Inactivity and Skeletal Muscle Metabolism: A Vicious Cycle in Old Age
Abstract
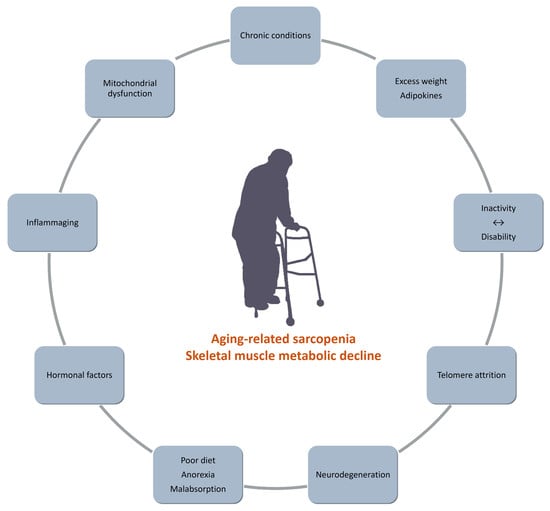
:1. Introduction
2. Senescence and Human Skeletal Muscles
2.1. Structural Decline
2.2. The Aging Mitochondrion
3. Body Composition and Physical Activity in Old Age
3.1. Skeletal Muscle and White Adipose Tissue in Old Age: AComplex Relationship
3.2. Exercise Versus Inactivity
4. Age-Related Hormonal Changes and Physical Activity
5. Inflammaging and Physical Activity
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gladyshev, T.V.; Gladyshev, V.N. A disease or not a disease? Aging as a pathology. Trends Mol. Med. 2016, 22, 966–995. [Google Scholar] [CrossRef] [Green Version]
- Seene, T.; Kaasik, P.; Riso, E.M. Review on aging, unloading and reloading: Changes in skeletal muscle quantity and quality. Arch. Gerontol. Geriatr. 2012, 54, 374–380. [Google Scholar] [CrossRef]
- Sanderson, W.C.; Scherbov, S. Remeasuring aging. Science 2010, 329, 1278–1287. [Google Scholar] [CrossRef]
- Harper, S. Economic and social implications of aging societies. Science 2014, 346, 587–591. [Google Scholar] [CrossRef]
- Dall, T.M.; Gallo, P.D.; Chakrabarti, R.; West, T.; Semilla, A.P.; Storm, M.V. An aging population and growing disease burden will require a large and specialized health care workforce by 2025. Health Aff. 2013, 32, 2013–2020. [Google Scholar] [CrossRef] [Green Version]
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Healthspan 2014, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Murgia, M.; Toniolo, L.; Nagaraj, N.; Ciciliot, S.; Vindigni, V.; Schiaffino, S.; Reggiani, C.; Mann, M. Single muscle fiber proteomics reveals fiber-type-specific features of human muscle aging. Cell Rep. 2017, 19, 2396–2409. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Gomez, D.; Bandinelli, S.; Del-Panta, V.; Patel, K.V.; Guralnik, J.M.; Ferrucci, L. Three-year changes in physical activity and decline in physical performance over 9 years of follow-up in older adults: The invecchiare in chianti study. J. Am. Geriatr. Soc. 2017, 65, 1176–1182. [Google Scholar] [CrossRef]
- Mendonca, G.V.; Pezarat-Correia, P.; Vaz, J.R.; Silva, L.; Heffernan, K.S. Impact of aging on endurance and neuromuscular physical performance: The role of vascular senescence. Sports Med. 2017, 47, 583–598. [Google Scholar] [CrossRef]
- Matsuda, P.N.; Verrall, A.M.; Finlayson, M.L.; Molton, I.R.; Jensen, M.P. Falls among adults aging with disability. Arch. Phys. Med. Rehabil. 2015, 96, 464–471. [Google Scholar] [CrossRef]
- Finlayson, M.L.; Peterson, E.W. Falls, aging, and disability. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 357–373. [Google Scholar] [CrossRef]
- Dobson, F.; Hinman, R.S.; Hall, M.; Marshall, C.J.; Sayer, T.; Anderson, C.; Newcomb, N.; Stratford, P.W.; Bennell, K.L. Reliability and measurement error of the Osteoarthritis Research Society International (OARSI) recommended performance-based tests of physical function in people with hip and knee osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1792–1796. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.E.; Maddocks, M.; Kon, S.S.; Canavan, J.L.; Nolan, C.M.; Clark, A.L.; Polkey, M.I.; Man, W.D. Sarcopenia in COPD: Prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015, 70, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Gianoudis, J.; Bailey, C.A.; Daly, R.M. Associations between sedentary behaviour and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos. Int. 2015, 26, 571–579. [Google Scholar] [CrossRef]
- Finkel, T. The metabolic regulation of aging. Nat. Med. 2015, 21, 1416–1423. [Google Scholar] [CrossRef]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Muller, M. Cellular senescence: Molecular mechanisms, in vivo significance, and redox considerations. Antioxid. Redox Signal 2009, 11, 59–98. [Google Scholar] [CrossRef]
- Bocklandt, S.; Lin, W.; Sehl, M.E.; Sánchez, F.J.; Sinsheimer, J.S.; Horvath, S.; Vilain, E. Epigenetic predictor of age. PLoS ONE 2011, 6, e14821. [Google Scholar] [CrossRef] [Green Version]
- Daniali, L.; Benetos, A.; Susser, E.; Kark, J.D.; Labat, C.; Kimura, M.; Desai, K.K.; Granick, M.; Aviv, A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 2013, 4, 1597. [Google Scholar] [CrossRef]
- Friedrich, U.; Griese, E.U.; Schwab, M.; Fritz, P.; Thon, K.P.; Klotz, U. Telomere length in different tissues of elderly patients. Mech. Ageing Dev. 2000, 119, 89–99. [Google Scholar] [CrossRef]
- Benetos, A.; Kimura, M.; Labat, C.; Buchoff, G.M.; Huber, S.; Labat, L.; Lu, X.; Aviv, A. A model of canine leukocyte telomere dynamics. Aging Cell 2011, 10, 991–995. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Gazitt, Y.; Cao, X.; Zhao, X.; Lansdorp, P.M.; Aviv, A. Synchrony of telomere length among hematopoietic cells. Exp. Hematol. 2010, 38, 854–859. [Google Scholar] [CrossRef] [Green Version]
- Gardner, J.P.; Kimura, M.; Chai, W.; Durrani, J.F.; Tchakmakjian, L.; Cao, X.; Lu, X.; Li, G.; Peppas, A.P.; Skurnick, J.; et al. Telomere dynamics in macaques and humans. J. Gerontol. Ser. A 2007, 62, 367. [Google Scholar] [CrossRef]
- Reichert, S.; Criscuolo, F.; Verinaud, E.; Zahn, S.; Massemin, S. Telomere length correlations among somatic tissues in adult zebra finches. PLoS ONE 2013, 8, e81496. [Google Scholar] [CrossRef] [Green Version]
- Boren, J.; Taskinen, M.R.; Olofsson, S.O.; Levin, M. Ectopic lipid storage and insulin resistance: A harmful relationship. J. Intern. Med. 2013, 274, 25–40. [Google Scholar] [CrossRef]
- Ferrucci, L.; Baroni, M.; Ranchelli, A.; Lauretani, F.; Maggio, M.; Mecocci, P.; Ruggiero, C. Interaction between bone and muscle in older persons with mobility limitations. Curr. Pharm. Des. 2014, 20, 3178–3197. [Google Scholar] [CrossRef] [Green Version]
- Miljkovic, I.; Kuipers, A.L.; Cvejkus, R.; Bunker, C.H.; Patrick, A.L.; Gordon, C.L.; Zmuda, J.M. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity 2016, 24, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Correa-de-Araujo, R.; Harris-Love, M.O.; Miljkovic, I.; Fragala, M.S.; Anthony, B.W.; Manini, T.M. The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscleof muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: A symposium report. Front. Physiol. 2017, 8, 87. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Atherton, P.J.; Williams, J.; Larvin, M.; Lund, J.N.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [Green Version]
- Moorthi, R.N.; Avin, K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiraoka, A.; Aibiki, T.; Okudaira, T.; Toshimori, A.; Kawamura, T.; Nakahara, H.; Suga, Y.; Azemoto, N.; Miyata, H.; Miyamoto, Y.; et al. Muscle atrophy as pre-sarcopenia in Japanese patients with chronic liver disease: Computed tomography is useful for evaluation. J. Gastroenterol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Topinkova, E.; Michel, J.P. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 1–7. [Google Scholar] [CrossRef]
- Narici, M.V.; Maganaris, C.N.; Reeves, N.D.; Capodaglio, P. Effect of aging on human muscle architecture. J. Appl. Physiol. 2003, 95, 2229–2234. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, E.; Chiles Shaffer, N.; Gonzalez-Freire, M.; Shardell, M.D.; Zoli, M.; Studenski, S.A.; Ferrucci, L. Early body composition, but not body mass, is associated with future accelerated decline in muscle quality. J. Cachexia Sarcopenia Muscle 2017, 8, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 2018, 105, 10–18. [Google Scholar] [CrossRef]
- Da Silva, A.P.; Matos, A.; Valente, A.; Gil, A.; Alonso, I.; Ribeiro, R.; Bicho, M.; Gorjão-Clara, J. Body composition assessment and nutritional status evaluation in men and women Portuguese centenarians. J. Nutr. Health Aging 2016, 20, 256–266. [Google Scholar] [CrossRef]
- Ferrucci, L.; Studenski, S. Clinical problems of aging. In Harrison’s Principles of Internal Medicine; Longo, D.L., Kasper, D.L., Hauser, S.L., Jameson, J.L., Loscalzo, J., Fauci, A.S., Eds.; McGraw-Hill: New York, NY, USA, 2012; Volume 18, pp. 570–585. [Google Scholar]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef]
- Howlett, K.F.; McGee, S. Epigenetic regulation of skeletal muscle metabolism. Clin. Sci. 2016, 130, 1051–1063. [Google Scholar] [CrossRef]
- Pasyukova, E.G.; Vaiserman, A.M. HDAC inhibitors: A new promising drug class in anti-aging research. Mech. Ageing Dev. 2017, 166, 6–15. [Google Scholar] [CrossRef]
- Tweedie, C.; Romestaing, C.; Burelle, Y.; Safdar, A.; Tarnopolsky, M.A.; Seadon, S.; Britton, S.L.; Koch, L.G.; Hepple, R.T. Lower oxidative DNA damage despite greater ROS production in muscles from rats selectively bred for high running capacity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R544–R553. [Google Scholar] [CrossRef] [Green Version]
- Radak, Z.; Suzuki, K.; Higuchi, M.; Balogh, L.; Boldogh, I.; Koltai, E. Physical exercise, reactive oxygen species and neuroprotection. Free Radic. Biol. Med. 2016, 98, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Sharples, A.P.; Stewart, C.E.; Seaborne, R.A. Does skeletal muscle have an ‘epi’-memory? The role of epigenetics in nutritional programming, metabolic disease, aging and exercise. Aging Cell 2016, 15, 603–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grazioli, E.; Dimauro, I.; Mercatelli, N.; Wang, G.; Pitsiladis, Y.; Di Luigi, L.; Caporossi, D. Physical activity in the prevention of human diseases: Role of epigenetic modifications. BMC Genom. 2017, 18 (Suppl. 8), 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, W.M. Exercise-associated DNA methylation change in skeletal muscle and the importance of imprinted genes: A bioinformatics meta-analysis. Br. J. Sports Med. 2015, 49, 1567–1578. [Google Scholar] [CrossRef] [Green Version]
- Kanzleiter, T.; Jähnert, M.; Schulze, G.; Selbig, J.; Hallahan, N.; Schwenk, R.W.; Schürmann, A. Exercise training alters DNA methylation patterns in genes related to muscle growth and differentiation in mice. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E912–E920. [Google Scholar] [CrossRef] [Green Version]
- Widmann, M.; Nieß, A.M.; Munz, B. Physical exercise and epigenetic modifications in skeletal muscle. Sports Med. 2019, 49, 509–523. [Google Scholar] [CrossRef]
- Drummond, M.J.; McCarthy, J.J.; Fry, C.S.; Esser, K.A.; Rasmussen, B.B. Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1333–E1340. [Google Scholar] [CrossRef] [Green Version]
- Ringholm, S.; Biensø, R.S.; Kiilerich, K.; Guadalupe-Grau, A.; Aachmann-Andersen, N.J.; Saltin, B.; Plomgaard, P.; Lundby, C.; Wojtaszewski, J.F.; Calbet, J.A.; et al. Bed rest reduces metabolic protein content and abolishes exercise-induced mRNA responses in exercise-induced mRNA responses in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E649–E658. [Google Scholar] [CrossRef]
- Andersen, J.L. Muscle fibre type adaptation in the elderly human muscle. Scan. J. Med. Sci. Sports 2003, 13, 40–47. [Google Scholar] [CrossRef]
- Vigelsø, A.; Prats, C.; Ploug, T.; Dela, F.; Helge, J.W. Higher muscle content of perilipin 5 and endothelial lipase protein in trained than untrained middle-aged men. Physiol. Res. 2016, 65, 293–302. [Google Scholar] [PubMed]
- Carter, C.S.; Justice, J.N.; Thompson, L. Lipotoxicity, aging, and muscle contractility: Does fiber type matter? Geroscience 2019, 41, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.; Newsom, S.A.; Strauss, A.; Kerege, A.; Kahn, D.E.; Harrison, K.A.; Snell-Bergeon, J.K.; Nemkov, T.; D’Alessandro, A.; Jackman, M.R.; et al. Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle. JCI Insight 2018, 3, 96805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Søgaard, D.; Baranowski, M.; Larsen, S.; Taulo Lund, M.; Munk Scheuer, C.; Vestergaard Abildskov, C.; Greve Dideriksen, S.; Dela, F.; Wulff Helge, J. Muscle-Saturated Bioactive Lipids Are Increased with Aging and Influenced by High-Intensity Interval Training. Int. J. Mol. Sci. 2019, 20, E1240. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.M.; Johannsen, D.L.; Ravussin, E. Skeletal muscle mitochondria and aging: A review. J. Aging Res. 2012, 194821, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Hu, P.; Huang, R.; Cao, Y.; Deng, J.; Yuan, W.; Liu, D.; Yang, J.; Gu, H.; et al. Aging-associated mitochondrial DNA mutations alter oxidative phosphorylation machinery and cause mitochondrial dysfunctions. Biochim. Biophys. Acta 2017, 1863, 2266–2273. [Google Scholar] [CrossRef]
- Ahlqvist, K.J.; Suomalainen, A.; Hämäläinen, R.H. Stem cells, mitochondria and aging. Biochim. Biophys. Acta 2015, 1847, 1380–1386. [Google Scholar] [CrossRef] [Green Version]
- Jing, E.; Emanuelli, B.; Hirschey, M.D.; Boucher, J.; Lee, K.Y.; Lombard, D.; Verdin, E.M.; Kahn, C.R. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc. Natl. Acad. Sci. USA 2011, 108, 14608–14613. [Google Scholar] [CrossRef] [Green Version]
- Guarente, L. Sirtuins, aging, and medicine. N. Engl. J. Med. 2011, 364, 2235–2244. [Google Scholar] [CrossRef]
- Brown, K.; Xie, S.; Qiu, X.; Mohrin, M.; Shin, J.; Liu, Y.; Zhang, D.; Scadden, D.T.; Chen, D. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013, 3, 319–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giralt, A.; Villarroya, F. SIRT3, a pivotal actor in mitochondrial functions: Metabolism, cell death and aging. Biochem. J. 2012, 444, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menshikova, E.V.; Ritov, V.B.; Dube, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.; Coen, P.M.; Goodpaster, B.H. Calorie restriction-induced weight loss and exercise have differential effects on skeletal muscle mitochondria despite similar effects on insulin sensitivity. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Cox, H.G. Later Life: The Realities of Aging, 6th ed.; Routledge, Taylor & Francis Group: New York, NY, USA, 2015; pp. 1–21. [Google Scholar]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, B.C.; Manini, T.M. Sarcopenia =/= dynapenia. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Manini, T.M. Functional consequences of sarcopenia and dynapenia in the elderly. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 271. [Google Scholar] [CrossRef] [Green Version]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Malafarina, V.; Uriz-Otano, F.; Iniesta, R.; Gil-Guerrero, L. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: A systematic review. J. Am. Med. Dir. Assoc. 2013, 14, 10–17. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Uchida, K.; Jeong, S.; Yamaga, M. Effects of nutritional supplements on muscle mass and activities of daily living in elderly rehabilitation patients with decreased muscle mass: A randomized controlled trial. J. Nutr. Health Aging 2016, 20, 185–191. [Google Scholar] [CrossRef]
- Artaza-Artabe, I.; Saez-Lopez, P.; Sánchez-Hernández, N.; Fernandez-Gutierrez, N.; Malafarina, V. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas 2016, 93, 89–99. [Google Scholar] [CrossRef]
- Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Degens, H.; Fuleihan, G.E.; Josse, R.; Lips, P.T.; Torres, J.M.; Rizzoli, R.; et al. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos. Int. 2013, 24, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Gougeon, R.; Nayar, K.; Morais, J.A. Frailty amplifies the effects of aging on protein metabolism: Role of protein intake. Am. J. Clin. Nutr. 2003, 78, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrucci, L.; Hesdorffer, C.; Bandinelli, S.; Simonsick, E.M. Frailty as a nexus between the biology of aging, environmental conditions and clinical geriatrics. Public Health Rev. 2010, 32, 475. [Google Scholar] [CrossRef] [Green Version]
- Azamar-Llamas, D.; Hernández-Molina, G.; Ramos-Ávalos, B.; Furuzawa-Carballeda, J. Adipokine contribution to the pathogenesis of osteoarthritis. Mediat. Inflamm. 2017, 5468023, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burlui, A.; Graur, M.; Gherasim, A.; Cardoneanu, A.; Rezuş, E. The role of adipokines in inflammation and connective tissue diseases: Can we face the challenge? Int. J. Med. Dent. 2018, 22, 132–139. [Google Scholar]
- Pessin, J.E.; Kwon, H. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Shintani, T.; Higashi, S.; Suzuki, R.; Takeuchi, Y.; Ikaga, R.; Yamazaki, T.; Kobayashi, K.; Noda, M. PTPRJ Inhibits Leptin Signaling, and Induction of PTPRJ in the Hypothalamus Is a Cause of the Development of Leptin Resistance. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Zabeau, L.; Jensen, C.J.; Seeuws, S.; Venken, K.; Verhee, A.; Catteeuw, D.; Van Loo, G.; Chen, H.; Walder, K.; Hollis, J.; et al. Leptin’s metabolic and immune functions can be uncoupled at the ligand/receptor interaction level. Cell. Mol. Life Sci. 2015, 72, 629–644. [Google Scholar] [CrossRef] [Green Version]
- Park, H.K.; Ahima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metab. Clin. Exp. 2015, 64, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Arch, J.R. The discovery of drugs for obesity, the metabolic effects of leptin and variable receptor pharmacology: Perspectives from β 3-adrenoceptor agonists. Naunyn Schmiedeberg’s Arch. Pharmacol. 2008, 378, 225–240. [Google Scholar] [CrossRef]
- Aguilar-Valles, A.; Inoue, W.; Rummel, C.; Luheshi, G.N. Obesity, adipokines and neuroinflammation. Neuropharmacology 2015, 96, 124–134. [Google Scholar] [CrossRef]
- Pareja-Galeano, H.; Santos-Lozano, A.; Sanchís-Gomar, F.; Fiuza-Luces, C.; Garatachea, N.; Gálvez, B.G.; Lucia, A.; Emanuele, E. Circulating leptin and adiponectin concentrations in healthy exceptional longevity. Mech. Ageing Dev. 2017, 162, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Park, A.J.; Battaglino, R.A.; Nguyen, N.M.; Morse, L.R. Associations between lean mass and leptin in men with chronic spinal cord injury: Results from the FRASCI-muscle study. PLoS ONE 2018, 13, e0198969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, B.; Santana, A.; Fuentes, T.; Delgado-Guerra, S.; Cabrera-Socorro, A.; Dorado, C.; Calbet, J.A. Leptin receptors in human skeletal muscle. J. Appl. Physiol. 2007, 102, 1786–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolsk, E.; Mygind, H.; Grøndahl, T.S.; Pedersen, B.K.; Van Hall, G. Human skeletal muscle releases leptin in vivo. Cytokine 2012, 60, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Nozhenko, Y.; Rodríguez, A.M.; Palou, A. Leptin rapidly induces the expression of metabolic and myokine genes in C2C12 muscle cells to regulate nutrient partition and oxidation. Cell. Physiol. Biochem. 2015, 35, 92–103. [Google Scholar] [CrossRef]
- Domingues-Faria, C.; Goncalves-Mendes, N.; Farges, M.C. Muscle Immune Cells, Obesity, and High-Fat Feeding. In Nutrition and Skeletal Muscle; Walrand, S., Ed.; Academic Press: New York, NY, USA; Elsevier: Amsterdam, The Netherlands, 2019; pp. 125–135. [Google Scholar]
- Ghamkhar, L.; Kahlaee, A.H. The effect of trunk muscle fatigue on postural control of upright stance: A systematic review. Gait Posture 2019, 72, 167–174. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Simar, D.; Chen, H.; Lambert, K.; Mercier, J.; Morris, M.J. Interaction between maternal obesity and post-natal over-nutrition on skeletal muscle metabolism. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 269–276. [Google Scholar] [CrossRef]
- Shelley, P.; Martin-Gronert, M.S.; Rowlerson, A.; Poston, L.; Heales, S.J.; Hargreaves, I.P.; McConnell, J.M.; Ozanne, S.E.; Fernandez-Twinn, D.S. Altered skeletal muscle insulin signaling and mitochondrial complex II-III linked activity in adult offspring of obese mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R675–R681. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Li, Q.; Liu, J.; Jia, S. Irisin, an exercise-induced myokine as a metabolic regulator: An updated narrative review. Diabetes Metab. Res. Rev. 2016, 32, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.E.; Séguin, C.; Parise, G.; Baker, S.K.; Phillips, S.M. Day-to-day changes in muscle protein synthesis in recovery from resistance, aerobic, and high-intensity interval exercise in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1024–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharmacal Res. 2018, 41, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Valet, P.; Dray, C.; Knauf, C.; Kunduzova, O.; Castan-Laurell, I. Pharmaceutical Composition for Use in the Treatment of Dysfunction Associated with Aging. U.S. Patent U.S. 2015/0290286 A1, 6 July 2016. [Google Scholar]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Hecksteden, A.; Wegmann, M.; Steffen, A.; Kraushaar, J.; Morsch, A.; Ruppenthal, S.; Kaestner, L.; Meyer, T. Irisin and exercise training in humans–results from a randomized controlled training trial. BMC Med. 2013, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A.; et al. Irisin–a myth rather than an exercise-inducible myokine. Sci. Rep. 2015, 5, 8889. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, R.A.; Gannon, N.P.; Mermier, C.M.; Conn, C.A. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J. Physiol. Biochem. 2015, 71, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.S.; Kim, N.; Kong, I.D. Circulating irisin levels as a predictive biomarker for sarcopenia: A cross-sectional community-based study. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, M.F.; Wallace, G.R.; Davis, E.T.; Murphy, D.P.; Nicholson, T.; Bennett, A.J.; Tsintzas, K.; Jones, S.W. Obese subcutaneous adipose tissue impairs human myogenesis, particularly in old skeletal muscle, via resistin-mediated activation of NFκB. Sci. Rep. 2018, 8, 15360. [Google Scholar] [CrossRef]
- Yoshiko, A.; Kaji, T.; Sugiyama, H.; Koike, T.; Oshida, Y.; Akima, H. Muscle quality characteristics of muscles in the thigh, upper arm and lower back in elderly men and women. Eur. J. Appl. Physiol. 2018, 118, 1385–1395. [Google Scholar] [CrossRef]
- Prestes, J.; da Cunha Nascimento, D.; de Sousa Neto, I.V.; Tibana, R.A.; Shiguemoto, G.E.; de Andrade Perez, S.E.; Botero, J.P.; Schoenfeld, B.J.; Pereira, G.B. The effects of muscle strength responsiveness to periodized resistance training on resistin, leptin, and cytokine in elderly postmenopausal women. J. Strength Cond. Res. 2018, 32, 113–120. [Google Scholar] [CrossRef]
- Van der Spoel, E.; Jansen, S.W.; Akintola, A.A.; Ballieux, B.E.; Cobbaert, C.M.; Slagboom, P.E.; Blauw, G.J.; Westendorp, R.G.; Pijl, H.; Roelfsema, F.; et al. Growth hormone secretion is diminished and tightly controlled in humans enriched for familial longevity. Aging Cell 2016, 15, 1126–1131. [Google Scholar] [CrossRef]
- Meyer, K.; Yankner, B.A. Slowing down aging. Cell Metabolism. 2017, 26, 592–593. [Google Scholar] [CrossRef] [Green Version]
- Vitale, G.; Cesari, M.; Mari, D. Aging of the endocrine system and its potential impact on sarcopenia. Eur. J. Inter. Med. 2016, 35, 10–15. [Google Scholar] [CrossRef]
- Janssen, J.A. Impact of Physical Exercise on Endocrine Aging. In Sports Endocrinology; Karger Publishers: Basel, Switzerland, 2016; Volume 47, pp. 68–81. [Google Scholar]
- Hayes, L.D.; Herbert, P.; Sculthorpe, N.F.; Grace, F.M. Exercise training improves free testosterone in lifelong sedentary aging men. Endocr. Connect. 2017, 6, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance training for older adults: Position statement from the National strength and conditioning association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Pharmacologic options for the treatment of sarcopenia. Calcif. Tissue Int. 2016, 98, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Neto, W.K.; Gama, E.F.; Rocha, L.Y.; Ramos, C.C.; Taets, W.; Scapini, K.B.; Ferreira, J.B.; Rodrigues, B.; Caperuto, É. Effects of testosterone on lean mass gain in elderly men: Systematic review with meta-analysis of controlled and randomized studies. Age 2015, 37, 9742. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Lee, B.M. Comparative safety evaluation of selective androgen receptor modulators and anabolic androgenic steroids. Expert Opin. Drug Saf. 2015, 14, 1773–1785. [Google Scholar] [CrossRef]
- Dalton, J.T. The long and winding road for selective androgen receptor modulators. Br. J. Clin. Pharmacol. 2017, 83, 2131–2133. [Google Scholar] [CrossRef]
- Neil, D.; Clark, R.V.; Magee, M.; Billiard, J.; Chan, A.; Xue, Z.; Russell, A. GSK2881078, a SARM, produces dose-dependent increases in lean mass in healthy older men and women. J. Clin. Endocrinol. Metab. 2018, 103, 3215–3224. [Google Scholar] [CrossRef]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef]
- Hayes, L.D.; Sculthorpe, N.; Herbert, P.; Baker, J.S.; Spagna, R.; Grace, F.M. Six weeks of conditioning exercise increases total, but not free testosterone in lifelong sedentary aging men. Aging Male 2015, 18, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Hackney, A.C.; Hooper, D.R. Reductions in testosterone are not indicative of exercise performance decrement in male endurance athletes. Aging Male 2019, 1–2. [Google Scholar] [CrossRef]
- Mandrup, C.M.; Egelund, J.; Nyberg, M.; Enevoldsen, L.H.; Kjær, A.; Clemmensen, A.E.; Christensen, A.N.; Suetta, C.; Frikke-Schmidt, R.; Steenberg, D.E.; et al. Effects of menopause and high-intensity training on insulin sensitivity and muscle metabolism. Menopause 2018, 25, 165–175. [Google Scholar] [CrossRef]
- Gorres, B.K.; Bomhoff, G.L.; Morris, J.K.; Geiger, P.C. In vivo stimulation of oestrogen receptor α increases insulin-stimulated skeletal muscle glucose uptake. J. Physiol. 2011, 589, 2041–2054. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.P.; Machado, U.F.; Warner, M.; Gustafsson, J.Å. Muscle GLUT4 regulation by estrogen receptors ERβ and ERα. Proc. Natl. Acad. Sci. USA 2006, 103, 1605–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, B.C.; Laakkonen, E.K.; Lowe, D.A. Aging of the musculoskeletal system: How the loss of estrogen impacts muscle strength. Bone 2019, 123, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.S.; Son, W.M. Combined exercise is a modality for improving insulin resistance and aging-related hormone biomarkers in elderly Korean women. Exp. Gerontol. 2018, 114, 13–18. [Google Scholar] [CrossRef]
- Im, J.Y.; Bang, H.S.; Seo, D.Y. The Effects of 12 Weeks of a Combined Exercise Program on Physical Function and Hormonal Status in Elderly Korean Women. Int. J. Environ. Res. Public Health 2019, 1, E4196. [Google Scholar] [CrossRef] [Green Version]
- Geerlings, M.I.; Gerritsen, L. Late-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: A systematic review and meta-analysis. Biol. Psychiatry 2017, 82, 339–350. [Google Scholar] [CrossRef]
- Savolainen, K.; Eriksson, J.G.; Kajantie, E.; Lahti, J.; Räikkönen, K. Telomere length and hypothalamic–pituitary–adrenal axis response to stress in elderly adults. Psychoneuroendocrinology 2015, 53, 179–184. [Google Scholar] [CrossRef]
- Bodine, S.C.; Furlow, J.D. Glucocorticoids and Skeletal Muscle. In Glucocorticoid Signaling; Springer: New York, NY, USA, 2015; pp. 145–176. [Google Scholar]
- Kraemer, R.R.; Castracane, V.D. Endocrine alterations from concentric vs. eccentric muscle actions: A brief review. Metabolism 2015, 64, 190–201. [Google Scholar] [CrossRef]
- Son, J.S.; Chae, S.A.; Testroet, E.D.; Du, M.; Jun, H.P. Exercise-induced myokines: A brief review of controversial issues of this decade. Expert Rev. Endocrinol. Metab. 2018, 13, 51–58. [Google Scholar] [CrossRef]
- Morley, J.E. Hormones and sarcopenia. Curr. Pharm. Des. 2017, 23, 4484–4492. [Google Scholar] [CrossRef]
- Chen, J.A.; Splenser, A.; Guillory, B.; Luo, J.; Mendiratta, M.; Belinova, B.; Halder, T.; Zhang, G.; Li, Y.P.; Garcia, J.M. Ghrelin prevents tumour-and cisplatin-induced muscle wasting: Characterization of multiple mechanisms involved. J. Cachexia Sarcopenia Muscle 2015, 6, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Gortan Cappellari, G.; Semolic, A.; Ruozi, G.; Vinci, P.; Guarnieri, G.; Bortolotti, F.; Barbetta, D.; Zanetti, M.; Giacca, M.; Barazzoni, R. Unacylated ghrelin normalizes skeletal muscle oxidative stress and prevents muscle catabolism by enhancing tissue mitophagy in experimental chronic kidney disease. FASEB J. 2017, 31, 5159–5171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillory, B.; Chen, J.A.; Patel, S.; Luo, J.; Splenser, A.; Mody, A.; Ding, M.; Baghaie, S.; Anderson, B.; Iankova, B.; et al. Deletion of ghrelin prevents aging-associated obesity and muscle dysfunction without affecting longevity. Aging Cell 2017, 16, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Gortan Cappellari, G.; Palus, S.; Vinci, P.; Ruozi, G.; Zanetti, M.; Semolic, A.; Ebner, N.; von Haehling, S.; Sinagra, G.; et al. Acylated ghrelin treatment normalizes skeletal muscle mitochondrial oxidative capacity and AKT phosphorylation in rat chronic heart failure. J. Cachexia Sarcopenia Muscle 2017, 8, 991–998. [Google Scholar] [CrossRef]
- Tremblay, A.; Dutheil, F.; Drapeau, V.; Metz, L.; Lesour, B.; Chapier, R.; Pereira, B.; Verney, J.; Baker, J.S.; Vinet, A.; et al. Long-term effects of high-intensity resistance and endurance exercise on plasma leptin and ghrelin in overweight individuals: The RESOLVE Study. Appl. Physiol. Nutr. Metab. 2019, 44, 1172–1179. [Google Scholar] [CrossRef]
- Serra-Prat, M.; Papiol, M.; Monteis, R.; Palomera, E.; Cabré, M. Relationship between plasma ghrelin levels and sarcopenia in elderly subjects: A cross-sectional study. J. Nutr. Health Aging 2015, 19, 669–672. [Google Scholar] [CrossRef]
- Bouchi, R.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; Hashimoto, K.; Yoshimoto, T.; Ogawa, Y. Insulin treatment attenuates decline of muscle mass in Japanese patients with type 2 diabetes. Calcif. Tissue Int. 2017, 101, 1–8. [Google Scholar] [CrossRef]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell. Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Canciglieri, P.H.; Kuga, G.K.; Muñoz, V.R.; Gaspar, R.C.; da Rocha, A.L.; Breda, L.; Anaruma, C.P.; Minuzzi, L.G.; da Silva, A.S.; Cintra, D.E.; et al. The reversal effect of physical exercise on aging-related increases in APPL2 content in skeletal muscle. Life Sci. 2018, 210, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef] [Green Version]
- Bucci, M.; Huovinen, V.; Guzzardi, M.A.; Koskinen, S.; Raiko, J.R.; Lipponen, H.; Ahsan, S.; Badeau, R.M.; Honka, M.J.; Koffert, J.; et al. Resistance training improves skeletal muscle insulin sensitivity in elderly offspring of overweight and obese mothers. Diabetologia 2016, 59, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, M.; Lithgow, H.; Hayes, L.; Florida-James, G. Potential Cellular and Biochemical Mechanisms of Exercise and Physical Activity on the Ageing Process. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science; Springer: Singapore, 2019; pp. 311–338. [Google Scholar]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Rezuș, E.; Cardoneanu, A.; Burlui, A.; Luca, A.; Codreanu, C.; Tamba, B.I.; Stanciu, G.D.; Dima, N.; Bădescu, C.; Rezuș, C. The link between inflammaging and degenerative joint diseases. Int. J. Mol. Sci. 2019, 20, 614. [Google Scholar] [CrossRef] [Green Version]
- Frasca, D.; Blomberg, B.B. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016, 17, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Howcroft, T.K.; Campisi, J.; Louis, G.B.; Smith, M.T.; Wise, B.; Wyss-Coray, T.; Augustine, A.D.; McElhaney, J.E.; Kohanski, R.; Sierra, F. The role of inflammation in age-related disease. Aging 2013, 5, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrisette-Thomas, V.; Cohen, A.A.; Fülöp, T.; Riesco, É.; Legault, V.; Li, Q.; Milot, E.; Dusseault-Bélanger, F.; Ferrucci, L. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech. Ageing Dev. 2014, 139, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Malafarina, V.; Úriz-Otano, F.; Iniesta, R.; Gil-Guerrero, L. Sarcopenia in the elderly: Diagnosis, physiopathology and treatment. Maturitas 2012, 71, 109–114. [Google Scholar] [CrossRef]
- Baylis, D.; Bartlett, D.B.; Patel, H.P.; Roberts, H.C. Understanding how we age: Insights into inflammaging. Longev. Healthspan 2013, 2, 8. [Google Scholar] [CrossRef]
- Mueller, C.; Compher, C.; Ellen, D.M. American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors.ASPEN clinical guidelines: Nutrition screening, assessment, and intervention in adults. J. Parenter. Enteral Nutr. 2011, 35, 16–24. [Google Scholar] [CrossRef]
- Girven, M.; Dugdale, H.F.; Owens, D.J.; Hughes, D.C.; Stewart, C.E.; Sharples, A.P. L-glutamine Improves Skeletal Muscle Cell Differentiation and Prevents Myotube Atrophy After Cytokine (TNF-α) Stress Via Reduced p38 MAPK Signal Transduction. J. Cell. Physiol. 2016, 231, 2720–2732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro-Junior, R.S.; de Tarso Maciel-Pinheiro, P.; da Matta Mello Portugal, E.; da Silva Figueiredo, L.F.; Terra, R.; Carneiro, L.S.; Rodrigues, V.D.; Nascimento, O.J.; Deslandes, A.C.; Laks, J. Effect of exercise on inflammatory profile of older persons: Systematic review and meta-analyses. J. Phys. Act. Health 2018, 15, 64–71. [Google Scholar] [CrossRef] [PubMed]
- De Lemos Muller, C.H.; De Matos, J.R.; Grigolo, G.B.; Schroeder, H.T.; Rodrigues-Krause, J.; Krause, M. Exercise Training for the Elderly: Inflammaging and the Central Role for HSP70. J. Sci. Sport Exerc. 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Ostan, R.; Lanzarini, C.; Pini, E.; Scurti, M.; Vianello, D.; Bertarelli, C.; Fabbri, C.; Izzi, M.; Palmas, G.; Biondi, F.; et al. Inflammaging and cancer: A challenge for the Mediterranean diet. Nutrients 2015, 7, 2589–2621. [Google Scholar] [CrossRef] [Green Version]
- Nicklas, B.J.; Hsu, F.C.; Brinkley, T.J.; Church, T.; Goodpaster, B.H.; Kritchevsky, S.B.; Pahor, M. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J. Am. Geriatr. Soc. 2008, 56, 2045–2052. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Kanaley, J.; Sadeghi, K. Long-term aerobic exercise and omega-3 supplementation modulate osteoporosis through inflammatory mechanisms in post-menopausal women: A randomized, repeated measures study. Nutr. Metab. 2011, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Martins, R.A.; Veríssimo, M.T.; Coehlo e Silva, M.J.; Cumming, S.P.; Teixeira, A.M. Effects of aerobic and strength-based training on metabolic health indicators in older adults. Lipids Health Dis. 2010, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Rea, I.M. Towards ageing well: Use it or lose it: Exercise, epigenetics and cognition. Biogerontology 2017, 18, 679–691. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezuş, E.; Burlui, A.; Cardoneanu, A.; Rezuş, C.; Codreanu, C.; Pârvu, M.; Rusu Zota, G.; Tamba, B.I. Inactivity and Skeletal Muscle Metabolism: A Vicious Cycle in Old Age. Int. J. Mol. Sci. 2020, 21, 592. https://doi.org/10.3390/ijms21020592
Rezuş E, Burlui A, Cardoneanu A, Rezuş C, Codreanu C, Pârvu M, Rusu Zota G, Tamba BI. Inactivity and Skeletal Muscle Metabolism: A Vicious Cycle in Old Age. International Journal of Molecular Sciences. 2020; 21(2):592. https://doi.org/10.3390/ijms21020592
Chicago/Turabian StyleRezuş, Elena, Alexandra Burlui, Anca Cardoneanu, Ciprian Rezuş, Cătălin Codreanu, Mirela Pârvu, Gabriela Rusu Zota, and Bogdan Ionel Tamba. 2020. "Inactivity and Skeletal Muscle Metabolism: A Vicious Cycle in Old Age" International Journal of Molecular Sciences 21, no. 2: 592. https://doi.org/10.3390/ijms21020592
APA StyleRezuş, E., Burlui, A., Cardoneanu, A., Rezuş, C., Codreanu, C., Pârvu, M., Rusu Zota, G., & Tamba, B. I. (2020). Inactivity and Skeletal Muscle Metabolism: A Vicious Cycle in Old Age. International Journal of Molecular Sciences, 21(2), 592. https://doi.org/10.3390/ijms21020592