Insights into Species Preservation: Cryobanking of Rabbit Somatic and Pluripotent Stem Cells
Abstract
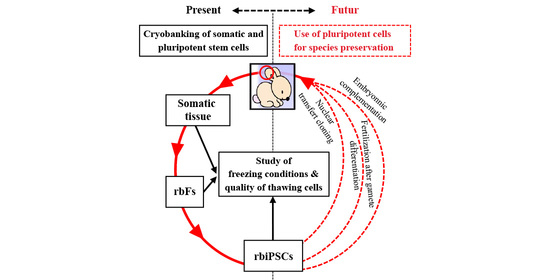
:1. Introduction
2. Results
2.1. Experimental Designs
2.2. Derivation of rbfs from Frozen Tissue
2.3. Quality of Frozen Tissue-Derived rbFs
2.4. Viability of Frozen–Thawed PSCs
2.5. Growth Curves of Frozen–Thawed PSCs
2.6. Gene Expression in Frozen–Thawed PSCs
2.7. Thermodynamic Properties of Freezing Media
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture
4.3. Cryopreservation
4.4. Flow Cytometry Assessment of Viability
4.5. Growth Curves
4.6. Infection Rate
4.7. Gene Expression
4.8. Differential Scanning Clorimetry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The State of the World’s Biodiversity for Food and Agriculture. In Commission on Genetic Resources for Food and Agriculture Assessments; Bélanger, J., Pilling, D., Eds.; FAO: Rome, Italy, 2019; pp. 65–111. Available online: http://www.fao.org/3/CA3129EN/CA3129EN.pdf (accessed on 1 October 2020).
- Blackburn, H.D. Genetic selection and conservation of genetic diversity. Reprod. Domest. Anim. 2012, 47 (Suppl. 4), 249–254. [Google Scholar] [CrossRef]
- Delaveau, A.M.S.; Tixier Boichard, M. CRB-Anim Website (INRAE) Biological Resource Centers for Domestic Animals of AgroBRC. 2018. Available online: https://www.crb-anim.fr/crb-anim_eng/ (accessed on 1 October 2020).
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, A.; Evans, M.; Kaufman, M.H.; Robertson, E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 1984, 309, 255–256. [Google Scholar] [CrossRef]
- Cai, H.; Xia, X.; Wang, L.; Liu, Y.; He, Z.; Guo, Q.; Xu, C. In vitro and in vivo differentiation of induced pluripotent stem cells into male germ cells. Biochem. Biophys. Res. Commun. 2013, 433, 286–291. [Google Scholar] [CrossRef]
- Hayashi, K.; Saitou, M. Generation of eggs from mouse embryonic stem cells and induced pluripotent stem cells. Nat. Protoc. 2013, 8, 1513–1524. [Google Scholar] [CrossRef]
- Kou, Z.; Kang, L.; Yuan, Y.; Tao, Y.; Zhang, Y.; Wu, T.; He, J.; Wang, J.; Liu, Z.; Gao, S. Mice cloned from induced pluripotent stem cells (iPSCs). Biol. Reprod. 2010, 83, 238–243. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Ding, C.; Zhao, X.; Wang, E.; Dai, X.; Liu, L.; Li, W.; Liu, Z.; Wan, H.; Feng, C.; et al. Successful generation of cloned mice using nuclear transfer from induced pluripotent stem cells. Cell Res. 2010, 20, 850–853. [Google Scholar] [CrossRef]
- Honda, A.; Choijookhuu, N.; Izu, H.; Kawano, Y.; Inokuchi, M.; Honsho, K.; Lee, A.R.; Nabekura, H.; Ohta, H.; Tsukiyama, T.; et al. Flexible adaptation of male germ cells from female iPSCs of endangered Tokudaia osimensis. Sci. Adv. 2017, 3, e1602179. [Google Scholar] [CrossRef] [Green Version]
- Stanton, M.M.; Tzatzalos, E.; Donne, M.; Kolundzic, N.; Helgason, I.; Ilic, D. Prospects for the Use of Induced Pluripotent Stem Cells in Animal Conservation and Environmental Protection. Stem Cells Transl. Med. 2019, 8, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, R.R. The rabbit as a research subject. Physiologist 1984, 27, 393–402. [Google Scholar] [PubMed]
- Houdebine, L.M. Transgenic animal models in biomedical research. Methods Mol. Biol. 2007, 360, 163–202. [Google Scholar] [CrossRef] [PubMed]
- Duranthon, V.; Beaujean, N.; Brunner, M.; Odening, K.E.; Santos, A.N.; Kacskovics, I.; Hiripi, L.; Weinstein, E.J.; Bosze, Z. On the emerging role of rabbit as human disease model and the instrumental role of novel transgenic tools. Transgenic Res. 2012, 21, 699–713. [Google Scholar] [CrossRef]
- Houdebine, L.M. Transgenic animal bioreactors. Transgenic Res. 2000, 9, 305–320. [Google Scholar] [CrossRef]
- Wang, S.; Tang, X.; Niu, Y.; Chen, H.; Li, B.; Li, T.; Zhang, X.; Hu, Z.; Zhou, Q.; Ji, W. Generation and characterization of rabbit embryonic stem cells. Stem Cells Dayt. Ohio 2007, 25, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Hirose, M.; Inoue, K.; Ogonuki, N.; Miki, H.; Shimozawa, N.; Hatori, M.; Shimizu, N.; Murata, T.; Hirose, M.; et al. Stable embryonic stem cell lines in rabbits: Potential small animal models for human research. Reprod. Biomed. Online 2008, 17, 706–715. [Google Scholar] [CrossRef]
- Intawicha, P.; Ou, Y.W.; Lo, N.W.; Zhang, S.C.; Chen, Y.Z.; Lin, T.A.; Su, H.L.; Guu, H.F.; Chen, M.J.; Lee, K.H.; et al. Characterization of embryonic stem cell lines derived from New Zealand white rabbit embryos. Cloning Stem Cells 2009, 11, 27–38. [Google Scholar] [CrossRef]
- Honda, A.; Hirose, M.; Hatori, M.; Matoba, S.; Miyoshi, H.; Inoue, K.; Ogura, A. Generation of induced pluripotent stem cells in rabbits: Potential experimental models for human regenerative medicine. J. Biol. Chem. 2010, 285, 31362–33136. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Shen, Y.; Yuan, X.; Chen, K.; Guo, X.; Chen, Y.; Niu, Y.; Li, J.; Xu, R.H.; Yan, X.; et al. Dissecting signaling pathways that govern self-renewal of rabbit embryonic stem cells. J. Biol. Chem. 2008, 283, 35929–35940. [Google Scholar] [CrossRef] [Green Version]
- Honda, A.; Hirose, M.; Ogura, A. Basic FGF and Activin/Nodal but not LIF signaling sustain undifferentiated status of rabbit embryonic stem cells. Exp. Cell Res. 2009, 315, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, T.; Suemori, H. Slow Cooling Cryopreservation Optimized to Human Pluripotent Stem Cells. Adv. Exp. Med. Biol. 2016, 951, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Assou, S.; Bouckenheimer, J.; De Vos, J. Concise Review: Assessing the Genome Integrity of Human Induced Pluripotent Stem Cells: What Quality Control Metrics? Stem Cells 2018, 36, 814–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Saha, D.; Niemann, H.; Gryshkov, O.; Glasmacher, B.; Hofmann, N. Effects of cryopreservation on the epigenetic profile of cells. Cryobiology 2017, 74, 1–7. [Google Scholar] [CrossRef]
- Salehi, M.; Mahdavi, A.H.; Sharafi, M.; Shahverdi, A. Cryopreservation of rooster semen: Evidence for the epigenetic modifications of thawed sperm. Theriogenology 2020, 142, 15–25. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, Y.; Gao, S. Epigenetic regulation of somatic cell reprogramming. Curr. Opin. Genet. Dev. 2017, 46, 146–163. [Google Scholar] [CrossRef]
- Hunt, C.J. Cryopreservation of Human Stem Cells for Clinical Application: A Review. Transfus. Med. Hemother. 2011, 38, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Porter, L.H.; Lawrence, M.G.; Wang, H.; Clark, A.K.; Bakshi, A.; Obinata, D.; Goode, D.; Papargiris, M.; Clouston, D.; Ryan, A.; et al. Establishing a cryopreservation protocol for patient-derived xenografts of prostate cancer. Prostate 2019, 79, 1326–1337. [Google Scholar] [CrossRef]
- Hunt, C.J. Cryopreservation: Vitrification and Controlled Rate Cooling. Methods Mol. Biol. 2017, 1590, 41–77. [Google Scholar] [CrossRef]
- Dalmazzo, A.; Losano, J.D.A.; Rocha, C.C.; Tsunoda, R.H.; Angrimani, D.S.R.; Mendes, C.M.; Assumpcao, M.; Nichi, M.; Barnabe, V.H. Effects of Soy Lecithin Extender on Dog Sperm Cryopreservation. Anim. Biotechnol. 2018, 29, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Ikebata, Y.; Onitsuka, T.; Do, L.T.; Sato, Y.; Taniguchi, M.; Otoi, T. Cryopreservation for bovine embryos in serum-free freezing medium containing silk protein sericin. Cryobiology 2013, 67, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Florentin, A.; Margalit, R. Hyaluronan is a key component in cryoprotection and formulation of targeted unilamellar liposomes. Biochim. Biophys. Acta 2003, 1612, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Thananurak, P.; Chuaychu-Noo, N.; Thelie, A.; Phasuk, Y.; Vongpralub, T.; Blesbois, E. Sucrose increases the quality and fertilizing ability of cryopreserved chicken sperms in contrast to raffinose. Poult. Sci. 2019, 98, 4161–4171. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Pramanik, K.; Sarangi, S.K.; Jain, N. Serum-free non-toxic freezing solution for cryopreservation of human adipose tissue-derived mesenchymal stem cells. Biotechnol. Lett. 2016, 38, 1397–1404. [Google Scholar] [CrossRef]
- Bruyere, P.; Baudot, A.; Joly, T.; Commin, L.; Pillet, E.; Guerin, P.; Louis, G.; Josson-Schramme, A.; Buff, S. A chemically defined medium for rabbit embryo cryopreservation. PLoS ONE 2013, 8, e71547. [Google Scholar] [CrossRef]
- Teixeira, M.; Commin, L.; Gavin-Plagne, L.; Bruyere, P.; Buff, S.; Joly, T. Rapid cooling of rabbit embryos in a synthetic medium. Cryobiology 2018, 85, 113–119. [Google Scholar] [CrossRef]
- Jurga, M.; Forraz, N.; Basford, C.; Atzeni, G.; Trevelyan, A.J.; Habibollah, S.; Ali, H.; Zwolinski, S.A.; McGuckin, C.P. Neurogenic properties and a clinical relevance of multipotent stem cells derived from cord blood samples stored in the biobanks. Stem Cells Dev. 2012, 21, 923–936. [Google Scholar] [CrossRef] [Green Version]
- Sarnowska, A.; Jablonska, A.; Jurga, M.; Dainiak, M.; Strojek, L.; Drela, K.; Wright, K.; Tripathi, A.; Kumar, A.; Jungvid, H.; et al. Encapsulation of mesenchymal stem cells by bioscaffolds protects cell survival and attenuates neuroinflammatory reaction in injured brain tissue after transplantation. Cell Transpl. 2013, 22 (Suppl. 1), S67–S82. [Google Scholar] [CrossRef]
- Mueller, A.A.; Forraz, N.; Gueven, S.; Atzeni, G.; Degoul, O.; Pagnon-Minot, A.; Hartmann, D.; Martin, I.; Scherberich, A.; McGuckin, C. Osteoblastic differentiation of Wharton jelly biopsy specimens and their mesenchymal stromal cells after serum-free culture. Plast. Reconstr. Surg. 2014, 134, 59e–69e. [Google Scholar] [CrossRef]
- Ducret, M.; Fabre, H.; Farges, J.C.; Degoul, O.; Atzeni, G.; McGuckin, C.; Forraz, N.; Mallein-Gerin, F.; Perrier-Groult, E. Production of Human Dental Pulp Cells with a Medicinal Manufacturing Approach. J. Endod. 2015, 41, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, P.; Baudot, A.; Guyader-Joly, C.; Guerin, P.; Louis, G.; Buff, S. Improved cryopreservation of in vitro-produced bovine embryos using a chemically defined freezing medium. Theriogenology 2012, 78, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Gavin-Plagne, L.; Commin, L.; Bruyere, P.; Buff, S.; Joly, T. Comparison Between an Animal-Derived Product Medium and a Chemically Defined Medium for Ram Sperm Cryopreservation. Biopreserv. Biobank. 2019, 17, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Abir, R.; Ben-Aharon, I.; Garor, R.; Yaniv, I.; Ash, S.; Stemmer, S.M.; Ben-Haroush, A.; Freud, E.; Kravarusic, D.; Sapir, O.; et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum. Reprod. 2016, 31, 750–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Ji, X.; Chen, T.; Qi, F.; Tian, F.; Yao, Q.; Tian, F. Deep hypothermic preservation of autologous skin in the treatment of large-area circumferential multi-plane degloving trauma: A pilot study of 2 cases. Cell Tissue Bank. 2019, 20, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Afanassieff, M.; Perold, F.; Bouchereau, W.; Cadiou, A.; Beaujean, N. Embryo-derived and induced pluripotent stem cells: Towards naive pluripotency and chimeric competency in rabbits. Exp. Cell Res. 2020, 389, 111908. [Google Scholar] [CrossRef]
- Li, Y.; Ma, T. Bioprocessing of cryopreservation for large-scale banking of human pluripotent stem cells. Biores. Open Access 2012, 1, 205–214. [Google Scholar] [CrossRef]
- Heng, W.L.; Madhavan, K.; Wee, P.; Seck, T.; Lim, Y.P.; Lim, C.H. Banking of cryopreserved iliac artery and vein homografts: Clinical uses in transplantation. Cell Tissue Bank. 2015, 16, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Hu, Z.; Song, H.; Chen, B.; Xie, D.; Zhou, L.; Zhang, Y. Cartilage storage at 4 degrees C with regular culture medium replacement benefits chondrocyte viability of osteochondral grafts in vitro. Cell Tissue Bank. 2016, 17, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Noguchi, H.; Yukawa, H.; Oishi, K.; Matsushita, K.; Iwata, H.; Hayashi, S. Cryopreservation of Induced Pluripotent Stem Cells. Cell Med. 2012, 3, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Sart, S.; Ma, T.; Li, Y. Cryopreservation of pluripotent stem cell aggregates in defined protein-free formulation. Biotechnol. Prog. 2013, 29, 143–153. [Google Scholar] [CrossRef]
- Liu, W.; Chen, G. Cryopreservation of human pluripotent stem cells in defined medium. Curr. Protoc. Stem. Cell Biol. 2014, 31, 1C.17.11–1C.17.13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, A.; Matsumura, K.; Lee, J.J.; Sumi, S.; Hyon, S.H. StemCell Keep Is Effective for Cryopreservation of Human Embryonic Stem Cells by Vitrification. Cell Transpl. 2017, 26, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Kayakabe, M.; Tsutsumi, S.; Watanabe, H.; Kato, Y.; Takagishi, K. Transplantation of autologous rabbit BM-derived mesenchymal stromal cells embedded in hyaluronic acid gel sponge into osteochondral defects of the knee. Cytotherapy 2006, 8, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Saw, K.Y.; Anz, A.; Merican, S.; Tay, Y.G.; Ragavanaidu, K.; Jee, C.S.; McGuire, D.A. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: A report of 5 cases with histology. Arthroscopy 2011, 27, 493–506. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Joly, T.; Nibart, M.; Thibier, M. Hyaluronic acid as a substitute for proteins in the deep-freezing of embryos from mice and sheep: An in vitro investigation. Theriogenology 1992, 37, 473–480. [Google Scholar] [CrossRef]
- Sbracia, M.; Grasso, J.; Sayme, N.; Stronk, J.; Huszar, G. Hyaluronic acid substantially increases the retention of motility in cryopreserved/thawed human spermatozoa. Hum. Reprod. 1997, 12, 1949–1954. [Google Scholar] [CrossRef] [Green Version]
- Lotfi, S.; Mehri, M.; Sharafi, M.; Masoudi, R. Hyaluronic acid improves frozen-thawed sperm quality and fertility potential in rooster. Anim. Reprod. Sci. 2017, 184, 204–210. [Google Scholar] [CrossRef]
- Adler, S.; Pellizzer, C.; Paparella, M.; Hartung, T.; Bremer, S. The effects of solvents on embryonic stem cell differentiation. Toxicol. Vitr. 2006, 20, 265–271. [Google Scholar] [CrossRef]
- Katkov, I.I.; Kim, M.S.; Bajpai, R.; Altman, Y.S.; Mercola, M.; Loring, J.F.; Terskikh, A.V.; Snyder, E.Y.; Levine, F. Cryopreservation by slow cooling with DMSO diminished production of Oct-4 pluripotency marker in human embryonic stem cells. Cryobiology 2006, 53, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Rowley, S.D.; Braine, H.G.; Piantadosi, S.; Santos, G.W. Clinical toxicity of cryopreserved bone marrow graft infusion. Blood 1990, 75, 781–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahamsen, J.F.; Bakken, A.M.; Bruserud, O. Cryopreserving human peripheral blood progenitor cells with 5-percent rather than 10-percent DMSO results in less apoptosis and necrosis in CD34+ cells. Transfusion 2002, 42, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Windrum, P.; Morris, T.C. Severe neurotoxicity because of dimethyl sulphoxide following peripheral blood stem cell transplantation. Bone Marrow Transpl. 2003, 31, 315. [Google Scholar] [CrossRef] [Green Version]
- Thirumala, S.; Goebel, W.S.; Woods, E.J. Clinical grade adult stem cell banking. Organogenesis 2009, 5, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.Y.; Jee, B.C.; Suh, C.S.; Kim, H.S.; Oh, S.K.; Kim, S.H.; Moon, S.Y. Cryopreservation of human embryonic stem cells without the use of a programmable freezer. Hum. Reprod. 2005, 20, 1779–1785. [Google Scholar] [CrossRef]
- Baran, S.W.; Ware, C.B. Cryopreservation of rhesus macaque embryonic stem cells. Stem Cells Dev. 2007, 16, 339–344. [Google Scholar] [CrossRef]
- Xu, X.; Cowley, S.; Flaim, C.J.; James, W.; Seymour, L.W.; Cui, Z. Enhancement of cell recovery for dissociated human embryonic stem cells after cryopreservation. Biotechnol. Prog. 2010, 26, 781–788. [Google Scholar] [CrossRef]
- Ntai, A.; La Spada, A.; De Blasio, P.; Biunno, I. Trehalose to cryopreserve human pluripotent stem cells. Stem Cell Res. 2018, 31, 102–112. [Google Scholar] [CrossRef]
- Aye, M.; Di Giorgio, C.; De Mo, M.; Botta, A.; Perrin, J.; Courbiere, B. Assessment of the genotoxicity of three cryoprotectants used for human oocyte vitrification: Dimethyl sulfoxide, ethylene glycol and propylene glycol. Food Chem. Toxicol. 2010, 48, 1905–1912. [Google Scholar] [CrossRef]
- Morris, J.G.; Acton, E. Controlled ice nucleation in cryopreservation—A review. Cryobiology 2013, 66, 85–92. [Google Scholar] [CrossRef]
- Ware, C.B.; Nelson, A.M.; Blau, C.A. Controlled-rate freezing of human ES cells. Biotechniques 2005, 38, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.F.; Hua, T.C.; Wu, J.; Chang, Z.H.; Tsung, H.C.; Cao, Y.L. Cryopreservation of human embryonic stem cells: A protocol by programmed cooling. CryoLetters 2006, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, J.C.; Li, L.S. Comparison of three methods for cryopreservation of human embryonic stem cells. Fertil. Steril. 2010, 93, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Afanassieff, M.; Osteil, P.; Savatier, P. Generation of Embryonic Stem Cells in Rabbits. Methods Mol. Biol. 2016, 1341, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Afanassieff, M.; Tapponnier, Y.; Savatier, P. Generation of Induced Pluripotent Stem Cells in Rabbits. Methods Mol. Biol. 2016, 1357, 149–172. [Google Scholar] [CrossRef]
- Osteil, P.; Moulin, A.; Santamaria, C.; Joly, T.; Jouneau, L.; Aubry, M.; Tapponnier, Y.; Archilla, C.; Schmaltz-Panneau, B.; Lecardonnel, J.; et al. A Panel of Embryonic Stem Cell Lines Reveals the Variety and Dynamic of Pluripotent States in Rabbits. Stem Cell Rep. 2016, 7, 383–398. [Google Scholar] [CrossRef] [Green Version]
- Osteil, P.; Tapponnier, Y.; Markossian, S.; Godet, M.; Schmaltz-Panneau, B.; Jouneau, L.; Cabau, C.; Joly, T.; Blachere, T.; Gocza, E.; et al. Induced pluripotent stem cells derived from rabbits exhibit some characteristics of naive pluripotency. Biol. Open 2013, 2, 613–628. [Google Scholar] [CrossRef] [Green Version]
- Toyooka, Y.; Shimosato, D.; Murakami, K.; Takahashi, K.; Niwa, H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 2008, 135, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Desnos, H.; Baudot, A.; Teixeira, M.; Louis, G.; Commin, L.; Buff, S.; Bruyere, P. Ice Induction in DSC experiments with Snomax. Thermochim. Acta 2018, 667, 193–206. [Google Scholar] [CrossRef]
- Johnson, J.B.; Omland, K.S. Model selection in ecology and evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Symonds, M.R.E.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2011, 65, 13–21. [Google Scholar] [CrossRef]






| Biopsy Treatments | Piece Sizes | Type of Tissues | Tissue Freezing | Quantity of Biopsies | Numbers of Derived rbF Lines | Freezing of rbF Lines at P2 * | Types of rbF Lines | |
|---|---|---|---|---|---|---|---|---|
| Media | % DMSO | |||||||
| Direct | 3 mm2 | Skin | / | / | 3 | 1; 3; 5 | N | rbF fresh controls |
| Cartilage | / | / | 3 | 2; 4; 6 | N | |||
| Skin | / | / | 2 | 71; 73 | Y | rbF frozen controls | ||
| Cartilage | / | / | 2 | 72; 74 | Y | |||
| Skin | FBS | 10 | 4 | 7; 15; 55; 63 | Y | Frozen tissue-derived rbFs | ||
| 4 | 4 | 8; 16; 56; 64 | Y | |||||
| CRYO3 | 10 | 4 | 9; 17; 57; 65 | Y | ||||
| 4 | 4 | 10; 18; 58; 66 | Y | |||||
| Cartilage | FBS | 10 | 4 | 11; 19; X; 67 | Y | |||
| 4 | 4 | 12; 20; 60; 68 | Y | |||||
| CRYO3 | 10 | 4 | 13; 21; 61; 69 | Y | ||||
| 4 | 4 | 14; 22; 62; 70 | Y | |||||
| 1 cm2 | Skin | FBS | 10 | 2 | 39; X | Y | ||
| 4 | 2 | 40; 48 | Y | |||||
| CRYO3 | 10 | 2 | 41; 49 | Y | ||||
| 4 | 2 | 42; 50 | Y | |||||
| Cartilage | FBS | 10 | 2 | 43; 51 | Y | |||
| 4 | 2 | 44; 52 | Y | |||||
| CRYO3 | 10 | 2 | 45; 53 | Y | ||||
| 4 | 2 | 46; 54 | Y | |||||
| 4 °C/48 h | 3 mm2 | Skin | FBS | 10 | 2 | 23; 31 | Y | |
| 4 | 2 | 24; 32 | Y | |||||
| CRYO3 | 10 | 2 | 25; 33 | Y | ||||
| 4 | 2 | 26; X | Y | |||||
| Cartilage | FBS | 10 | 2 | 27; 35 | Y | |||
| 4 | 2 | 28; 36 | Y | |||||
| CRYO3 | 10 | 2 | 29; 37 | Y | ||||
| 4 | 2 | 30; 38 | Y | |||||
| 1 cm2 | Skin | FBS | 10 | 2 | X | / | No rbF derivation from frozen tissues | |
| 4 | 2 | X | / | |||||
| CRYO3 | 10 | 2 | X | / | ||||
| 4 | 2 | X | / | |||||
| Cartilage | FBS | 10 | 2 | X | / | |||
| 4 | 2 | X | / | |||||
| CRYO3 | 10 | 2 | X | / | ||||
| 4 | 2 | X | / | |||||
| Cell Types | Media | DMSO Concentrations | Tc (°C) * | Tm (°C) ** | ∆H (J/g) *** |
|---|---|---|---|---|---|
| mESCs | FBS | 0% | −16.73 ± 1.01 | 0.99 ± 0.04 | −280.27 ± 4.01 |
| 5% | −15.97 ± 4.33 | −1.56 ± 0.08 | −224.78 ± 4.31 | ||
| 10% | −18.68 ± 1.81 | −3.61 ± 0.34 | −185.06 ± 3.11 | ||
| rbESCs | FBS | 0% | −17.95 ± 1.25 | 0.71 ± 0.28 | −274.05 ± 5.10 |
| 5% | −19.65 ± 1.01 | −2.53 ± 0.42 | −205.53 ± 0.98 | ||
| 10% | −19.19 ± 4.23 | −3.82 ± 0.12 | −184.05 ± 3.11 | ||
| rbiPSCs | KOSR | 0% | −18.22 ± 4.58 | 0.45 ± 0.06 | −256.32 ± 5.51 |
| 5% | −21.74 ± 3.35 | −1.70 ± 0.27 | −215.06 ± 1.20 | ||
| 10% | −19.50 ± 2.23 | −3.99 ± 0.14 | −175.71 ± 0.11 | ||
| All cell types | CRYO3 | 0% | −16.61 ± 1.96 | 0.51 ± 0.10 | −263.15 ± 1.32 |
| 5% | −15.66 ± 2.83 | −1.69 ± 0.28 | −224.34 ± 9.40 | ||
| 10% | −21.32 ± 3.35 | −4.20 ± 0.16 | −183.70 ± 7.28 | ||
| All cell types | CryoStor | 10% | −22.42 ± 1.63 | −4.45 ± 0.35 | −158.61 ± 1.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavin-Plagne, L.; Perold, F.; Osteil, P.; Voisin, S.; Moreira, S.C.; Combourieu, Q.; Saïdou, V.; Mure, M.; Louis, G.; Baudot, A.; et al. Insights into Species Preservation: Cryobanking of Rabbit Somatic and Pluripotent Stem Cells. Int. J. Mol. Sci. 2020, 21, 7285. https://doi.org/10.3390/ijms21197285
Gavin-Plagne L, Perold F, Osteil P, Voisin S, Moreira SC, Combourieu Q, Saïdou V, Mure M, Louis G, Baudot A, et al. Insights into Species Preservation: Cryobanking of Rabbit Somatic and Pluripotent Stem Cells. International Journal of Molecular Sciences. 2020; 21(19):7285. https://doi.org/10.3390/ijms21197285
Chicago/Turabian StyleGavin-Plagne, Lucie, Florence Perold, Pierre Osteil, Sophie Voisin, Synara Cristina Moreira, Quitterie Combourieu, Véronique Saïdou, Magali Mure, Gérard Louis, Anne Baudot, and et al. 2020. "Insights into Species Preservation: Cryobanking of Rabbit Somatic and Pluripotent Stem Cells" International Journal of Molecular Sciences 21, no. 19: 7285. https://doi.org/10.3390/ijms21197285
APA StyleGavin-Plagne, L., Perold, F., Osteil, P., Voisin, S., Moreira, S. C., Combourieu, Q., Saïdou, V., Mure, M., Louis, G., Baudot, A., Buff, S., Joly, T., & Afanassieff, M. (2020). Insights into Species Preservation: Cryobanking of Rabbit Somatic and Pluripotent Stem Cells. International Journal of Molecular Sciences, 21(19), 7285. https://doi.org/10.3390/ijms21197285