Brain-immune interactions. Ann. Med. 2003, 35, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Moffitt, T.E.; Pariante, C.M.; Ambler, A.; Poulton, R.; Caspi, A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry 2008, 65, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Licinio, J.; Wong, M. The role of inflammatory mediators in the biology of major depression: Central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol. Psychiatry 1999, 4, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef]
- Vaváková, M.; Ďuračková, Z.; Trebatická, J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.-G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Turnbull, D.M.; Reeve, A.K. Mitochondrial Dysfunction in Parkinson’s Disease—Cause or Consequence? Biology 2019, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Akundi, R.S. Mitochondria: A connecting link in the major depressive disorder jigsaw. Curr. Neuropharmacol. 2019, 17, 550–562. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef]
- Tanila, H. The role of BDNF in Alzheimer’s disease. Neurobiol. Dis. 2017, 97, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a promising therapeutic agent in Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Li, W.; Lv, L.; Zhang, Z.; Zhan, X. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov. Med. 2018, 26, 127–136. [Google Scholar]
- Schwab, A.D.; Thurston, M.J.; Machhi, J.; Olson, K.E.; Namminga, K.L.; Gendelman, H.E.; Mosley, R.L. Immunotherapy for Parkinson’s disease. Neurobiol. Dis. 2020, 137, 104760. [Google Scholar] [CrossRef]
- Jankovic, J.; Aguilar, L.G. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2008, 4, 743. [Google Scholar] [CrossRef] [Green Version]
- Mrazek, D.A.; Hornberger, J.C.; Altar, C.A.; Degtiar, I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr. Serv. 2014, 65, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Moongkarndi, P.; Srisawat, C.; Saetun, P.; Jantaravinid, J.; Peerapittayamongkol, C.; Soi-ampornkul, R.; Junnu, S.; Sinchaikul, S.; Chen, S.T.; Charoensilp, P.; et al. Protective effect of mangosteen extract against beta-amyloid-induced cytotoxicity, oxidative stress and altered proteome in SK-N-SH cells. J. Proteome Res. 2010, 9, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.; Kim, Y.; Chin, Y.; Cho, J. Inhibition of Excitotoxic and Aβ (25-35)-induced Neuronal Damage by Fractions Prepared from Mangosteen Pericarp in Primary Cultured Rat Cortical Cells. In Proceedings of the 2012 Fall International Convention of The Pharmaceutical Society of Korea, Seoul, Korea, 23–24 October 2012; p. 208. [Google Scholar]
- Cho, J.; Chin, Y.-W. Neuroprotective And Memory-Enhancing Effects of The Water Extract From Pericarp of Mangosteen Fruit. Clin. Ther. 2016, 38, e31. [Google Scholar] [CrossRef] [PubMed]
- Weecharangsan, W.; Opanasopit, P.; Sukma, M.; Ngawhirunpat, T.; Sotanaphun, U.; Siripong, P. Antioxidative and neuroprotective activities of extracts from the fruit hull of mangosteen (Garcinia mangostana Linn.). Med. Princ. Pract. 2006, 15, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Sattayasai, J.; Chaonapan, P.; Arkaravichie, T.; Soi-ampornkul, R.; Junnu, S.; Charoensilp, P.; Samer, J.; Jantaravinid, J.; Masaratana, P.; Suktitipat, B. Protective effects of mangosteen extract on H2O2-induced cytotoxicity in SK-N-SH cells and scopolamine-induced memory impairment in mice. PLoS ONE 2013, 8, e85053. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.J.; Chen, W.L.; Hsieh, R.H.; Hsieh-Li, H.M. Multifunctional Effects of Mangosteen Pericarp on Cognition in C57BL/6J and Triple Transgenic Alzheimer’s Mice. Evid. Based Complement. Alternat. Med. 2014, 2014, 813672. [Google Scholar] [CrossRef]
- Avinash, P.; Reddy, A.; Begum, N.; Bakshi, V. Neuroprotective Effect of Garcinia mangostana on Streptozotocin Induced Sporadic Type Alzheimer’s Disease in Mice. Int. J. Appl. Pharm. Sci. Res. 2016, 1, 8–15. [Google Scholar]
- Wang, S.N.; Li, Q.; Jing, M.H.; Alba, E.; Yang, X.H.; Sabate, R.; Han, Y.F.; Pi, R.B.; Lan, W.J.; Yang, X.B.; et al. Natural Xanthones from Garcinia mangostana with Multifunctional Activities for the Therapy of Alzheimer’s Disease. Neurochem. Res. 2016, 41, 1806–1817. [Google Scholar] [CrossRef]
- Zhao, L.X.; Wang, Y.; Liu, T.; Wang, Y.X.; Chen, H.Z.; Xu, J.R.; Qiu, Y. alpha-Mangostin decreases beta-amyloid peptides production via modulation of amyloidogenic pathway. CNS Neurosci. Ther. 2017, 23, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xia, Z.; Xu, J.R.; Wang, Y.X.; Hou, L.N.; Qiu, Y.; Chen, H.Z. Alpha-mangostin, a polyphenolic xanthone derivative from mangosteen, attenuates beta-amyloid oligomers-induced neurotoxicity by inhibiting amyloid aggregation. Neuropharmacology 2012, 62, 871–881. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.; Oh, Y.; Kim, Y.M.; Chin, Y.W.; Cho, J. Inhibition of Oxidative Neurotoxicity and Scopolamine-Induced Memory Impairment by gamma-Mangostin: In Vitro and In Vivo Evidence. Oxid. Med. Cell. Longev. 2019, 2019, 3640753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaw, K.Y.; Choi, S.B.; Tan, S.C.; Wahab, H.A.; Chan, K.L.; Murugaiyah, V. Prenylated xanthones from mangosteen as promising cholinesterase inhibitors and their molecular docking studies. Phytomedicine 2014, 21, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa)(TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971. [Google Scholar] [PubMed]
- Janhom, P.; Dharmasaroja, P. Neuroprotective Effects of Alpha-Mangostin on MPP(+)-Induced Apoptotic Cell Death in Neuroblastoma SH-SY5Y Cells. J. Toxicol. 2015, 2015, 919058. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.M.; Li, L.D.; Duan, C.L.; Li, Y.J. Neuroprotective effect of alpha-mangostin on mitochondrial dysfunction and alpha-synuclein aggregation in rotenone-induced model of Parkinson’s disease in differentiated SH-SY5Y cells. J. Asian Nat. Prod. Res. 2017, 19, 833–845. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, W.; Ling, J.; Jiang, C. Alpha-Mangostin Inhibits alpha-Synuclein-Induced Microglial Neuroinflammation and Neurotoxicity. Cell. Mol. Neurobiol. 2016, 36, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Parkhe, A.; Parekh, P.; Nalla, L.V.; Sharma, N.; Sharma, M.; Gadepalli, A.; Kate, A.; Khairnar, A. Protective effect of alpha mangostin on rotenone induced toxicity in rat model of Parkinson’s disease. Neurosci. Lett. 2020, 716, 134652. [Google Scholar] [CrossRef]
- Jaisin, Y.; Ratanachamnong, P.; Kuanpradit, C.; Khumpum, W.; Suksamrarn, S. Protective effects of gamma-mangostin on 6-OHDA-induced toxicity in SH-SY5Y cells. Neurosci. Lett. 2018, 665, 229–235. [Google Scholar] [CrossRef]
- Ulusoy, A.; Bjorklund, T.; Buck, K.; Kirik, D. Dysregulated dopamine storage increases the vulnerability to alpha-synuclein in nigral neurons. Neurobiol. Dis. 2012, 47, 367–377. [Google Scholar] [CrossRef]
- Oberholzer, I.; Möller, M.; Holland, B.; Dean, O.M.; Berk, M.; Harvey, B.H. Garcinia mangostana Linn displays antidepressant-like and pro-cognitive effects in a genetic animal model of depression: A bio-behavioral study in the flinders sensitive line rat. Metab. Brain Dis. 2018, 33, 467–480. [Google Scholar] [CrossRef]
- Dazzi, L.; Ladu, S.; Spiga, F.; Vacca, G.; Rivano, A.; Pira, L.; Biggio, G. Chronic treatment with imipramine or mirtazapine antagonizes stress- and FG7142-induced increase in cortical norepinephrine output in freely moving rats. Synapse 2002, 43, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Omoi, N.-O.; Hayasaka, T.; Shinnkai, T.; Suzuki, S.; Abe, K.; Urano, S. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann. N. Y. Acad. Sci. 2002, 959, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.L.; Harvey, B.H.; Viljoen, F.; Ellis, S.M.; Brink, C.B. Ozone exposure of Flinders Sensitive Line rats is a rodent translational model of neurobiological oxidative stress with relevance for depression and antidepressant response. Psychopharmacology 2015, 232, 2921–2938. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Liu, X.; Liu, J.; Cai, E.; Zhao, Y.; Li, H.; Zhang, L.; Li, P.; Gao, Y. α-Mangostin exhibits antidepressant-like effects mediated by the modification of GABAergic, serotonergic and dopaminergic systems. Nat. Prod. Res. 2018, 1–4. [Google Scholar] [CrossRef]
- Lotter, J.; Möller, M.; Dean, O.; Berk, M.; Harvey, B.H. Studies on Haloperidol and Adjunctive α-Mangostin or Raw Garcinia mangostana Linn Pericarp on Bio-Behavioral Markers in an Immune-Inflammatory Model of Schizophrenia in Male Rats. Front. Psychiatry 2020, 11, 121. [Google Scholar] [CrossRef]
- Chang, C.-W.; Huang, T.-Z.; Chang, W.-H.; Tseng, Y.-C.; Wu, Y.-T.; Hsu, M.-C. Acute Garcinia mangostana (mangosteen) supplementation does not alleviate physical fatigue during exercise: A randomized, double-blind, placebo-controlled, crossover trial. J. Int. Soc. Sports Nutr. 2016, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Bae, J.K.; Chae, H.S.; Kim, Y.M.; Sreymom, Y.; Han, L.; Jang, H.Y.; Chin, Y.W. α-Mangostin Regulates Hepatic Steatosis and Obesity through SirT1-AMPK and PPARγ Pathways in High-Fat Diet-Induced Obese Mice. J. Agric. Food Chem. 2015, 63, 8399–8406. [Google Scholar] [CrossRef]
- Kudiganti, V.; Kodur, R.R.; Kodur, S.R.; Halemane, M.; Deep, D.K. Efficacy and tolerability of Meratrim for weight management: A randomized, double-blind, placebo-controlled study in healthy overweight human subjects. Lipids Health Dis. 2016, 15, 136. [Google Scholar] [CrossRef] [Green Version]
- Laupu, W. Interpreting outcomes from the supplementation of mangosteen rind powder capsules in schizophrenia and schizoaffective disorders. Br. J. Med. Health Res. 2016, 3, 15–25. [Google Scholar]
- Ashton, M.M.; Berk, M.; Ng, C.H.; Hopwood, M.; Dodd, S.; Turner, A.; Brown, E.; Jacka, F.N.; Cotton, S.M.; Khoo, J.-P. Efficacy of adjunctive Garcinia mangostana Linn (mangosteen) pericarp for bipolar depression: Study protocol for a proof-of-concept trial. Braz. J. Psychiatry 2019, 41, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Brunner, I.; Han, A.R.; Hamburger, M.; Kinghorn, A.D.; Frye, R.; Butterweck, V. Pharmacokinetics of alpha-mangostin in rats after intravenous and oral application. Mol. Nutr. Food Res. 2011, 55 (Suppl. 1), S67–S74. [Google Scholar] [CrossRef]
- Han, S.Y.; You, B.H.; Kim, Y.C.; Chin, Y.W.; Choi, Y.H. Dose-Independent ADME Properties and Tentative Identification of Metabolites of alpha-Mangostin from Garcinia mangostana in Mice by Automated Microsampling and UPLC-MS/MS Methods. PLoS ONE 2015, 10, e0131587. [Google Scholar]
- Ramaiya, A.; Li, G.; Petiwala, S.M.; Johnson, J.J. Single dose oral pharmacokinetic profile of alpha-mangostin in mice. Curr. Drug Targets 2012, 13, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Foti, R.S.; Pearson, J.T.; Rock, D.A.; Wahlstrom, J.L.; Wienkers, L.C. In vitro inhibition of multiple cytochrome P450 isoforms by xanthone derivatives from mangosteen extract. Drug Metab. Dispos. 2009, 37, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, A.R.; Kinghorn, A.D.; Frye, R.F.; Derendorf, H.; Butterweck, V. Pharmacokinetic properties of pure xanthones in comparison to a mangosteen fruit extract in rats. Planta Med. 2013, 79, 646–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.H.; Han, S.Y.; Kim, Y.J.; Kim, Y.M.; Chin, Y.W. Absorption, tissue distribution, tissue metabolism and safety of alpha-mangostin in mangosteen extract using mouse models. Food Chem. Toxicol. 2014, 66, 140–146. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Riedl, K.M.; Suksumrarn, S.; Clinton, S.K.; Kinghorn, A.D.; Failla, M.L. Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults. J. Nutr. 2012, 142, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Bumrungpert, A.; Kalpravidh, R.W.; Suksamrarn, S.; Chaivisuthangkura, A.; Chitchumroonchokchai, C.; Failla, M.L. Bioaccessibility, biotransformation, and transport of alpha-mangostin from Garcinia mangostana (Mangosteen) using simulated digestion and Caco-2 human intestinal cells. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S54–S61. [Google Scholar] [CrossRef]
- Kondo, M.; Zhang, L.; Ji, H.; Kou, Y.; Ou, B. Bioavailability and antioxidant effects of a xanthone-rich Mangosteen (Garcinia mangostana) product in humans. J. Agric. Food. Chem. 2009, 57, 8788–8792. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Sintara, M.; Chang, T.; Ou, B. Functional beverage of Garcinia mangostana (mangosteen) enhances plasma antioxidant capacity in healthy adults. Food Sci. Nutr. 2015, 3, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Huang, M.; Wang, X.R.; Fu, J.; Han, M.; Shen, Y.Q.; Xia, Z.; Gao, J.Q. Transferrin-modified liposome promotes alpha-mangostin to penetrate the blood-brain barrier. Nanomedicine 2016, 12, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.K.; Jiang, H.; Yang, K.; Wang, Y.Q.; Zhang, Q.; Zuo, J. Development and in vivo evaluation of self-microemulsion as delivery system for alpha-mangostin. Kaohsiung J. Med. Sci. 2017, 33, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Aisha, A.F.; Ismail, Z.; Abu-Salah, K.M.; Majid, A.M. Solid dispersions of alpha-mangostin improve its aqueous solubility through self-assembly of nanomicelles. J. Pharm. Sci. 2012, 101, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Yamin, B.M.; Mat Lazim, A. A study on dispersion and characterisation of alpha-mangostin loaded pH sensitive microgel systems. Chem. Cent. J. 2013, 7, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Tang, G.; Tang, Q.; Zhang, J.; Hou, Y.; Cai, E.; Liu, S.; Lei, D.; Zhang, L.; Wang, S. A Method of Effectively Improved alpha-Mangostin Bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Wojcik, E.; Szwajgier, D. Alzheimer’s disease: Review of current nanotechnological therapeutic strategies. Expert. Rev. Neurother. 2020, 20, 271–279. [Google Scholar] [CrossRef]
- Basak, J.M.; Verghese, P.B.; Yoon, H.; Kim, J.; Holtzman, D.M. Low-density lipoprotein receptor represents an apolipoprotein E-independent pathway of Abeta uptake and degradation by astrocytes. J. Biol. Chem. 2012, 287, 13959–13971. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Gu, X.; Song, Q.; Wang, X.; Huang, M.; Hu, M.; Hou, L.; Kang, T.; Chen, J.; Chen, H.; et al. Nanoformulated alpha-mangostin ameliorates Alzheimer’s disease neuropathology by elevating LDLR expression and accelerating amyloid-beta clearance. J. Control. Release 2016, 226, 1–14. [Google Scholar] [CrossRef]
- Song, Q.; Song, H.; Xu, J.; Huang, J.; Hu, M.; Gu, X.; Chen, J.; Zheng, G.; Chen, H.; Gao, X. Biomimetic ApoE-Reconstituted High Density Lipoprotein Nanocarrier for Blood-Brain Barrier Penetration and Amyloid Beta-Targeting Drug Delivery. Mol. Pharm. 2016, 13, 3976–3987. [Google Scholar] [CrossRef]
- Rahmayanti, F.; Suniarti, D.; Masud, Z.; Bachtiar, B.; Wimardhani, Y.S.; Subita, G. Acute oral toxicity testing of ethyl acetate fraction from Garcinia mangostana Linn extract in sprague-dawley rats. Res. J. Med. Plant. 2016, 10, 261–264. [Google Scholar] [CrossRef]
- Jujun, P.; Pootakham, K.; Pongpaibul, Y.; Duangrat, C.; Tharavichitkul, P. Acute and repeated dose 28-day oral toxicity study of Garcinia mangostana Linn. rind extract. Chiang Mai Univ. J. Nat. Sci. 2008, 7, 199–208. [Google Scholar]
- Hutadilok-Towatana, N.; Reanmongkol, W.; Wattanapiromsakul, C.; Bunkrongcheap, R. Acute and subchronic toxicity evaluation of the hydroethanolic extract of mangosteen pericarp. J. Med. Plant. Res. 2010, 4, 969–974. [Google Scholar]
- Bunyong, R.; Chaijaroenkul, W.; Plengsuriyakarn, T.; Na-Bangchang, K. Antimalarial activity and toxicity of Garcinia mangostana Linn. Asian Pac. J. Trop. Med. 2014, 7, 693–698. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Bhatt, P.C.; Kaithwas, G.; Rashid, M.; Al-Abbasi, F.; Khan, J.A.; Anwar, F.; Verma, A. α-Mangostin mediated pharmacological modulation of hepatic carbohydrate metabolism in diabetes induced Wistar rat. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 255–276. [Google Scholar] [CrossRef] [Green Version]
- Nelli, G.B.; K, A.S.; Kilari, E.K. Antidiabetic effect of alpha-mangostin and its protective role in sexual dysfunction of streptozotocin induced diabetic male rats. Syst. Biol. Reprod. Med. 2013, 59, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Saiyed, Z.M.; Sengupta, K.; Krishnaraju, A.V.; Trimurtulu, G.; Lau, F.C.; Lugo, J.P. Safety and toxicological evaluation of Meratrim(R): An herbal formulation for weight management. Food Chem. Toxicol. 2015, 78, 122–129. [Google Scholar] [CrossRef]
- Kittipaspallop, W.; Taepavarapruk, P.; Chanchao, C.; Pimtong, W.J.E.B. Acute toxicity and teratogenicity of α-mangostin in zebrafish embryos. Exp. Biol. Med. 2018, 243, 1212–1219. [Google Scholar] [CrossRef]
- Gutierrez-Orozco, F.; Thomas-Ahner, J.M.; Berman-Booty, L.D.; Galley, J.D.; Chitchumroonchokchai, C.; Mace, T.; Suksamrarn, S.; Bailey, M.T.; Clinton, S.K.; Lesinski, G.B. Dietary α-mangostin, a xanthone from mangosteen fruit, exacerbates experimental colitis and promotes dysbiosis in mice. Mol. Nutr. Food Res. 2014, 58, 1226–1238. [Google Scholar] [CrossRef]
- Gutierrez-Orozco, F.; Thomas-Ahner, J.M.; Galley, J.D.; Bailey, M.T.; Clinton, S.K.; Lesinski, G.B.; Failla, M.L. Intestinal microbial dysbiosis and colonic epithelial cell hyperproliferation by dietary α-mangostin is independent of mouse strain. Nutrients 2015, 7, 764–784. [Google Scholar] [CrossRef]
- Udani, J.K.; Singh, B.B.; Barrett, M.L.; Singh, V.J. Evaluation of Mangosteen juice blend on biomarkers of inflammation in obese subjects: A pilot, dose finding study. Nutr. J. 2009, 8, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, J.S.; Peerson, J.; Mishra, A.T.; Sadasiva Rao, M.V.; Rajeswari, K.P. Efficacy and tolerability of a novel herbal formulation for weight management. Obesity 2013, 21, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.S.; Peerson, J.; Mishra, A.T.; Mathukumalli, V.S.; Konda, P.R. Efficacy and tolerability of an herbal formulation for weight management. J. Med. Food. 2013, 16, 529–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Sintara, M.; Chang, T.; Ou, B. Daily consumption of a mangosteen-based drink improves in vivo antioxidant and anti-inflammatory biomarkers in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Food Sci. Nutr. 2015, 3, 342–348. [Google Scholar] [CrossRef]
- Wong, L.P.; Klemmer, P.J. Severe lactic acidosis associated with juice of the mangosteen fruit Garcinia mangostana. Am. J. Kidney Dis. 2008, 51, 829–833. [Google Scholar] [CrossRef]
- Lobb, A.L. Science in liquid dietary supplement promotion: The misleading case of mangosteen juice. Hawaii J. Med. Public Health 2012, 71, 46. [Google Scholar]


| No. | Agents | Experimental Models | Experimental Conditions | Results | References |
|---|---|---|---|---|---|
| 1 | Water-soluble partition of methanol MPE | SK-N-SH cells | Aβ1-42 | ↓ Neurotoxicity ↓ Caspase-3 ↓ ROS | [73] |
| 2 | Butanol fraction of methanol MPE | Primary cultured rat cortical neurons | NMDA Aβ25-35 | ↓ Neurotoxicity & apoptotic events ↓ ROS | [74] |
| Rat brain homogenates | Fe2+/ascorbic acid | ↓ Lipid peroxidation | |||
| Cell-free bioassay | ↓ β-Secretase activity | ||||
| 3 | Water MPE | Primary cultured rat cortical neurons | NMDA Aβ25-35 | ↓ Neurotoxicity & apoptotic events ↓ ROS | [75] |
| Rat brain homogenates | Fe2+/ascorbic acid | ↓ Lipid peroxidation | |||
| Cell-free bioassay | ↓ β-Secretase activity ↓ AChE activity | ||||
| ICR mice | Scopolamine | ↓ Memory impairment | |||
| 4 | Water/50% ethanol MPE | NG108-15 cells | H2O2 | ↓Oxidative neurotoxicity Free radical scavenging activity | [76] |
| 5 | Water-soluble partition of ethanol MPE | SK-N-SH cells | H2O2 PCBs | ↓Oxidative neurotoxicity ↓ Caspase-3 ↓ ROS ↓ AChE activity | [77] |
| ICR mice | Scopolamine | ↓ Memory impairment ↓ Brain ROS ↓ Caspase-3 | |||
| 6 | MP diet | 3×Tg-AD mice | NA | ↓ Aβ deposition ↓ Phosphorylated tau ↓ Memory impairment | [78] |
| B6 mice | ↓ Systemic IL-6 ↑ BDNF level ↓ Phosphorylated tau ↓ Cognitive impairment | ||||
| MP | OHSC | ↓ Neurotoxicity ↑ BDNF level | |||
| 7 | 50% Ethanol MPE | Male SA mice | Streptozotocin | ↑ Antioxidant parameters: superoxide dismutase, glutathione peroxidase, glutathione, and catalase ↓ AChE levels ↓ Cognitive & memory impairment | [79] |
| 8 | α-MG, γ-MG, gartanin, garcinone C | HT22 cells | Glutamate | ↓ Neurotoxicity ↑ HO-1 level ↓ DPPH radicals | [80] |
| E. coli/Cell-free bioassay | ↓ Self-induced Aβ aggregation | ||||
| Cell-free bioassay | ↓ β-Secretase activity | ||||
| 9 | α-MG | Primary cultured rat cortical neurons | NA | ↓ β- & γ-Secretase activity ↓ Aβ1-40 & Aβ1-42 production | [81] |
| 10 | α-MG | Primary cultured rat cortical neurons | Aβ1-40 or Aβ1-42 oligomers | ↓ Neurotoxicity ↓ Aβ fibril formation & pre-formed fibrils | [82] |
| 11 | γ-MG | Primary cultured rat cortical neurons | H2O2 or xanthine/xanthine oxidase | ↓ Oxidative neurotoxicity ↓ ROS ↓ DNA fragmentation ↓ Caspases-3 & 9 | [83] |
| Rat brain homogenates | Fe2+/ascorbic acid | ↓ Lipid peroxidation & DPPH radicals | |||
| Cell-free bioassay | ↓ β-Secretase activity | ||||
| ICR mice | Scopolamine | ↓ Memory impairment | |||
| 12 | α-MG, γ-MG, mangostanol, 3-isomangostin, & garcinone C | Cell-free bioassay | NA | ↓ AChE activity | [84] |
| 13 | α-MG | Female B6 mice | LPS | ↓ IL-6, COX-2 & TSPO | [20] |
| No. | Agents | Experimental Models | Experimental Conditions | Results | References |
|---|---|---|---|---|---|
| 1 | α-MG | SH-SY5Y cells | MPP+ | ↓ Apoptosis ↓ ROS | [86] |
| 2 | α-MG | SH-SY5Y cells | Rotenone | ↓ Cell death ↓ Caspases-3 & 8 ↓ ROS ↓ Mitochondrial dysfunction ↓ Aggregation of α-syn and TH loss | [87] |
| 3 | α-MG | Primary rat microglia cells | α-Syn | ↓ ROS ↓ TNF-α, IL-1β, IL-6, NO, NF-κB & NADPH oxidase | [88] |
| Primary rat mesencephalic neuron-glia co-culture | α-Syn | ↓ DAergic neuronal cell death | |||
| 4 | α-MG | Female B6 mice | LPS | ↓ IL-6, COX-2 & TSPO | [20] |
| 5 | α-MG | Adult male SD rats | Rotenone | ↓ MDA, nitrite ↑ GSH ↓ Phosphorylated α-syn ↓ TH+-DAergic neuronal loss in SNpc ↑ Locomotor activity ↑ Neuromuscular function | [89] |
| 6 | γ-MG | SH-SY5Y cells | 6-OHDA | ↓ Neurotoxicity ↓ Apoptosis ↑ Antioxidant potential | [90] |
| Agents | Experimental Models | Experimental Conditions | Results | References |
|---|---|---|---|---|
| Ethyl acetate MPE | FSL rats | Acute treatment Chronic treatment | Antidepressant-like effect Antidepressant & pro-cognitive effects Prominent serotonergic action ↓ Hippocampal lipid peroxidation | [92] |
| Ethyl acetate MPE/α-MG | Pregnant female SD rats | MIA | Antidepressant-like effect in schizophrenia | [98] |
| Mangosteen-based products | Healthy adults | RCT | Positive impact on POMS scores | [99] |
| Mangosteen-based products (Meratrim) | Overweight subjects | RCT | ↓ Body weight ↓ Total mood disturbance | [101] |
| Encapsulated MP powder | Patients with schizophrenia or schizoaffective disorder | RCT | ↓ PANSS ↓ MADRS | [102] |
| Water MPE | Patients with bipolar depression | RCT | No published results | [103] |
| α-MG | Mice | TST | Antidepressant-like activity (reversed by pretreatment with HAL, bicuculline & p-CPA) ↑ Brain DA, 5-HT & GABA levels | [97] |
| α-MG | Female B6 mice | LPS | ↓ IL-6, COX-2 & TSPO | [20] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, H.T.T.; Cho, J. Mangosteen Pericarp and Its Bioactive Xanthones: Potential Therapeutic Value in Alzheimer’s Disease, Parkinson’s Disease, and Depression with Pharmacokinetic and Safety Profiles. Int. J. Mol. Sci. 2020, 21, 6211. https://doi.org/10.3390/ijms21176211
Do HTT, Cho J. Mangosteen Pericarp and Its Bioactive Xanthones: Potential Therapeutic Value in Alzheimer’s Disease, Parkinson’s Disease, and Depression with Pharmacokinetic and Safety Profiles. International Journal of Molecular Sciences. 2020; 21(17):6211. https://doi.org/10.3390/ijms21176211
Chicago/Turabian StyleDo, Ha Thi Thu, and Jungsook Cho. 2020. "Mangosteen Pericarp and Its Bioactive Xanthones: Potential Therapeutic Value in Alzheimer’s Disease, Parkinson’s Disease, and Depression with Pharmacokinetic and Safety Profiles" International Journal of Molecular Sciences 21, no. 17: 6211. https://doi.org/10.3390/ijms21176211
APA StyleDo, H. T. T., & Cho, J. (2020). Mangosteen Pericarp and Its Bioactive Xanthones: Potential Therapeutic Value in Alzheimer’s Disease, Parkinson’s Disease, and Depression with Pharmacokinetic and Safety Profiles. International Journal of Molecular Sciences, 21(17), 6211. https://doi.org/10.3390/ijms21176211