Molecular and Functional Characterization of Grapevine NIPs through Heterologous Expression in aqy-Null Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Results and Discussion
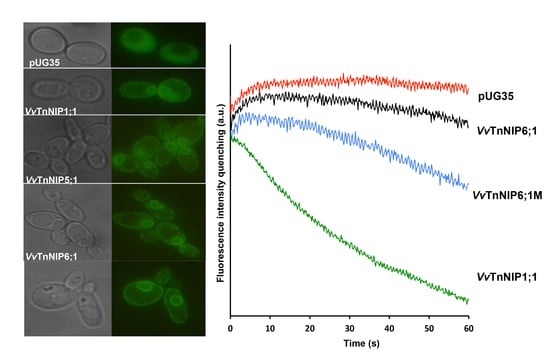
2.1. Sequence Analysis and Expression of Grapevine NIPs in S. cerevisiae
2.2. Water and Glycerol Transport Assays by Stopped-Flow Spectroscopy
2.3. pH-Dependent Gating of Grapevine NIPs
2.4. Growth Assays of Transformed Yeast Strains for Screening the Substrate Selectivity Profile of Grapevine NIPs
2.4.1. Metalloids
Arsenium
Selenium (Se)
Boron (B)
2.4.2. H2O2
2.4.3. Heavy Metals
3. Materials and Methods
3.1. Yeast Strain, Vector, and Growth Conditions
3.2. Sequence Analysis, Cloning, and Expression of Grapevine NIPs in S. cerevisiae
3.3. Water and Glycerol Transport Assays by Stopped-Flow Spectroscopy
3.4. Effect of pH on Gating of Grapevine NIPs
3.5. Growth Assays for Screening of Substrate Selectivity Profile of Grapevine NIPs
3.5.1. Substrates Other Than Water and Glycerol
3.5.2. Low-Phosphate Media
3.5.3. Drop-Test and Growth Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abascal, F.; Irisarri, I.; Zardoya, R. Diversity and evolution of membrane intrinsic proteins. Biochim. Biophys. Acta 2014, 1840, 1468–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurel, C.; Chrispeels, M.J. Aquaporins. A molecular entry into plant water relations. Plant Physiol. 2001, 125, 135–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortin, M.G.; Morrison, N.A.; Verma, D.P.S. Nodulin-26, a peribacteroid membrane nodulin is expressed independently of the development of the peribacteroid compartment. Nucleic Acids Res. 1987, 15, 813–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, I.S.; Choi, W.G.; Roberts, D.M. The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim. Biophys. Acta 2006, 1758, 1165–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pommerrenig, B.; Diehn, T.A.; Bienert, G.P. Metalloido-porins: Essentiality of Nodulin 26-like intrinsic proteins in metalloid transport. Plant Sci. 2015, 238, 212–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zardoya, R.; Ding, X.; Kitagawa, Y.; Chrispeels, M.J. Origin of plant glycerol transporters by horizontal gene transfer and functional recruitment. Proc. Natl. Acad. Sci. USA 2002, 99, 14893–14896. [Google Scholar] [CrossRef] [Green Version]
- Pommerrenig, B.; Diehn, T.A.; Bernhardt, N.; Bienert, M.D.; Mitani-Ueno, N.; Fuge, J.; Bieber, A.; Spitzer, C.; Bräutigam, A.; Ma, J.F. Functional evolution of Nodulin 26-like Intrinsic Proteins: From bacterial arsenic detoxification to plant nutrient transport. New Phytol. 2020, 225, 1383–1396. [Google Scholar] [CrossRef] [Green Version]
- Hove, R.M.; Bhave, M. Plant aquaporins with non-aqua functions: Deciphering the signature sequences. Plant Mol. Biol. 2011, 75, 413–430. [Google Scholar] [CrossRef]
- Rouge, P.; Barre, A. A molecular modeling approach defines a new group of Nodulin 26-like aquaporins in plants. Biochem. Biophys. Res. Commun. 2008, 367, 60–66. [Google Scholar] [CrossRef]
- Wang, Y.; Schulten, K.; Tajkhorshid, E. What makes an aquaporin a glycerol channel? A comparative study of AqpZ and GlpF. Structure 2005, 13, 1107–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Beitz, E. Aquaporins with selectivity for unconventional permeants. Cell. Mol. Life Sci. 2007, 64, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, O.; Aury, J.-M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar]
- Corso, M.; Bonghi, C. Grapevine rootstock effects on abiotic stress tolerance. Plant Sci. Today 2014, 1, 108–113. [Google Scholar] [CrossRef]
- Sabir, F.; Leandro, M.J.; Martins, A.P.; Loureiro-Dias, M.C.; Moura, T.F.; Soveral, G.; Prista, C. Exploring three PIPs and three TIPs of grapevine for transport of water and atypical substrates through heterologous expression in aqy-null yeast. PLoS ONE 2014, 9, e102087. [Google Scholar] [CrossRef]
- Noronha, H.; Araújo, D.; Conde, C.; Martins, A.P.; Soveral, G.; Chaumont, F.; Delrot, S.; Gerós, H. The grapevine uncharacterized intrinsic protein 1 (VvXIP1) is regulated by drought stress and transports glycerol, hydrogen peroxide, heavy metals but not water. PLoS ONE 2016, 11, e0160976. [Google Scholar] [CrossRef] [Green Version]
- Gambetta, G.A.; Manuck, C.M.; Drucker, S.T.; Shaghasi, T.; Fort, K.; Matthews, M.A.; Walker, M.A.; McElrone, A.J. The relationship between root hydraulics and scion vigour across Vitis rootstocks: What role do root aquaporins play? J. Exp. Bot. 2012, 63, 6445–6455. [Google Scholar] [CrossRef]
- Leitão, L.; Prista, C.; Moura, T.F.; Loureiro-Dias, M.C.; Soveral, G. Grapevine aquaporins: Gating of a tonoplast intrinsic protein (TIP2;1) by cytosolic pH. PLoS ONE 2012, 7, e33219. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.P.; Thorsen, M.; Schussler, M.D.; Nilsson, H.R.; Wagner, A.; Tamas, M.J.; Jahn, T.P. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Wallace, I.S.; Roberts, D.M. Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry 2005, 44, 16826–16834. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Zhang, Z.; Wu, J.; Feng, Y.; Zhu, Z. Divergence in function and expression of the NOD26-like intrinsic proteins in plants. BMC Genom. 2009, 10, 313. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, I.S.; Wills, D.M.; Guenther, J.F.; Roberts, D.M. Functional selectivity for glycerol of the nodulin 26 subfamily of plant membrane intrinsic proteins. FEBS Lett. 2002, 523, 109–112. [Google Scholar] [CrossRef]
- Tamás, M.J.; Karlgren, S.; Bill, R.M.; Hedfalk, K.; Allegri, L.; Ferreira, M.; Thevelein, J.M.; Rydström, J.; Mullins, J.G.L.; Hohmann, S. A short regulatory domain restricts glycerol transport through yeast Fps1p. J. Biol. Chem. 2003, 278, 6337–6345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, H.; Han, B.G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural basis of water-specific transport through the AQP1 water channel. Nature 2001, 414, 872–878. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, S.; Krantz, M.; Nordlander, B. Yeast osmoregulation. Methods Enzymol. 2007, 428, 29–45. [Google Scholar]
- Aubert, S.; Gout, E.; Bligny, R.; Douce, R. Multiple effects of glycerol on plant cell metabolism. Phosphorus-31 nuclear magnetic resonance studies. J. Biol. Chem. 1994, 269, 21420–21427. [Google Scholar]
- Hu, J.; Zhang, Y.; Wang, J.; Zhou, Y. Glycerol affects root development through regulation of multiple pathways in Arabidopsis. PLoS ONE 2014, 9, e86269. [Google Scholar] [CrossRef]
- Niemietz, C.M.; Tyerman, S.D. Channel-mediated permeation of ammonia gas through the peribacteroid membrane of soybean nodules. FEBS Lett. 2000, 465, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.A.; Zakharenko, A.M.; Zemchenko, I.V.; Haider, M.S.; Ali, M.A.; Imtiaz, M.; Chung, G.; Tsatsakis, A.; Sun, S.; Golokhvast, K.S. Phytolith formation in plants: From soil to cell. Plants 2019, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, R.K.; Vivancos, J.; Ramakrishnan, G.; Guerin, V.; Carpentier, G.; Sonah, H.; Labbe, C.; Isenring, P.; Belzile, F.J.; Belanger, R.R. A precise spacing between the NPA domains of aquaporins is essential for silicon permeability in plants. Plant J. 2015, 83, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Prak, S.; Hem, S.; Boudet, J.; Viennois, G.; Sommerer, N.; Rossignol, M.; Maurel, C.; Santoni, V. Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins role in subcellular trafficking of AtPIP2; 1 in response to salt stress. Mol. Cell. Proteom. 2008, 7, 1019–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, I.; Larsson, C.; Ek, B.; Kjellbom, P. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 1996, 8, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Daniels, M.J.; Yeager, M. Phosphorylation of aquaporin PvTIP3; 1 defined by mass spectrometry and molecular modeling. Biochemistry 2005, 44, 14443–14454. [Google Scholar] [CrossRef]
- Miao, G.H.; Hong, Z.; Verma, D.P. Topology and phosphorylation of soybean nodulin-26, an intrinsic protein of the peribacteroid membrane. J. Cell Biol. 1992, 118, 481–490. [Google Scholar] [CrossRef]
- Guenther, J.F.; Chanmanivone, N.; Galetovic, M.P.; Wallace, I.S.; Cobb, J.A.; Roberts, D.M. Phosphorylation of soybean nodulin 26 on serine 262 enhances water permeability and is regulated developmentally and by osmotic signals. Plant Cell 2003, 15, 981–991. [Google Scholar] [CrossRef] [Green Version]
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef] [Green Version]
- Tournaire-Roux, C.; Sutka, M.; Javot, H.; Gout, E.; Gerbeau, P.; Luu, D.-T.; Bligny, R.; Maurel, C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 2003, 425, 393–397. [Google Scholar] [CrossRef]
- Bellati, J.; Alleva, K.; Soto, G.; Vitali, V.; Jozefkowicz, C.; Amodeo, G. Intracellular pH sensing is altered by plasma membrane PIP aquaporin co-expression. Plant Mol. Biol. 2010, 74, 105–118. [Google Scholar] [CrossRef]
- Frick, A.; Järvå, M.; Törnroth-Horsefield, S. Structural basis for pH gating of plant aquaporins. FEBS Lett. 2013, 587, 989–993. [Google Scholar] [CrossRef]
- Zeuthen, T.; Klaerke, D.A. Transport of water and glycerol in aquaporin 3 is gated by H+. J. Biol. Chem. 1999, 274, 21631–21636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangi, R.; Filella, M. Transport routes of metalloids into and out of the cell: A review of the current knowledge. Chem. Biol. Interact. 2012, 197, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Maciaszczyk-Dziubinska, E.; Wawrzycka, D.; Wysocki, R. Arsenic and antimony transporters in eukaryotes. Int. J. Mol. Sci. 2012, 13, 3527–3548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothstein, A. Interactions of arsenate with the phosphate-transporting system of yeast. J. Gen. Physiol. 1963, 46, 1075–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Dai, W.; Yan, H.; Li, S.; Shen, H.; Chen, Y.; Xu, H.; Sun, Y.; He, Z.; Ma, M. Arabidopsis NIP3; 1 plays an important role in arsenic uptake and root-to-shoot translocation under arsenite stress conditions. Mol. Plant 2015, 8, 722–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiya, T.; Tanaka, M.; Mitani, N.; Ma, J.F.; Maeshima, M.; Fujiwara, T. NIP1;1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. J. Biol. Chem. 2009, 284, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Isayenkov, S.V.; Maathuis, F.J.M. The Arabidopsis thaliana aquaglyceroporin AtNIP7; 1 is a pathway for arsenite uptake. FEBS Lett. 2008, 582, 1625–1628. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, E.R.; Maathuis, F.J. Arabidopsis thaliana NIP7;1 is involved in tissue arsenic distribution and tolerance in response to arsenate. FEBS Lett. 2016, 590, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, F.; Shi, W.; Li, Y.; Miao, Y. Physiological characteristics of selenite uptake by maize roots in response to different pH levels. J. Plant Nutr. Soil Sci. 2010, 173, 417–422. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Mitani, N.; Yamaji, N.; Shen, R.F.; Ma, J.F. Involvement of silicon influx transporter OsNIP2;1 in selenite uptake in rice. Plant Physiol. 2010, 153, 1871–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitani, N.; Yamaji, N.; Ma, J.F. Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflug. Arch. 2008, 456, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.W.; Pilon-Smits, E.A.H.; Schiavon, M. Mechanisms of selenium hyperaccumulation in plants: A survey of molecular, biochemical and ecological cues. Biochim. Biophys. Acta 2018, 1862, 2343–2353. [Google Scholar] [CrossRef]
- Yoshinari, A.; Takano, J. Insights into the mechanisms underlying boron homeostasis in plants. Front. Plant Sci. 2017, 8, 1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, K.; Fujiwara, T. Boron transport in plants: Co-ordinated regulation of transporters. Ann. Bot. 2010, 105, 1103–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dordas, C.; Chrispeels, M.J.; Brown, P.H. Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol. 2000, 124, 1349–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, J.; Wada, M.; Ludewig, U.; Schaaf, G.; Von Wiren, N.; Fujiwara, T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 2006, 18, 1498–1509. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Wallace, I.S.; Takano, J.; Roberts, D.M.; Fujiwara, T. NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 2008, 20, 2860–2875. [Google Scholar] [CrossRef] [Green Version]
- Durbak, A.R.; Phillips, K.A.; Pike, S.; O’Neill, M.A.; Mares, J.; Gallavotti, A.; Malcomber, S.T.; Gassmann, W.; McSteen, P. Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 2014, 26, 2978–2995. [Google Scholar] [CrossRef] [Green Version]
- Hanaoka, H.; Uraguchi, S.; Takano, J.; Tanaka, M.; Fujiwara, T. O s NIP 3; 1, a rice boric acid channel, regulates boron distribution and is essential for growth under boron-deficient conditions. Plant J. 2014, 78, 890–902. [Google Scholar] [CrossRef] [Green Version]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Dynowski, M.; Schaaf, G.; Loque, D.; Moran, O.; Ludewig, U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem. J. 2008, 414, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C.; Verdoucq, L. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA-and pathogen-triggered stomatal closure. Procs. Natl. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef] [Green Version]
- Katsuhara, M.; Sasano, S.; Horie, T.; Matsumoto, T.; Rhee, J.; Shibasaka, M. Functional and molecular characteristics of rice and barley NIP aquaporins transporting water, hydrogen peroxide and arsenite. Plant Biotechnol. 2014, 31, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Chai, T.; Wen, Z.; Zhang, H. Indian mustard aquaporin improves drought and heavy-metal resistance in tobacco. Mol. Biotechnol. 2008, 40, 280–292. [Google Scholar] [CrossRef]
- Afzal, Z.; Howton, T.C.; Sun, Y.; Mukhtar, M.S. The roles of aquaporins in plant stress responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Przedpelska-Wasowicz, E.M.; Wierzbicka, M. Gating of aquaporins by heavy metals in Allium cepa L. epidermal cells. Protoplasma 2011, 248, 663–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güldener, U.; Hegemann, J. A Second Generation of GFP-Vectors for Subcellular Localization Studies in Budding Yeast; Heinrich Heine Universität: Düsseldorf, Germany, 1998. [Google Scholar]
- Pronk, J.T. Auxotrophic yeast strains in fundamental and applied research. Appl. Environ. Microbiol. 2002, 68, 2095–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krogh, A.; Larsson, B.; Von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tusnady, G.E.; Simon, I. The HMMTOP transmembrane topology prediction server. Bioinformatics 2001, 17, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.A.W.S. TMbase—A database of membrane spanning proteins segments. Biol. Chem. Hoppe Seyler 1993, 374, 166. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. In BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef]
- Soveral, G.; Madeira, A.; Loureiro-Dias, M.C.; Moura, T.F. Water transport in intact yeast cells as assessed by fluorescence self-quenching. Appl. Environ. Microbiol. 2007, 73, 2341–2343. [Google Scholar] [CrossRef] [Green Version]
- Pampulha, M.E.; Loureiro-Dias, M.C. Combined effect of acetic acid, pH and ethanol on intracellular pH of fermenting yeast. Appl. Microbiol. Biotechnol. 1989, 31, 547–550. [Google Scholar] [CrossRef]
- Rubin, G.M. Three forms of the 5.8-S ribosomal RNA species in Saccharomyces cerevisiae. Eur. J. Biochem. 1974, 41, 197–202. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]









| Strains | Activation Energy for Water Transport Ea (kcal mol−1) | Activation Energy for Glycerol Transport Ea (kcal mol−1) | ||||
|---|---|---|---|---|---|---|
| pHout 5.0 | pHout 5.0 + BA 1 | pHout 6.8 | pHout 5.0 | pHout 5.0 + BA 1 | pHout 6.8 | |
| (pHin 6.1) | (pHin 4.8) | (pHin 6.8) | (pHin 6.1) | (pHin 4.8) | (pHin 6.8) | |
| pUG35 | 14.05 ± 0.01 | 13.80 ± 0.2 | 13.67 ± 0.4 | 24.30 ± 1.2 | 25.10 ± 0.8 | 24.20 ± 0.98 |
| VvTnNIP1;1 | 9.80 ± 0.15 | 12.78 ± 0.16 | 9.53 ± 0.1 | 6.93 ± 0.22 | 11.69 ± 0.34 | 9.11 ± 0.023 |
| VvTnNIP5;1 | 14.60 ± 0.8 | nd | nd | 24.50 ± 1.1 | nd | nd |
| VvTnNIP6;1 | 13.54 ± 0.2 | nd | nd | 12.55 ± 0.8 | nd | nd |
| VvTnNIP6;1M | 11.3 ± 0.4 | 14.34 ± 0.26 | 11.93 ± 0.93 | 8.6 ± 0.5 | 12.11 ± 1.9 | 8.54 ± 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabir, F.; Gomes, S.; Loureiro-Dias, M.C.; Soveral, G.; Prista, C. Molecular and Functional Characterization of Grapevine NIPs through Heterologous Expression in aqy-Null Saccharomyces cerevisiae. Int. J. Mol. Sci. 2020, 21, 663. https://doi.org/10.3390/ijms21020663
Sabir F, Gomes S, Loureiro-Dias MC, Soveral G, Prista C. Molecular and Functional Characterization of Grapevine NIPs through Heterologous Expression in aqy-Null Saccharomyces cerevisiae. International Journal of Molecular Sciences. 2020; 21(2):663. https://doi.org/10.3390/ijms21020663
Chicago/Turabian StyleSabir, Farzana, Sara Gomes, Maria C. Loureiro-Dias, Graça Soveral, and Catarina Prista. 2020. "Molecular and Functional Characterization of Grapevine NIPs through Heterologous Expression in aqy-Null Saccharomyces cerevisiae" International Journal of Molecular Sciences 21, no. 2: 663. https://doi.org/10.3390/ijms21020663
APA StyleSabir, F., Gomes, S., Loureiro-Dias, M. C., Soveral, G., & Prista, C. (2020). Molecular and Functional Characterization of Grapevine NIPs through Heterologous Expression in aqy-Null Saccharomyces cerevisiae. International Journal of Molecular Sciences, 21(2), 663. https://doi.org/10.3390/ijms21020663