Influence of the Core Formulation on Features and Drug Delivery Ability of Carbamate-Based Nanogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
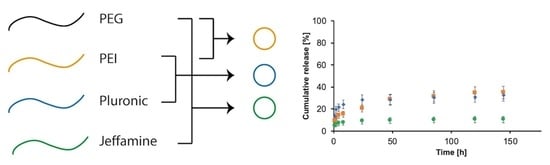
2.2. Synthesis of PEG-PEI Nanogels
2.3. Synthesis of Pluronic F-127-PEI Nanogels
2.4. Synthesis of PEG-Jeffamine Nanogels
2.5. Chemical Characterization of Polymers and Nanogels
2.6. Physical Characterization of Nanogels
2.7. Loading of Nanogels with Different Drug Mimetics
2.8. Drug Loading Evaluation and In Vitro Drug Delivery
2.9. Primary Cell Cultures
2.10. Biocompatibility Tests
3. Results and Debate
3.1. Nanogel Chemical Characterization
3.2. Nanogel Physical Characterization
3.3. Drug Loading and Release Profiles
3.4. Biocompatibility Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CDI | 1,1′-carbonyldiimidazole |
| DLS | Dynamic Light Scattering |
| Jef | Jeffammina (Elastamine® Re-2000 amine) |
| NGs | NanoGels |
| PBS | Phosphate-Buffered Saline solution |
| PDI | PolyDispersity Index |
| PEG | PolyEthylene Glycol |
| PEI | PolyEthylImine |
| RhB | Rhodamine B |
| Plu | Pluronic F-127 |
| SF | Sodium Fluorescein |
References
- Soni, K.S.; Desale, S.S.; Bronichm, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Khatoon, F. Different types of smart nanogel for targeted delivery. J. Sci. Adv. Mater. Dev. 2019, 4, 201–212. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnabas, W. Drug targeting strategies into the brain for treating neurological diseases. J. Neurosci. Method. 2019, 311, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Kandil, R.; Merkel, O.M. Recent progress of polymeric nanogels for gene delivery. Curr. Opin. Colloid Interface Sci. 2019, 39, 11–23. [Google Scholar] [CrossRef]
- Shah, S.; Rangaraj, N.; Laxmikeshav, K.; Sampathi, S. Nanogels as drug carriers – Introduction, chemical aspects, release mechanisms and potential applications. Int. J. Pharm. 2020, 581, 119268. [Google Scholar] [CrossRef]
- Mauri, E.; Perale, G.; Rossi, F. Nanogel Functionalization: A Versatile Approach to Meet the Challenges of Drug and Gene Delivery. ACS Appl. Nano Mater. 2018, 1, 6525–6541. [Google Scholar] [CrossRef]
- Vicario-de-la-Torre, M.; Forcada, J. The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy. Gels 2017, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Ghaeini-Hesaroeiye, S.; Bagtash, H.R.; Boddohi, S.; Vasheghani-Farahani, E.; Jabbari, E. Thermoresponsive Nanogels Based on Different Polymeric Moieties for Biomedical Applications. Gels 2020, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.; Batrakova, E.; Kabanov, A. Poly(ethylene glycol)-polyethyleneimine NanoGel(TM) particles: Novel drug delivery systems for antisense oligonucleotides. Colloids Surf. B 1999, 16, 291–304. [Google Scholar] [CrossRef]
- Sabatino, M.A.; Ditta, L.A.; Conigliaro, A.; Dispenza, C. A multifuctional nanoplatform for drug targeted delivery based on radiation-engineered nanogels. Radiat. Phys. Chem. 2020, 169, 108059. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, Y.; Zhao, Y.; Yang, M.; Li, L. Preparation and evaluation of highly biocompatible nanogels with pH-sensitive charge-convertible capability based on doxorubicin prodrug. Mater. Sci. Eng. C 2019, 98, 161–176. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Blanco, E.R.O.; Gugliotta, L.M.; Alvarez Igarzabal, C.I.; Calderón, M. Crossing biological barriers with nanogels to improve drug delivery performance. J. Control Release 2019, 307, 221–246. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Romanski, J.; Krug, P.; Mazur, M.; Stojek, Z.; Karbarz, M. Tunable environmental sensitivity and degradability of nanogels based on derivatives of cystine and poly(ethylene glycols) of various length for biocompatible drug carrier. Eur. Polym. J. 2019, 118, 606–613. [Google Scholar] [CrossRef]
- Mauri, E.; Moroni, I.; Magagnin, L.; Masi, M.; Sacchetti, A.; Rossi, F. Comparison between two different click strategies to synthesize fluorescent nanogels for therapeutic applications. React. Funct. Polym. 2016, 105, 35–44. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; De, B.; Li, L. Folate-modified pluronic-polyethylenimine and cholic acid polyion complex micelles as targeted drug delivery system for paclitaxel. J. Microencapsul. 2014, 31, 805–814. [Google Scholar] [CrossRef]
- Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A.; Loftsson, T. Cyclodextrin-based nanogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2012, 428, 152–163. [Google Scholar] [CrossRef]
- Argenziano, M.; Dianzani, C.; Ferrara, B.; Swaminathan, S.; Manfredi, A.; Ranucci, E.; Cavalli, R.; Ferruti, P. Cyclodextrin-Based Nanohydrogels Containing Polyamidoamine Units: A New Dexamethasone Delivery System for Inflammatory Diseases. Gels 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinelli, F.; Perale, G.; Rossi, F. Coating and functionalization strategies for nanogels and nanoparticles for selective drug delivery. Gels 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinelli, F.; Sacchetti, A.; Perale, G.; Rossi, F. Is nanoparticle functionalization a versatile approach to meet the challenges of drug and gene delivery? Ther. Deliv. 2020, 11, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Vismara, I.; Papa, S.; Veneruso, V.; Mauri, E.; Mariani, A.; De Paola, M.; Affatato, R.; Rossetti, A.; Sponchioni, M.; Moscatelli, D.; et al. Selective Modulation of A1 Astrocytes by Drug-Loaded Nano-Structured Gel in Spinal Cord Injury. ACS Nano 2020, 14, 360–371. [Google Scholar] [CrossRef]
- Mauri, E.; Veglianese, P.; Papa, S.; Rossetti, A.; De Paola, M.; Mariani, A.; Posel, Z.; Possocco, P.; Sacchetti, A.; Rossi, F. Effects of primary amine-based coatings on microglia internalization of nanogels. Colloids Surf. B 2020, 185, 110574. [Google Scholar] [CrossRef]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 419–433. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gorain, B.; Low, S.Y.; Tan, S.A.; Ling, E.C.S.; Lim, Y.K.; Chin, C.M.; Lee, P.Y.; Ooi, C.H.; Choudhury, H. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res. Clin. Pract. 2018, 136, 52–77. [Google Scholar] [CrossRef]
- Ahmed, S.; Alhareth, K.; Mignet, N. Advancement in nanogel formulations provides controlled drug release. Int. J. Pharm. 2020, 584, 119435. [Google Scholar] [CrossRef]
- Javanmardi, S.; Tamaddon, A.M.; Aghamaali, M.R.; Ghahramani, L.; Abolmaali, S.S. Redox-sensitive, PEG-shielded carboxymethyl PEI nanogels silencing MicroRNA-21, sensitizes resistant ovarian cancer cells to cisplatin. Asian J. Pharm. Sci. 2020, 15, 69–82. [Google Scholar] [CrossRef]
- Yoon, D.Y.; Kim, J.C. In vivo residence duration of human growth hormone loaded in nanogels comprising cinnamoyl alginate, cinnamoyl Pluronic F127 and cinnamoyl poly(ethylene glycol). Int. J. Pharm. 2016, 509, 229–236. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.; Chen, Y.; Ye, J.; Sha, X.; Fang, X. Multifunctional Pluronic P123/F127 mixed polymeric micelles loaded with paclitaxel for the treatment of multidrug resistant tumors. Biomaterials 2011, 32, 2894–2906. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Rabiee, N.; Bagherzadeh, M.; Elmi, F.; Fatahi, Y.; Farjadian, F.; Baheiraei, N.; Nasseri, B.; Rabiee, M.; Dastjerd, N.T.; et al. Stimulus-responsive sequential release systems for drug and gene delivery. Nano Today 2020, 34, 100914. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH Responsive 5-Fluorouracil Loaded Biocompatible Nanogels For Topical Chemotherapy of Aggressive Melanoma. Colloids. Surf. B 2019, 174, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Bardajee, G.R.; Khamooshi, N.; Nasri, S.; Vancaeyzeele, C. Multi-stimuli responsive nanogel/hydrogel nanocomposites based on κ-carrageenan for prolonged release of levodopa as model drug. Int. J. Biol. Macromol. 2020, 153, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Phan, Q.T.; Patil, M.P.; Tu, T.T.K.; Kim, G.-D.; Lim, K.T. Synthesis of zwitterionic redox-responsive nanogels by one-pot amine-thiol-ene reaction for anticancer drug release application. React. Funct. Polym. 2020, 147, 104463. [Google Scholar] [CrossRef]
- Mauri, E.; Rossi, F.; Sacchetti, A. Tunable drug delivery using chemoselective functionalization of hydrogels. Mater. Sci. Eng. C. 2016, 61, 851–857. [Google Scholar] [CrossRef]
- Mauri, E.; Veglianese, P.; Papa, S.; Mariani, A.; De Paola, M.; Rigamonti, R.; Chincarini, G.M.F.; Rimondo, S.; Saccheti, A.; Rossi, F. Chemoselective functionalization of nanogels for microglia treatment. Eur. Polym. J. 2017, 94, 143–151. [Google Scholar] [CrossRef]
- Mauri, E.; Cappella, F.; Masi, M.; Rossi, F. PEGylation influences drug delivery from nanogels. J. Drug Deliv. Sci. Technol. 2018, 46, 87–92. [Google Scholar] [CrossRef]
- Vinogradov, S.V.; Kohli, E.; Zeman, A.D. Comparison of nanogel drug carriers and their formulations with nucleoside 5′-triphosphates. Pharm. Res. 2006, 23, 920–930. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Yang, X.; Zhai, G.; Li, L. Multifunctional pluronic/poly(ethylenimine) nanoparticles for anticancer drug. J. Colloid Interface Sci. 2010, 350, 117–125. [Google Scholar] [CrossRef]
- Mauri, E.; Naso, D.; Rossetti, A.; Borghi, E.; Ottaviano, E.; Griffini, G.; Masi, M.; Saccheti, A.; Rossi, F. Design of polymer-based antimicrobial hydrogels through physico-chemical transition. Mater. Sci. Eng. C 2019, 103, 109791. [Google Scholar] [CrossRef] [PubMed]
- Sponchioni, M.; Rodrigues Bassam, P.; Moscatelli, D.; Arosio, P.; Capasso Palmiero, U. Biodegradable zwitterionic nanoparticles with tunable UCST-type phase separation under physiological conditions. Nanoscale 2019, 11, 16582–16591. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, H.D.; Stansbury, J.W.; Nair, D.P. Photoreactive nanogels as versatile polymer networks with tunable in situ drug release kinetics. J. Mech. Behav. Biomed. Mater. 2020, 108, 103755. [Google Scholar] [CrossRef] [PubMed]







| Nanogel | Diameter (nm) | Polydispersity Index (−) | ζ-Potential (mV) |
|---|---|---|---|
| PEG-PEI NGs | 180 | 0.15 | 0.01 |
| Plu-PEI NGs | 103 | 0.271 | −0.1 |
| PEG-Jef NGs | 165 | 0.252 | −0.08 |
| Nanogel | SF Loading (%) | RhB Loading (%) | Pyrene Loading (%) |
|---|---|---|---|
| PE-PEI NGs | 51 | 56 | 96 |
| Plu-PEI NGs | 58 | 59 | 92 |
| PEG-Jef NGs | 69 | 76 | 98 |
| Nanogel | D-SF (m2/s) | D-RhB (m2/s) |
|---|---|---|
| PEG-PEI NGs | 2.00 × 10−9 | 2.00 × 10−9 |
| Plu-PEI NGs | 2.70 × 10−9 | 3.20 × 10−9 |
| PEG-Jef NGs | 3.30 × 10−9 | 3.00 × 10−9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinelli, F.; Pizzetti, F.; Ortolà, Ó.F.; Marchetti, A.; Rossetti, A.; Sacchetti, A.; Rossi, F. Influence of the Core Formulation on Features and Drug Delivery Ability of Carbamate-Based Nanogels. Int. J. Mol. Sci. 2020, 21, 6621. https://doi.org/10.3390/ijms21186621
Pinelli F, Pizzetti F, Ortolà ÓF, Marchetti A, Rossetti A, Sacchetti A, Rossi F. Influence of the Core Formulation on Features and Drug Delivery Ability of Carbamate-Based Nanogels. International Journal of Molecular Sciences. 2020; 21(18):6621. https://doi.org/10.3390/ijms21186621
Chicago/Turabian StylePinelli, Filippo, Fabio Pizzetti, Óscar Fullana Ortolà, Alessandro Marchetti, Arianna Rossetti, Alessandro Sacchetti, and Filippo Rossi. 2020. "Influence of the Core Formulation on Features and Drug Delivery Ability of Carbamate-Based Nanogels" International Journal of Molecular Sciences 21, no. 18: 6621. https://doi.org/10.3390/ijms21186621
APA StylePinelli, F., Pizzetti, F., Ortolà, Ó. F., Marchetti, A., Rossetti, A., Sacchetti, A., & Rossi, F. (2020). Influence of the Core Formulation on Features and Drug Delivery Ability of Carbamate-Based Nanogels. International Journal of Molecular Sciences, 21(18), 6621. https://doi.org/10.3390/ijms21186621