Resveratrol Pretreatment Ameliorates Concanavalin A-Induced Advanced Renal Glomerulosclerosis in Aged Mice through Upregulation of Sirtuin 1-Mediated Klotho Expression
Abstract
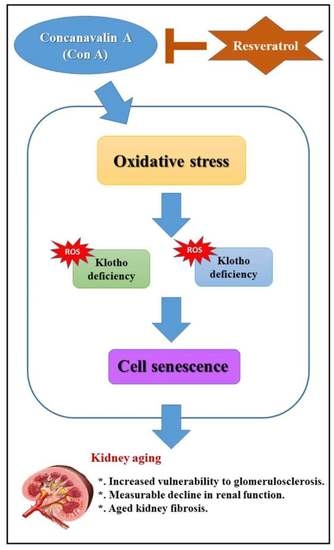
:1. Introduction
2. Results
2.1. Effects of Resveratrol on Con A-Induced Renal Dysfunctions and Histological Changes in Aged Mice
2.2. Effect of Resveratrol on Con A-Induced Glomerulosclerosis in Aged Mice
2.3. Effect of Resveratrol on Klotho and SIRT1 in Con A-Challenged Aged Mice
2.4. Effect of Resveratrol on Con A-Induced Oxidative Stress in the Kidney Tissues of Aged Mice
2.5. Effects of Resveratrol and Klotho Gene Silencing on the Con A-Mediated Reduction of Klotho and SIRT1 Levels and the Increased Expression of Glomerulosclerosis-Related Factors and ROS in Mesangial Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Mouse Glomerular Mesangial Cell Culture
4.3. Measurements of Blood Urea Nitrogen (BUN) and Urinary Concentrations of Albumin and Creatinine
4.4. Histological Staining
4.5. Reverse Transcription Quantitative Polymerase Chain Reaction
4.6. Lipid Peroxidation Assay
4.7. Measurements of SOD Activity and Glutathione (GSH) Levels
4.8. Flow Cytometry for ROS
4.9. Klotho Small Interfering (Si) RNA Transient Transfection
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Materials
Abbreviations
| 8-OH-dG | 8-hydroxydeoxyguanosine |
| AKI | acute kidney injury |
| BUN | blood urea nitrogen |
| CKD | chronic kidney disease |
| Col1 | collagen type I |
| Con A | concanavalin A |
| ECM | extracellular matrix |
| FN | fibronectin |
| GSH | glutathione |
| GST | glutathione S-transferase |
| IHC | immunohistochemistry |
| MDA | malondialdehyde |
| MME | mesangial matrix expansion |
| PIIINP | procollagen III propeptide |
| ROS | reactive oxygen species |
| siRNA | small interfering RNA |
| SIRT1 | sirtuin 1 |
| SOD | superoxide dismutase |
| TF | tubulointerstitial fibrosis |
| TGF-β1 | transforming growth factor-β1 |
| UACR | urinary albumin/creatinine ratio |
| α-SMA | α-smooth muscle actin |
References
- Yoon, H.E.; Kim, E.N.; Kim, M.Y.; Lim, J.H.; Jang, I.A.; Ban, T.H.; Shin, S.J.; Park, C.W.; Chang, Y.S.; Choi, B.S. Age-associated changes in the vascular renin-angiotensin system in mice. Oxid. Med. Cell. Longev. 2016, 2016, 6731093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, M. Aging and the kidney. J. Am. Soc. Nephrol. 1996, 7, 1106–1122. [Google Scholar]
- Yang, H.; Fogo, A.B. Cell senescence in the aging kidney. J. Am. Soc. Nephrol. 2010, 21, 1436–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.H.; Kim, E.N.; Kim, M.Y.; Chung, S.; Shin, S.J.; Kim, H.W.; Yang, C.W.; Kim, Y.S.; Chang, Y.S.; Park, C.W.; et al. Age-associated molecular changes in the kidney in aged mice. Oxid. Med. Cell Longev. 2012, 2012, 171383. [Google Scholar] [CrossRef] [Green Version]
- Razzaque, M.S. Does renal ageing affect survival? Ageing Res. Rev. 2007, 6, 211–222. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Levey, A.S. CKD in the elderly—Old questions and new challenges: World Kidney Day 2008. Am. J. Kidney Dis. 2008, 51, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Simão, S.; Silva, E.; Pinto, V.; Amaral, J.S.; Afonso, J.; Serrão, M.P.; Pinho, M.J.; Soares-da-Silva, P. Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure. Oxid. Med. Cell Longev. 2009, 2, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.S.; Song, M.F.; Kasai, H.; Kawai, K. 8-hydroxyguanine in urine and serum as an oxidative stress marker: Effects of diabetes and aging. J. Uoeh 2013, 35, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.H. Mesangial cells and renal fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 165–194. [Google Scholar]
- Goldstein, I.J.; Hollerman, C.E.; Smith, E.E. Protein-carbohydrate interaction. II. Inhibition studies on the interaction of concanavalin A with polysaccharides. Biochemistry 1965, 4, 876–883. [Google Scholar] [CrossRef]
- Tiegs, G.; Hentschel, J.; Wendel, A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Invest. 1992, 90, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Chen, C.C.; Liu, H.M.; Lee, T.Y.; Shieh, S.H. Resveratrol pretreatment attenuates concanavalin A-induced hepatitis through reverse of aberration in the immune response and regenerative capacity in aged mice. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Cheng, Z.; Wang, Y.; Dai, X.; Zhang, J.; Xue, D. Paeoniflorin exerts a nephroprotective effect on concanavalin A-induced damage through inhibition of macrophage infiltration. Diagn. Pathol. 2015, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Dali-Youcef, N.; Lagouge, M.; Froelich, S.; Koehl, C.; Schoonjans, K.; Auwerx, J. Sirtuins: The ‘magnificent seven’, function, metabolism and longevity. Ann. Med. 2007, 39, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.Y.; Cai, W.; Li, X.; Fang, L.; Xu, J.; Yacoub, R.; He, J.C.; Lee, K. Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am. J. Physiol. Ren. Physiol. 2017, 313, F621–F628. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, S.R.; Huang, X.Z.; Xie, Q.H.; Xu, Y.Y.; Shang, D.; Hao, C.M. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1–dependent manner. J. Am. Soc. Nephrol. 2017, 28, 2337–2352. [Google Scholar] [CrossRef] [Green Version]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Huang, C.L.; Moe, O.W. Klotho: A novel regulator of calcium and phosphorus homeostasis. Pflügers Arch. 2011, 462, 185–193. [Google Scholar] [CrossRef]
- Sugiura, H.; Yoshida, T.; Shiohira, S.; Kohei, J.; Mitobe, M.; Kurosu, H.; Kuro-o, M.; Nitta, K.; Tsuchiya, K. Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am. J. Physiol. Ren. Physiol. 2012, 302, F1252–F1264. [Google Scholar] [CrossRef]
- Saldanha, J.F.; Leal, V.dO.; Stenvinkel, P.; Carraro-Eduardo, J.C.; Mafra, D. Resveratrol: Why is it a promising therapy for chronic kidney disease patients? Oxid. Med. Cell. Longev. 2013, 2013, 963217. [Google Scholar] [CrossRef]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagul, P.K.; Banerjee, S.K. Application of resveratrol in diabetes: Rationale, strategies and challenges. Curr. Mol. Med. 2015, 15, 312–330. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, JL.; Kurokawa, Y.; Takami, A. Rationale for assessing the therapeutic potential of resveratrol in hematological malignancies. Blood Rev. 2019, 33, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Halter, J.B.; Hazzard, W.R.; Himmelfarb, J.; Horne, F.M.; Kaysen, G.A.; Kusek, J.W.; Nayfield, S.G.; Schmader, K.; Tian, Y.; et al. Prediction, progression, and outcomes of chronic kidney disease in older adults. J. Am. Soc. Nephrol. 2009, 20, 1199–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, R.; Melk, A. Molecular mechanisms of renal aging. Kidney Int. 2017, 92, 569–579. [Google Scholar] [CrossRef]
- Wiggins, J.E. Aging in the glomerulus. J. Gerontol. A 2012, 67, 1358–1364. [Google Scholar] [CrossRef] [Green Version]
- Pavenstadt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef] [Green Version]
- Higashiyama, H.; Yoshimoto, D.; Kaise, T.; Matsubara, S.; Fujiwara, M.; Kikkawa, H.; Asano, S.; Kinoshita, M. Inhibition of activin receptor-like kinase 5 attenuates bleomycin-induced pulmonary fibrosis. Exp. Mol. Pathol. 2007, 83, 39–46. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, R.; Torreggiani, M.; Ting, A.; Xiong, H.; Striker, GE.; Vlassara, H.; Zheng, F. Induction of diabetes in aged C57B6 mice results in severe nephropathy: An association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am. J. Pathol. 2010, 176, 2163–2176. [Google Scholar] [CrossRef]
- Kasai, H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat. Res. 1997, 387, 147–163. [Google Scholar] [CrossRef]
- Jang, I.A.; Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Effects of resveratrol on the renin-angiotensin system in the aging kidney. Nutrients 2018, 10, 1741. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Ma, X.; Zhou, Y.; Liu, Y.; Shao, Y.; Wang, Q. Klotho restraining Egr1/TLR4/mTOR axis to reducing the expression of fibrosis and inflammatory cytokines in high glucose cultured rat mesangial cells. Exp. Clin. Endocrinol. Diabetes 2019, 127, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Huang, S.M.; Chen, A.; Sun, C.Y.; Lin, S.H.; Chen, J.S.; Liu, S.T.; Hsu, Y.J. Resveratrol increases anti-aging Klotho gene expression via the activating transcription factor 3/c-Jun complex-mediated signaling pathway. Int. J. Biochem. Cell Biol. 2014, 53, 361–371. [Google Scholar] [CrossRef]
- Ergür, B.U.; Mıcılı, S.Ç.; Yılmaz, O.; Akokay, P. The effects of α-lipoic acid on aortic injury and hypertension in the rat remnant kidney (5/6 nephrectomy) model. Anatol. J. Cardiol. 2015, 15, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Mao, S.; Yu, J.; Song, J.; Jia, Z.; Huang, S.M.; Zhang, A.H. NLRP3 deletion protects against renal fibrosis and attenuates mitochondrial abnormality in mouse with 5/6 nephrectomy. Am. J. Physiol. Renal Physiol. 2016, 310, F1081–F1088. [Google Scholar] [CrossRef] [Green Version]
- Yokozawa, T.; Zheng, P.D.; Oura, H.; Koizumi, F. Animal model of adenine-induced chronic renal failure in rats. Nephron 1986, 44, 230–234. [Google Scholar] [CrossRef]
- López-Novoa, J.M.; Rodríguez-Peña, A.B.; Ortiz, A.; Martínez-Salgado, C.; López Hernández, F.J. Etiopathology of chronic tubular, glomerular and renovascular nephropathies: Clinical implications. J. Transl. Med. 2011, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, R.L.; Forbes, M.S.; Thornhill, B.A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009, 75, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- Tsaih, S.W.; Pezzolesi, M.G.; Yuan, R.; Warram, J.H.; Krolewski, A.S.; Korstanje, R. Genetic analysis of albuminuria in aging mice and concordance with loci for human diabetic nephropathy found in a genome-wide association scan. Kidney Int. 2010, 77, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Chang, Z.Y.; Lee, T.Y.; Huang, T.H.; Wen, C.K.; Chien, R.N.; Chang, H.H. Hepatoprotective effects of Ger-Gen-Chyn-Lian-Tang in thioacetamide-induced fibrosis in mice. J. Chin. Med. Assoc. 2014, 77, 360–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Gene | Forward | Reverse |
|---|---|---|
| Klotho | AGACCTCCCGATGTATGTGAC | CGAGATGAAGACCAGCAAAG |
| SIRT-1 | GCAACAGCATCTTGCCTGA | GTGCTACTGGTCTCACTT |
| TGF-β | TGCCCTCTACAACCAACACAACCCG | AACTGCTCCACCTTGGGCTTGCGAC |
| Fibronectin | TAGGAGAACAGTGGCAGAAAG | CCATCGGGACTGGGTTCA |
| Procollagen-III | CCCCTGGTCCCTGCTGTGG | GAGGCCCGGCTGGAAAGAA |
| Collagen I | GAGAGGTGAACAAGGTCCCG | AAACCTCTCTCGCCTCTTGC |
| GAPDH | TCACCACCATGGAGAAGGC | GCTAAGCAGTTGGTGGTGCA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Chang, Z.-Y.; Tsai, F.-J.; Chen, S.-Y. Resveratrol Pretreatment Ameliorates Concanavalin A-Induced Advanced Renal Glomerulosclerosis in Aged Mice through Upregulation of Sirtuin 1-Mediated Klotho Expression. Int. J. Mol. Sci. 2020, 21, 6766. https://doi.org/10.3390/ijms21186766
Chen C-C, Chang Z-Y, Tsai F-J, Chen S-Y. Resveratrol Pretreatment Ameliorates Concanavalin A-Induced Advanced Renal Glomerulosclerosis in Aged Mice through Upregulation of Sirtuin 1-Mediated Klotho Expression. International Journal of Molecular Sciences. 2020; 21(18):6766. https://doi.org/10.3390/ijms21186766
Chicago/Turabian StyleChen, Chin-Chang, Zi-Yu Chang, Fuu-Jen Tsai, and Shih-Yin Chen. 2020. "Resveratrol Pretreatment Ameliorates Concanavalin A-Induced Advanced Renal Glomerulosclerosis in Aged Mice through Upregulation of Sirtuin 1-Mediated Klotho Expression" International Journal of Molecular Sciences 21, no. 18: 6766. https://doi.org/10.3390/ijms21186766
APA StyleChen, C. -C., Chang, Z. -Y., Tsai, F. -J., & Chen, S. -Y. (2020). Resveratrol Pretreatment Ameliorates Concanavalin A-Induced Advanced Renal Glomerulosclerosis in Aged Mice through Upregulation of Sirtuin 1-Mediated Klotho Expression. International Journal of Molecular Sciences, 21(18), 6766. https://doi.org/10.3390/ijms21186766