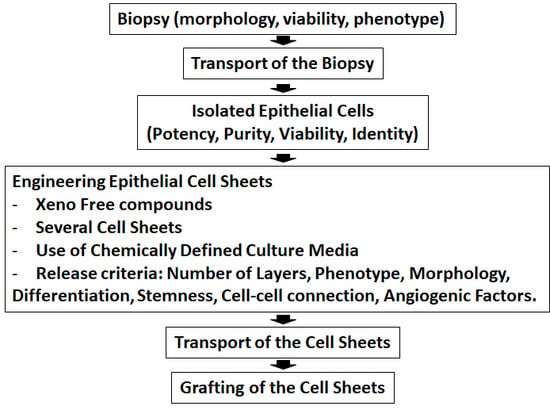
Clinical Trials of Limbal Stem Cell Deficiency Treated with Oral Mucosal Epithelial Cells
Abstract
:1. Introduction
2. Tissue Engineering Techniques
2.1. Autologous Graft and Allograft to Treat LSCD
2.2. LSCD Treatment with Stem Cells
2.3. Treatment of Limbal Stem Cells with Oral Mucosa Epithelial Cell Sheets
2.4. Carrier for Cell Sheet Grafting
3. Steps Prior to Cell Sheet Graft
3.1. Treated Patients
3.2. Characterization of Biopsy and Cell Sheet
4. Transplantation and Post-Surgery
4.1. Adverse Events and Severe Adverse Events
4.2. Follow-Up and Visual Acuity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lagali, N.; Germundsson, J.; Fagerholm, P. The Role of Bowman’s Layer in Corneal Regeneration after Phototherapeutic Keratectomy: A Prospective Study Using In Vivo Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4192–4198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, S.E.; Hong, J.W. Bowman’s layer structure and function: Critical or dispensable to corneal function? A hypothesis. Cornea 2000, 19, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Wang, S.; Liu, X.; He, Y.; Li, Y.; Zhang, Y. Proteins of the corneal stroma: Importance in visual function. Cell Tissue Res. 2016, 364, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Funderburgh, M.L.; Mann, M.M.; Sundarraj, N.; Funderburgh, J.L. Multipotent stem cells in human corneal stroma. Stem Cells 2005, 23, 1266–1275. [Google Scholar] [CrossRef] [Green Version]
- Bogerd, B.V.D.; Ni Dhubhghaill, S.; Koppen, C.; Tassignon, M.J.; Zakaria, N. A review of the evidence for in vivo corneal endothelial regeneration. Surv. Ophthalmol. 2018, 63, 149–165. [Google Scholar] [CrossRef] [Green Version]
- Faragher, R.G.A.; Mulholland, B.; Tuft, S.J.; Sandeman, S.; Khaw, P.T. Aging and the cornea. Br. J. Ophthalmol. 1997, 81, 814–817. [Google Scholar] [CrossRef] [Green Version]
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Yoon, J.J.; Ismail, S.; Sherwin, T. Limbal stem cells: Central concepts of corneal epithelial homeostasis. World J. Stem Cells 2014, 6, 391–403. [Google Scholar] [CrossRef]
- Goldberg, M.F.; Bron, A.J. Limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1982, 80, 155–171. [Google Scholar]
- Notara, M.; Lentzsch, A.; Coroneo, M.; Cursiefen, C. The Role of Limbal Epithelial Stem Cells in Regulating Corneal (Lymph)angiogenic Privilege and the Micromilieu of the Limbal Niche following UV Exposure. Stem Cells Int. 2018, 2018, 8620172. [Google Scholar] [CrossRef] [Green Version]
- Abdelfattah, N.S.; Amgad, M.; Zayed, A.A.; Salem, H.; Elkhanany, A.E.; Hussein, H.; El-Baky, N.A. Clinical correlates of common corneal neovascular diseases: A literature review. Int. J. Ophthalmol. 2015, 8, 182–193. [Google Scholar] [PubMed]
- Cursiefen, C.; Colin, J.; Dana, R.; Diaz-Llopis, M.; Faraj, L.A.; Garcia-Delpech, S.; Geerling, G.; Price, F.W.; Remeijer, L.; Rouse, B.T.; et al. Consensus statement on indications for anti-angiogenic therapy in the management of corneal diseases associated with neovascularisation: Outcome of an expert roundtable. Br. J. Ophthalmol. 2012, 96, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Azar, D.T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an american ophthalmological society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 264–302. [Google Scholar] [PubMed]
- Sacchetti, M.; Rama, P.; Bruscolini, A.; Lambiase, A. Limbal Stem Cell Transplantation: Clinical Results, Limits, and Perspectives. Stem Cells Int. 2018, 2018, 8086269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E.; The International Limbal Stem Cell Deficiency Working, Group. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea 2019, 38, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Miri, A.; Said, D.G. Contemporary limbal stem cell transplantation—A review. Clin. Exp. Ophthalmol. 2010, 38, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.X.; Sejpal, K.; Bakhtiari, P. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr. J. Ophthalmol. 2013, 20, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Buenaga, R.; Aiello, F.; Zaher, S.S.; Grixti, A.; Ahmad, S. Twenty years of limbal epithelial therapy: An update on managing limbal stem cell deficiency. BMJ Open Ophthalmol. 2018, 3, e000164. [Google Scholar] [CrossRef]
- Ahmad, S. Concise Review: Limbal Stem Cell Deficiency, Dysfunction, and Distress. Stem Cells Transl. Med. 2012, 1, 110–115. [Google Scholar] [CrossRef]
- Armitage, W.J.; Tullo, A.B.; Larkin, D.F.P. The first successful full-thickness corneal transplant: A commentary on Eduard Zirm’s landmark paper of 1906. Br. J. Ophthalmol. 2006, 90, 1222–1223. [Google Scholar] [CrossRef]
- Daya, S.M. Conjunctival-limbal autograft. Curr. Opin. Ophthalmol. 2017, 28, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Azuara-Blanco, A. Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br. J. Ophthalmol. 2000, 84, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amescua, G.; Atallah, M.R.; Palioura, S.; Perez, V.L. Limbal stem cell transplantation: Current perspectives. Clin. Ophthalmol. 2016, 10, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Cheung, A.Y.; Holland, E.J. Keratolimbal allograft. Curr. Opin. Ophthalmol. 2017, 28, 377–381. [Google Scholar] [CrossRef]
- Bhalekar, S.; Basu, S.; Sangwan, V.S. Successful management of immunological rejection following allogeneic simple limbal epithelial transplantation (SLET) for bilateral ocular burns. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazirani, J.; Mariappan, I.; Ramamurthy, S.; Fatima, S.; Basu, S.; Sangwan, V.S. Surgical Management of Bilateral Limbal Stem Cell Deficiency. Ocul. Surf. 2016, 14, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Röck, T.; Bramkamp, M.; Bartz-Schmidt, K.U.; Röck, D. Organ transplantation scandal influencing corneal donation rate. Int. J. Ophthalmol. 2017, 10, 1001–1003. [Google Scholar] [PubMed]
- Niederkorn, J.Y. Immunology of Corneal Allografts: Insights from Animal Models. J. Clin. Exp. Ophthalmol. 2015, 6, 429. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, M.; Welder, J.D.; Pandya, H.K.; Nassiri, N.; Djalilian, A.R. Adverse Effects of Systemic Immunosuppression in Keratolimbal Allograft. J. Ophthalmol. 2012, 2012, 576712. [Google Scholar] [CrossRef] [Green Version]
- Keivyon, K.R.; Tseng, S.C. Limbal Autograft Transplantation for Ocular Surface Disorders. Ophthalmology 1989, 96, 709–723. [Google Scholar] [CrossRef]
- Chen, J.J.; Tseng, S.C. Corneal epithelial wound healing in partial limbal deficiency. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1301–1314. [Google Scholar]
- Espana, E.M.; Grueterich, M.; Romano, A.C.; Touhami, A.; Tseng, S.C.G. Idiopathic limbal stem cell deficiency. Ophthalmology 2002, 109, 2004–2010. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, R.J.F.; Li, L.M.; Chen, J.K. Reconstruction of Damaged Corneas by Transplantation of Autologous Limbal Epithelial Cells. N. Engl. J. Med. 2000, 343, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Theng, J.T.; Tan, D.T. Combined penetrating keratoplasty and limbal allograft transplantation for severe corneal burns. Ophthalmic Surg. Lasers Imaging Retin. 1997, 28, 765–768. [Google Scholar]
- Tsai, R.J.F.; Tseng, S.C. Human Allograft Limbal Transplantation for Corneal Surface Reconstruction. Cornea 1994, 13, 389–400. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, R.; Ishikawa, Y.; Ito, M.; Kageyama, T.; Takashiba, K.; Fujioka, T.; Tsujikawa, M.; Miyoshi, H.; Yamato, M.; Nakamura, Y.; et al. Generation of Corneal Epithelial Cells from Induced Pluripotent Stem Cells Derived from Human Dermal Fibroblast and Corneal Limbal Epithelium. PLoS ONE 2012, 7, e45435. [Google Scholar] [CrossRef] [Green Version]
- Dyrlund, T.F.; Poulsen, E.T.; Scavenius, C.; Nikolajsen, C.L.; Thøgersen, I.B.; Vorum, H.; Enghild, J.J. Human Cornea Proteome: Identification and Quantitation of the Proteins of the Three Main Layers Including Epithelium, Stroma, and Endothelium. J. Proteome Res. 2012, 11, 4231–4239. [Google Scholar] [CrossRef]
- Joyce, N.C.; Harris, D.L.; Markov, V.; Zhang, Z.; Saitta, B. Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol. Vis. 2012, 18, 547–564. [Google Scholar]
- Bharti, K.; Miller, S.S.; Arnheiter, H. The new paradigm: Retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res. 2011, 24, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augello, A.; De Bari, C. The Regulation of Differentiation in Mesenchymal Stem Cells. Hum. Gene Ther. 2010, 21, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; Monteiro, B.G.; Da Silva, M.C.P.; Kerkis, A.; Cerruti, H.; Kerkis, I.; Melo, G.B.; Smith, R.L.; Lizier, N.F. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Human Immature Dental Pulp Stem Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Xing, C.; Han, J.; Tso, M.O.; Hong, J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol. Vis. 2009, 15, 99–107. [Google Scholar]
- Choong, P.F.; Mok, P.L.; Cheong, S.K.; Then, K.Y. Mesenchymal stromal cell-like characteristics of corneal keratocytes. Cytotherapy 2007, 9, 252–258. [Google Scholar] [CrossRef]
- Keller, G. Embryonic stem cell differentiation: Emergence of a new era in biology and medicine. Genes Dev. 2005, 19, 1129–1155. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; De Luca, M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Vliet, P.; Goumans, M.J.; Doevendans, P.A.; Sluijter, J.P.G. Human cardiomyocyte progenitor cells: A short history of nearly everything. J. Cell. Mol. Med. 2012, 16, 1669–1673. [Google Scholar] [CrossRef] [Green Version]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.D.; Guerquin-Kern, J.L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, P.C.H.; Segers, V.F.M.; Davis, M.E.; MacGillivray, C.; Gannon, J.; Molkentin, J.D.; Robbins, J.; Lee, R.T. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat. Med. 2007, 13, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Tchao, J.; Tobita, K. Concise review: Skeletal muscle stem cells and cardiac lineage: Potential for heart repair. Stem Cells Transl. Med. 2014, 3, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Takagi, R.; Murakami, D.; Kondo, M.; Ohki, T.; Sasaki, R.; Mizutani, M.; Yamato, M.; Nishida, K.; Namiki, H.; Yamamoto, M.; et al. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest. Endosc. 2010, 72, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Kremer, M.J. Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 2001, 2001, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Autologous Oral Mucosal Epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Gipson, I.K.; Geggel, H.S.; Spurr-Michaud, S.J. Transplant of Oral Mucosal Epithelium to Rabbit Ocular Surface Wounds In Vivo. Arch. Ophthalmol. 1986, 104, 1529–1533. [Google Scholar] [CrossRef]
- Nakamura, T.; Kinoshita, S. Ocular surface reconstruction using cultivated mucosal epithelial stem cells. Cornea 2003, 22, S75–S80. [Google Scholar] [CrossRef]
- Liu, J.; Sheha, H.; Fu, Y.; Giegengack, M.; Tseng, S.C. Oral Mucosal Graft with Amniotic Membrane Transplantation for Total Limbal Stem Cell Deficiency. Am. J. Ophthalmol. 2011, 152, 739–747. [Google Scholar] [CrossRef]
- Sotozono, C.; Inatomi, T.; Nakamura, T.; Koizumi, N.; Yokoi, N.; Ueta, M.; Matsuyama, K.; Miyakoda, K.; Kaneda, H.; Fukushima, M.; et al. Visual Improvement after Cultivated Oral Mucosal Epithelial Transplantation. Ophthalmology 2013, 120, 193–200. [Google Scholar] [CrossRef]
- Utheim, T.P.; Utheim, Ø.A.; Salvanos, P.; Jackson, C.J.; Schrader, S.; Geerling, G.; Sehic, A. Concise Review: Altered Versus Unaltered Amniotic Membrane as a Substrate for Limbal Epithelial Cells. Stem Cells Transl. Med. 2018, 7, 415–427. [Google Scholar] [CrossRef]
- Utheim, T.P.; Utheim, Ø.A.; Khan, Q.E.S.; Sehic, A. Culture of Oral Mucosal Epithelial Cells for the Purpose of Treating Limbal Stem Cell Deficiency. J. Funct. Biomater. 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehic, A.; Utheim, Ø.A.; Ommundsen, K.; Utheim, T.P. Pre-Clinical Cell-Based Therapy for Limbal Stem Cell Deficiency. J. Funct. Biomater. 2015, 6, 863–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhira, S.L.; Vemuganti, G.; Bhaduri, A.; Gaddipati, S.; Sangwan, V.S.; Ghanekar, Y. Culture and characterization of oral mucosal epithelial cells on human amniotic membrane for ocular surface reconstruction. Mol. Vis. 2008, 14, 189–196. [Google Scholar] [PubMed]
- Yang, A.; Schweitzer, R.; Sun, D.; Kaghad, M.; Walker, N.; Bronson, R.T.; Tabin, C.; Sharpe, A.; Caput, D.; Crum, C.; et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999, 398, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, H.J.; Ryu, J.S.; Kim, Y.H.; Jeon, S.; Oh, J.Y.; Choung, H.K.; Khwarg, S.I.; Wee, W.R.; Kim, M.K. Prospective Clinical Trial of Corneal Reconstruction with Biomaterial-Free Cultured Oral Mucosal Epithelial Cell Sheets. Cornea 2018, 37, 76–83. [Google Scholar] [CrossRef]
- Burillon, C.; Huot, L.; Justin, V.; Nataf, S.; Chapuis, F.; Decullier, E.; Damour, O. Cultured Autologous Oral Mucosal Epithelial Cell Sheet (CAOMECS) Transplantation for the Treatment of Corneal Limbal Epithelial Stem Cell Deficiency. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1325–1331. [Google Scholar] [CrossRef] [Green Version]
- Satake, Y.; Higa, K.; Tsubota, K.; Shimazaki, J. Long-term Outcome of Cultivated Oral Mucosal Epithelial Sheet Transplantation in Treatment of Total Limbal Stem Cell Deficiency. Ophthalmology 2011, 118, 1524–1530. [Google Scholar] [CrossRef]
- Inatomi, T.; Nakamura, T.; Kojyo, M.; Koizumi, N.; Sotozono, C.; Kinoshita, S. Ocular Surface Reconstruction with Combination of Cultivated Autologous Oral Mucosal Epithelial Transplantation and Penetrating Keratoplasty. Am. J. Ophthalmol. 2006, 142, 757–764. [Google Scholar] [CrossRef]
- Inatomi, T.; Nakamura, T.; Koizumi, N.; Sotozono, C.; Yokoi, N.; Kinoshita, S. Midterm Results on Ocular Surface Reconstruction Using Cultivated Autologous Oral Mucosal Epithelial Transplantation. Am. J. Ophthalmol. 2006, 141, 267–275. [Google Scholar] [CrossRef]
- Kanayama, S.; Nishida, K.; Yamato, M.; Hayashi, R.; Sugiyama, H.; Soma, T.; Maeda, N.; Okano, T.; Tano, Y. Analysis of angiogenesis induced by cultured corneal and oral mucosal epithelial cell sheets in vitro. Exp. Eye Res. 2007, 85, 772–781. [Google Scholar] [CrossRef]
- Chen, H.C.J.; Yeh, L.K.; Tsai, Y.J.; Lai, C.H.; Chen, C.C.; Lai, J.Y.; Sun, C.C.; Chang, G.; Hwang, T.L.; Chen, J.K.; et al. Expression of Angiogenesis-Related Factors in Human Corneas after Cultivated Oral Mucosal Epithelial Transplantation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5615–5623. [Google Scholar] [CrossRef] [PubMed]
- Canonico, S. The use of human fibrin glue in the surgical operations. Acta Biomed. 2003, 74, 21–25. [Google Scholar] [PubMed]
- Di Girolamo, N.; Chui, J.; Wakefield, D.; Coroneo, M.T. Cultured human ocular surface epithelium on therapeutic contact lenses. Br. J. Ophthalmol. 2007, 91, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vemuganti, G.K.; Balasubramanian, D.; Gaddipati, S.; Muralidhar, R.; Sangwan, V.S.; Mariappan, I. Oral epithelial cells transplanted on to corneal surface tend to adapt to the ocular phenotype. Indian J. Ophthalmol. 2014, 62, 644–648. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, D.H.; Shin, E.J.; Lee, H.J.; Wee, W.R.; Jeon, S.; Kim, M.K. Comparative Analysis of Substrate-Free Cultured Oral Mucosal Epithelial Cell Sheets from Cells of Subjects with and without Stevens-Johnson Syndrome for Use in Ocular Surface Reconstruction. PLoS ONE 2016, 11, e0147548. [Google Scholar] [CrossRef]
- Shukla, I.M. Amniotic membrane grafts in corneal ulcer. J. All India Ophthalmol. Soc. 1962, 10, 55–60. [Google Scholar]
- Tseng, S.C.G.; Prabhasawat, P.; Barton, K.; Gray, T.; Meller, D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch. Ophthalmol. 1998, 116, 431–441. [Google Scholar] [CrossRef]
- Koizumi, N.; Inatomi, T.; Quantock, A.J.; Fullwood, N.J.; Dota, A.; Kinoshita, S. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea 2000, 19, 65–71. [Google Scholar] [CrossRef]
- Koizumi, N.; Fullwood, N.J.; Bairaktaris, G.; Inatomi, T.; Kinoshita, S.; Quantock, A.J. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2506–2513. [Google Scholar]
- Prabhasawat, P.; Ekpo, P.; Uiprasertkul, M.; Chotikavanich, S.; Tesavibul, N.; Pornpanich, K.; Luemsamran, P. Long-term result of autologous cultivated oral mucosal epithelial transplantation for severe ocular surface disease. Cell Tissue Bank. 2016, 17, 491–503. [Google Scholar] [CrossRef]
- Avila, M.; España, M.; Moreno, C.; Peña, C. Reconstruction of ocular surface with heterologous limbal epithelium and amniotic membrane in a rabbit model. Cornea 2001, 20, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.P.K.; Nakamura, T.; Inatomi, T.; Sotozono, C.; Koizumi, N.; Yokoi, N.; Kinoshita, S. Autologous Serum–Derived Cultivated Oral Epithelial Transplants for Severe Ocular Surface Disease. Arch. Ophthalmol. 2006, 124, 1543–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baradaran-Rafii, A.; Delfazayebaher, S.; Aghdami, N.; Taghiabadi, E.; Bamdad, S.; Roshandel, D. Midterm outcomes of penetrating keratoplasty after cultivated oral mucosal epithelial transplantation in chemical burn. Ocul. Surf. 2017, 15, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Satake, Y.; Higa, K.; Yamaguchi, T.; Shimazaki, J. Transplantation of Cultivated Oral Mucosal Epithelium Prepared in Fibrin-Coated Culture Dishes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.K.; Kuo, M.T.; Tsai, Y.J.; Chen, H.C.J.; Chen, X.L.; Wang, S.F.; Li, L.; Hsiao, C.H.; Lin, K.K. Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye 2009, 23, 1442–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Amemiya, T.; Kanamura, N.; Kinoshita, S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1280–1284. [Google Scholar] [CrossRef]
- Priya, C.G.; Arpitha, P.; Vaishali, S.; Prajna, N.V.; Usha, K.; Sheetal, K.; Muthukkaruppan, V. Adult human buccal epithelial stem cells: Identification, ex-vivo expansion, and transplantation for corneal surface reconstruction. Eye 2011, 25, 1641–1649. [Google Scholar] [CrossRef]
- Satake, Y.; Dogru, M.; Yamane, G.Y.; Kinoshita, S.; Tsubota, K.; Shimazaki, J. Barrier Function and Cytologic Features of the Ocular Surface Epithelium After Autologous Cultivated Oral Mucosal Epithelial Transplantation. Arch. Ophthalmol. 2008, 126, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Nakamura, T.; Inatomi, T.; Sotozono, C.; Watanabe, A.; Kinoshita, S. Ocular Surface Reconstruction Using the Combination of Autologous Cultivated Oral Mucosal Epithelial Transplantation and Eyelid Surgery for Severe Ocular Surface Disease. Am. J. Ophthalmol. 2011, 152, 195–201. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-isopropylacrylamide). J. Macromol. Sci. Part A Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Ohashi, K.; Yokoyama, T.; Yamato, M.; Kuge, H.; Kanehiro, H.; Tsutsumi, M.; Amanuma, T.; Iwata, H.; Yang, J.; Okano, T.; et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat. Med. 2007, 13, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Maeda, N.; Watanabe, H.; Yamamoto, K.; Nagai, S.; Kikuchi, A.; Tano, Y.; et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 2004, 77, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Yamato, M.; Akutsu, T.; Shibata, T.; Isoi, Y.; Kikuchi, A.; Umezu, M.; Okano, T. Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J. Biomed. Mater. Res. 2002, 60, 110–117. [Google Scholar] [CrossRef]
- Yamato, M.; Utsumi, M.; Kushida, A.; Konno, C.; Kikuchi, A.; Okano, T. Thermo-Responsive Culture Dishes Allow the Intact Harvest of Multilayered Keratinocyte Sheets without Dispase by Reducing Temperature. Tissue Eng. 2001, 7, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Okuhara, M.; Karikusa, F.; Kikuchi, A.; Sakurai, Y.; Okano, T. Signal transduction and cytoskeletal reorganization are required for cell detachment from cell culture surfaces grafted with a temperature-responsive polymer. J. Biomed. Mater. Res. 1999, 44, 44–52. [Google Scholar] [CrossRef]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.H.K. Aging of mesenchymal stem cells: Implication in regenerative medicine. Regen. Ther. 2018, 9, 120–122. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Zhou, S.; Greenberger, J.S.; Epperly, M.W.; Goff, J.P.; Adler, C.; LeBoff, M.S.; Glowacki, J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 2008, 7, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Abu Eid, R.; Sawair, F.; Landini, G.; Saku, T. Age and the architecture of oral mucosa. Age 2012, 34, 651–658. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med. 2014, 12, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blázquez-Prunera, A.; Díez, J.M.; Gajardo, R.; Grancha, S. Human mesenchymal stem cells maintain their phenotype, multipotentiality, and genetic stability when cultured using a defined xeno-free human plasma fraction. Stem Cell Res. Ther. 2017, 8, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grezella, C.; Fernandez-Rebollo, E.; Franzen, J.; Ferreira, M.S.V.; Beier, F.; Wagner, W. Effects of senolytic drugs on human mesenchymal stromal cells. Stem Cell Res. Ther. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Wang, H.; Yang, Y.J.; Dong, Q.T.; Wang, T.J.; Qian, H.Y.; Li, N.; Wang, X.M.; Jin, C. Atorvastatin treatment improves the effects of mesenchymal stem cell transplantation on acute myocardial infarction: The role of the RhoA/ROCK/ERK pathway. Int. J. Cardiol. 2014, 176, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J. Association of preoperative tear function with surgical outcome in severe Stevens-Johnson syndrome. Ophthalmology 2000, 107, 1518–1523. [Google Scholar] [CrossRef]
- Hos, D.; Saban, D.R.; Bock, F.; Regenfuss, B.; Onderka, J.; Masli, S.; Cursiefen, C. Suppression of Inflammatory Corneal Lymphangiogenesis by Application of Topical Corticosteroids. Arch. Ophthalmol. 2011, 129, 445–452. [Google Scholar] [CrossRef]
- Foodand Drug Administration. Bovine Derived Materials Used in Vaccine Manufacturing Questions and Answers. 2018. Available online: https://www.fda.gov/vaccines-blood-biologics/questions-about-vaccines/bovine-derived-materials-used-vaccine-manufacturing-questions-and-answers (accessed on 23 March 2018).
- Kolli, S.; Ahmad, S.; Mudhar, H.S.; Meeny, A.; Lako, M.; Figueiredo, F.C. Successful Application of Ex Vivo Expanded Human Autologous Oral Mucosal Epithelium for the Treatment of Total Bilateral Limbal Stem Cell Deficiency. Stem Cells 2014, 32, 2135–2146. [Google Scholar] [CrossRef]
- Sotozono, C.; Inatomi, T.; Nakamura, T.; Koizumi, N.; Yokoi, N.; Ueta, M.; Matsuyama, K.; Kaneda, H.; Fukushima, M.; Kinoshita, S. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol. 2014, 92, e447–e453. [Google Scholar] [CrossRef]
- Jayme, D.W.; Smith, S.R. Media formulation options and manufacturing process controls to safeguard against introduction of animal origin contaminants in animal cell culture. Cytotechnology 2000, 33, 27–36. [Google Scholar] [CrossRef]
- Stein, A. Decreasing variability in your cell culture. Biotechniques 2007, 43, 228–229. [Google Scholar] [CrossRef]
- Oliva, J.; Florentino, A.; Bardag-Gorce, F.; Niihara, Y. Vitrification and storage of oral mucosa epithelial cell sheets. J. Tissue Eng. Regen. Med. 2019, 13, 1153–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michcik, A.; Cichorek, M.; Daca, A.; Chomik, P.; Wojcik, S.; Zawrocki, A.; Wlodarkiewicz, A. Tobacco smoking alters the number of oral epithelial cells with apoptotic features. Folia Histochem. Cytobiol. 2014, 52, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Wang, L. Effects of alcohol on the morphological and structural changes in oral mucosa. Pak. J. Med. Sci. 2013, 29, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Grewal, H.K.; Jindal, S.; Chauhan, I. Alteration in buccal mucosal cells due to the effect of tobacco and alcohol by assessing the silver-stained nucleolar organiser regions and micronuclei. J. Cytol. 2013, 30, 174–178. [Google Scholar] [CrossRef]
- Boyle, J.O.; Gümüş, Z.H.; Kacker, A.; Choksi, V.L.; Bocker, J.M.; Zhou, X.K.; Yantiss, R.K.; Hughes, D.B.; Du, B.; Judson, B.L.; et al. Effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev. Res. 2010, 3, 266–278. [Google Scholar] [CrossRef] [Green Version]
- Maier, H.; Weidauer, H.; Zöller, J.; Seitz, H.K.; Flentje, M.; Mall, G.; Born, I.A. Effect of Chronic Alcohol Consumption on the Morphology of the Oral Mucosa. Alcohol. Clin. Exp. Res. 1994, 18, 387–391. [Google Scholar] [CrossRef]
- Romano, R.A.; Smalley, K.; Magraw, C.; Serna, V.A.; Kurita, T.; Raghavan, S.; Sinha, S. DeltaNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development 2012, 139, 772–782. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Wahlin, Y.B.; Coates, P.J.; Sjöström, B.; Nylander, K. Decreased expression of p63 in oral lichen planus and graft-vs.host disease associated with oral inflammation. J. Oral Pathol. Med. 2006, 35, 46–50. [Google Scholar] [CrossRef]
- Candi, E.; Dinsdale, D.; Rufini, A.; Salomoni, P.; Knight, R.A.; Mueller, M.; Krammer, P.H.; Melino, G. TAp63 and DeltaNp63 in cancer and epidermal development. Cell Cycle 2007, 6, 274–285. [Google Scholar] [CrossRef]
- Fukumoto, J.; Patil, S.S.; Krishnamurthy, S.; Saji, S.; John, I.; Narala, V.R.; Hernández-Cuervo, H.; Alleyn, M.; Breitzig, M.T.; Galam, L.; et al. Altered expression of p63 isoforms and expansion of p63- and club cell secretory protein-positive epithelial cells in the lung as novel features of aging. Am. J. Physiol. Physiol. 2019, 316, C492–C508. [Google Scholar] [CrossRef]
- Katzman, L.R.; Jeng, B.H. Management strategies for persistent epithelial defects of the cornea. Saudi J. Ophthalmol. 2014, 28, 168–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Inatomi, T.; Cooper, L.J.; Rigby, H.; Fullwood, N.J.; Kinoshita, S. Phenotypic Investigation of Human Eyes with Transplanted Autologous Cultivated Oral Mucosal Epithelial Sheets for Severe Ocular Surface Diseases. Ophthalmology 2007, 114, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Takeda, K.; Inatomi, T.; Sotozono, C.; Kinoshita, S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br. J. Ophthalmol. 2011, 95, 942–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.H.K.; Chen, J.K.; Zhang, F.; Lin, K.Y.; Yao, J.Y.; Yu, J.S. Regulation of corneal angiogenesis in limbal stem cell deficiency. Prog. Retin. Eye Res. 2006, 25, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Ochiai, K.; Florentino, A.; Bardag-Gorce, F.; Wood, A.; Niihara, Y. Feeder Cells Free Rabbit Oral Mucosa Epithelial Cell Sheet Engineering. Tissue Eng. Regen. Med. 2018, 15, 321–332. [Google Scholar] [CrossRef]
- Ilmarinen, T.; Laine, J.; Juuti-Uusitalo, K.; Numminen, J.; Seppanen-Suuronen, R.; Uusitalo, H.; Skottman, H. Towards a defined, serum- and feeder-free culture of stratified human oral mucosal epithelium for ocular surface reconstruction. Acta Ophthalmol. 2013, 91, 744–750. [Google Scholar] [CrossRef]
- Gill, O.N.; Spencer, Y.; Richard-Loendt, A.; Kelly, C.; Dabaghian, R.; Boyes, L.; Linehan, J.; Simmons, M.; Webb, P.; Bellerby, P.; et al. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: Large scale survey. BMJ 2013, 347, 5675. [Google Scholar] [CrossRef] [Green Version]
- Bardag-Gorce, F.; Hoft, R.H.; Wood, A.; Oliva, J.; Niihara, H.; Makalinao, A.; Thropay, J.; Pan, D.; Meepe, I.; Tiger, K.; et al. The Role of E-Cadherin in Maintaining the Barrier Function of Corneal Epithelium after Treatment with Cultured Autologous Oral Mucosa Epithelial Cell Sheet Grafts for Limbal Stem Deficiency. J. Ophthalmol. 2016, 2016, 4805986. [Google Scholar] [CrossRef] [Green Version]
- Higa, K.; Shimmura, S.; Kato, N.; Kawakita, T.; Miyashita, H.; Itabashi, Y.; Fukuda, K.; Shimazaki, J.; Tsubota, K. Proliferation and Differentiation of Transplantable Rabbit Epithelial Sheets Engineered with or without an Amniotic Membrane Carrier. Investig. Ophthalmol. Vis. Sci. 2007, 48, 597–604. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, J.; Bardag-Gorce, F.; Niihara, Y. Clinical Trials of Limbal Stem Cell Deficiency Treated with Oral Mucosal Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 411. https://doi.org/10.3390/ijms21020411
Oliva J, Bardag-Gorce F, Niihara Y. Clinical Trials of Limbal Stem Cell Deficiency Treated with Oral Mucosal Epithelial Cells. International Journal of Molecular Sciences. 2020; 21(2):411. https://doi.org/10.3390/ijms21020411
Chicago/Turabian StyleOliva, Joan, Fawzia Bardag-Gorce, and Yutaka Niihara. 2020. "Clinical Trials of Limbal Stem Cell Deficiency Treated with Oral Mucosal Epithelial Cells" International Journal of Molecular Sciences 21, no. 2: 411. https://doi.org/10.3390/ijms21020411
APA StyleOliva, J., Bardag-Gorce, F., & Niihara, Y. (2020). Clinical Trials of Limbal Stem Cell Deficiency Treated with Oral Mucosal Epithelial Cells. International Journal of Molecular Sciences, 21(2), 411. https://doi.org/10.3390/ijms21020411