Synthesis of Novel Tetra(µ3-Methoxo) Bridged with [Cu(II)-O-Cd(II)] Double-Open-Cubane Cluster: XRD/HSA-Interactions, Spectral and Oxidizing Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cluster Preparation
2.2. Single Crystal X-ray Diffraction (SC-XRD) Investigation
2.3. HS and 2D-FP Investigation
2.4. FT-IR and EDX Investigations
2.5. Electronic Transfer and Optical Energy Gap
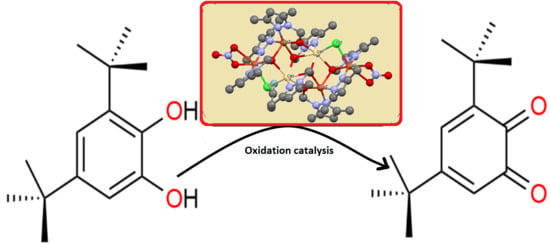
2.6. Cluster Oxidation Potential toward Catecholase of Catechol
3. Materials and Methods
3.1. Synthesis of [Cl2Cu4Cd2(NNO)6(NN)2(NO3)2].CH3CN Bimetallic Cluster
3.2. Catechol Oxidation Reaction
3.3. X-Ray
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paula, A.; Mistria, S.; Bertolasib, V.; Manna, S.C. DNA/protein binding and molecular docking studies of two tetranuclear Cu (II) complexes with double-open-cubane core like structure. Inorg. Chim. Acta 2019, 495, 119005. [Google Scholar] [CrossRef]
- Zheng, S.-R.; Pan, M.; Wu, K.; Chen, L.; Jiang, J.-J.; Wang, D.-W.; Shi, J.-Y.; Su, C.-Y. Assembly of Binuclear, Tetranuclear, and Multinuclear Complexes from Pincer-Like Mononuclear Metallotectons: Structural Diversity Dependent on Precursors. Cryst. Growth Des. 2015, 15, 625–634. [Google Scholar] [CrossRef]
- Aronica, C.; Chumakov, Y.; Jeanneau, E.; Luneau, D.; Neugebauer, P.; Barra, A.-L.; Gillon, B.; Goujon, A.; Cousson, A.; Tercero, J.; et al. Structure, Magnetic Properties, Polarized Neutron Diffraction, and Theoretical Study of a Copper(II) Cubane. Chem. Eur. J. 2008, 14, 9540. [Google Scholar] [CrossRef] [PubMed]
- Than, R.; Feldmann, A.A.; Krebs, B. Structural and functional studies on model compounds of purple acid phosphatases and catechol oxidases.Coord. Chem. Rev. 1999, 182, 211. [Google Scholar]
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic Copper-Catalyzed Organic Reactions. Chem. Rev. 2013, 113, 6234. [Google Scholar] [CrossRef] [Green Version]
- Bilyachenko, A.N.; Dronova, M.S.; Yalymov, A.I.; Lamaty, F.; Bantreil, X.; Martinez, J.; Bizet, C.; Shul’pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; et al. Cage-like Copper(II) Silsesquioxanes: Transmetalation Reactions and Structural, Quantum Chemical, and Catalytic Studies. Chem. Eur. J. 2015, 21, 8758. [Google Scholar] [CrossRef]
- Dias, S.S.P.; Kirillova, M.V.; Andre, V.; Kłak, J.; Kirillov, A.M. New tricopper(II) cores self-assembled from aminoalcohol biobuffers and homophthalic acid: Synthesis, structural and topological features, magnetic properties and mild catalytic oxidation of cyclic and linear C5–C8 alkanes. Inorg. Chem. Front. 2015, 2, 525. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Santos, C.I.M.; Andre, V.; Kłak, J.; Kirillova, M.V.; Kirillov, A.M. Copper(II) Coordination Polymers Self-Assembled from Aminoalcohols and Pyromellitic Acid: Highly Active Precatalysts for the Mild Water-Promoted Oxidation of Alkanes. Inorg. Chem. 2016, 55, 125. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Yasui, H. Zinc Complexes Developed as Metallopharmaceutics for Treating Diabetes Mellitus based on the Bio-Medicinal Inorganic Chemistry. Curr. Top. Med. Chem. 2012, 12, 210. [Google Scholar] [CrossRef]
- Muche, S.; Levacheva, I.; Samsonova, O.; Pham, L.; Christou, G.; Bakowsky, U.; Hołyńska, M. A Chiral, Low-Cytotoxic [Ni15]-Wheel Complex. Inorg. Chem. 2014, 53, 7642. [Google Scholar] [CrossRef]
- Vančo, J.; Marek, J.; Travniček, Z.; Račanska, E.; Muselik, J.; Švajlenova, O. Synthesis, structural characterization, antiradical and antidiabetic activities of copper(II) and zinc(II) Schiff base complexes derived from salicylaldehyde and β-alanine. J. Inorg. Biochem. 2008, 102, 595. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.-D.; Jiang, Y.-L.; Shan, L. Synthesis, characterization of diorganotin (IV) schiff base complexes and their in vitro antitumor activity. Chin. J. Chem. 2001, 19, 1136. [Google Scholar] [CrossRef]
- Vanco, J.; Svajlenova, O.; Racanska, E.; Muselik, J.; Valentova, J.; Elem, J. Antiradical activity of different copper(II) Schiff base complexes and their effect on alloxan-induced diabetes. Med. Biol. 2004, 18, 155. [Google Scholar]
- Serna, Z.; De la Pinta, N.; Urtiaga, M.K.; Lezama, L.; Madariaga, G.; Juan, J.M.; Coronado, E.; Cortes, R. Defective Dicubane-like Tetranuclear Nickel(II) Cyanate and Azide Nanoscale Magnets. Inorg. Chem. 2010, 49, 11541. [Google Scholar] [CrossRef]
- Wang, H.; Wan, C.-Q.; Mak, T.C.W. High-nuclearity silver(I) cluster-based coordination polymers assembled with multidentate oligo-α-heteroarylsulfanyl ligands. Dalton Trans. 2014, 43, 7254. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, J.; Li, G.; Shen, P.; Jin, H.; Zhang, N. Two coordination polymers constructed from a multidentate carboxylic acid ligand with a tertiary amine serve as acid–base catalysts for the synthesis of chloropropene carbonate from CO2 under atmospheric pressure. Dalton Trans. 2014, 43, 13965. [Google Scholar] [CrossRef] [PubMed]
- Penney, M.K.; Giang, R.; Burroughs, M.A., Jr.; Klausmeyer, K.K. Structure and luminescence of discrete and polymeric Ag(I) complexes formed by the multidentate pyridylphosphine (PPh2CH2)2N(3-CH2C5H4N). Polyhedron 2015, 87, 43. [Google Scholar] [CrossRef]
- Landers, A.E.; Phillips, D.J. Alkoxy-bridged complexes of copper(II) and nickel(II) with schiff bases of alcoholamines-including effects of structural distortion on the electronic spectra of Six-coordinate nickel(II) Complexes. Inorg. Chim. Acta 1979, 32, 53. [Google Scholar] [CrossRef]
- Si, S.-F.; Tang, J.-K.; Liao, D.-Z.; Jiang, Z.-H.; Yan, S.-P. Synthesis, structure, and characterization of dicopper(II) complex with a new amidate ligand. Inorg. Chem. Commun. 2002, 5, 76. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, H.; Chan, A.S.C.; Liu, Q.L.; Xu, X.L.; Du, C.X.; Zhu, Y. Structural and magnetic properties of O-bridged tetranuclear and binuclear Cu(II) complexes. Inorg. Chim. Acta 2002, 333, 138. [Google Scholar] [CrossRef]
- Koikawa, M.; Yamashita, H.; Tokii, T. Crystal structures and magnetic properties of tetranuclear copper(II) complexes of N-(2-hydroxymethylphenyl)salicylideneimine with a defective double-cubane core. Inorg. Chim. Acta 2004, 357, 2635. [Google Scholar] [CrossRef]
- Titi, A.; Shiga, T.; Oshio, H.; Touzani, R.; Hammouti, B.; Mouslim, M.; Warad, I. Synthesis of novel Cl2Co4L6 clusterusing 1-hydroxymethyl-3,5-dimethylpyrazole (LH) ligand: Crystal structure, spectral, thermal, Hirschfeld surface analysis and catalytic oxidation evaluation. J. Mol. Struct. 2020, 1199, 126995. [Google Scholar] [CrossRef]
- Titi, A.; Oshio, H.; Touzani, R.; Mouslim, M.; Zarrouk, A.; Hammouti, B.; Al-Zaqri, N.L.; Alsalme, A.; Warad, I. Synthesis and XRD of Novel Ni4(µ3-O)4 Twist Cubane Cluster Using Three NNO Mixed Ligands: Hirshfeld, Spectral, Thermal and Oxidation Properties. J. Clust. Sci. 2020, 26, 1. [Google Scholar] [CrossRef]
- Zienkiewicz, M.; Jabłonska-Wawrzycka, A.; Szlachetko, J.; Kayser, Y.; Stadnicka, K.; Sawka-Dobrowolska, W.; Jezierska, J.; Barszcz, B.; Sá, J. Effective catalytic disproportionation of aqueous H2O2 with di- and mono-nuclear manganese(ii) complexes containing pyridine alcohol ligands. Dalton Trans. 2014, 43, 8599. [Google Scholar] [CrossRef]
- Zienkiewicz, M.; Szlachetko, J.; Lothschütz, C.; Hodorowicz, M.; Wawrzycka, A.; Sá, J.; Barszcz, B. A novel single-site manganese(II) complex of a pyridine derivative as a catalase mimetic for disproportionation of H2O2 in water. Dalton Trans. 2013, 42, 7761. [Google Scholar] [CrossRef]
- El Kodadi, M.; Malek, F.; Touzani, R.; Ramdani, A. Synthesis of new tripodal ligand 5-(bis(3,5-dimethyl-1H-pyrazol-1-ylmethyl)amino)pentan-1-ol, catecholase activities studies of three functional tripodal pyrazolyl N-donor ligands, with different copper (II) salts. Catal. Commun. 2008, 9, 966. [Google Scholar] [CrossRef]
- Kim, E.; Woo, H.Y.; Kim, S.; Lee, H.; Kim, D.; Lee, H. Synthesis and X-ray crystal structure of derivatives from the N,N-bis(1H-pyrazolyl-1-methyl)aniline(dichloro)Zn(II) complex: Substituent effects on the phenyl ring versus the pyrazole ring. Polyhedron 2012, 42, 135. [Google Scholar] [CrossRef]
- Otero, A.; Fernández-Baeza, J.; Lara-Sánchez, A.; Sánchez-Barba, L.F. Metal complexes with heteroscorpionate ligands based on the bis(pyrazol-1-yl)methane moiety: Catalytic chemistry. Coord. Chem. Rev. 2013, 257, 1806. [Google Scholar] [CrossRef]
- Xue, F.; Zhao, J.; Hor, A.T.S. Cross-coupling of alkyl halides with aryl or alkyl Grignards catalyzed by dinuclear Ni(ii) complexes containing functionalized tripodal amine-pyrazolyl ligands. Dalton Trans. 2013, 42, 5150. [Google Scholar] [CrossRef]
- Han, X.-B.; Li, Y.-G.; Zhang, Z.-M.; Tan, H.-Q.; Lu, Y.; Wang, E.-B. Polyoxometalate-Based Nickel Clusters as Visible Light-Driven Water Oxidation Catalysts. J. Am. Chem. Soc. 2015, 137, 5486. [Google Scholar] [CrossRef]
- Asadi, Z.; Zarei, L.; Golchin, M.; Skorepova, E.; Eigner, V.; Amirghofran, Z. A novel Cu(II) distorted cubane complex containing Cu4O4 core as the first tetranuclear catalyst for temperature dependent oxidation of 3,5-di-tert-butyl catechol and in interaction with DNA & protein (BSA). Spectrochim. Acta A 2020, 227, 117593. [Google Scholar]
- Driessen, W.L. Synthesis of some new pyrazole-containing chelating agents. Recl. Trav. Chim. Pays-Bas 1982, 101, 441. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer2. 1. University of Western Australia. In Crystal Explorer 2.1; University of Western Australia: Perth, Australia, 2007. [Google Scholar]
- Sheldrick, G.M. A magic triangle for experimental phasing of macromolecules. Acta Cryst. 2008, 64, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, C.K.; Tam, T.L.D.; Wei, F.; Lub, X.; Wu, J. Anion–π and anion–π-radical interactions in bis(triphenylphosphonium)-naphthalene diimide salts. Org. Chem. Front. 2019, 6, 110. [Google Scholar] [CrossRef]
- Dorn, T.; Janiak, C.; Abu-Shandi, K. Hydrogen-bonding, pi-stacking and Cl-anioninteractions of linear bipyridinium cations with phosphate, chloride and [CoCl4] 2-anions. CrystEngComm 2005, 7, 633–641. [Google Scholar] [CrossRef]
- Warad, I.; Awwadi, F.F.; Al-Ghani, B.A.; Sawafta, A.; Shivalingegowda, N.; Lokanath, N.K.; Mubarak, M.S.; Hadda, T.B.; Zarrouk, A.; Al-Rimawi, F.; et al. Ultrasound-assisted synthesis of two novel [CuBr(diamine)2·H2O]Br complexes: Solvatochromism, crystal structure, physicochemical, Hirshfeld surface thermal, DNA/binding, antitumor and antibacterial activities. Ultrason. Sonochem. 2018, 48, 1. [Google Scholar] [CrossRef]
- Warad, I.; Al-Demeri, Y.; Al-Nuri, M.; Shahwan, S.; Abdoh, M.; Naveen, S.; Lokanath, N.K.; Mubarak, M.S.; Hadda, T.B.; Mabkhot, Y.N. Crystal structure, Hirshfeld surface, physicochemical, thermal and DFT studies of (N1E, N2E)-N1,N2-bis((5-bromothiophen-2-yl)methylene)ethane-1,2-diamine N2S2 ligand and its [CuBr(N2S2)]Br complex. J. Mol. Struct. 2017, 1142, 217. [Google Scholar] [CrossRef]
- Saleem, F.A.; Musameh, S.; Sawafta, A.; Brandao, P.; Tavares, C.J.; Ferdov, S.; Barakat, A.; Al Ali, A.; Al-Noaimi, M.; Warad, I. Diethylenetriamine/diamines/copper (II) complexes [Cu(dien)(NN)]Br2: Synthesis, solvatochromism, thermal, electrochemistry, single crystal, Hirshfeld surface analysis and antibacterial activity. Arab. J. Chem. 2017, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Warad, I.; Khan, A.A.; Al-Resayes, S.I.; Haddad, S.F. Design and structural studies of diimine/CdX2 (X = Cl, I) complexes based on 2,2-dimethyl-1,3-diaminopropane ligand. J. Mol. Struct. 2014, 1062, 167. [Google Scholar] [CrossRef]
- Aouad, M.R.; Messali, M.; Rezki, N.; Al-Zaqri, N.; Warad, I. Single proton intramigration in novel 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione: XRD-crystal interactions, physicochemical, thermal, Hirshfeld surface, DFT realization of thiol/thione tautomerism. J. Molec. Liq. 2018, 264, 621. [Google Scholar] [CrossRef]
- Warad, I.; Eftaiha, A.A.F.; Al-Nuri, M.A.; Husein, A.I.; Assal, M.; Abu-Obaid, A.; Al-Zaqri, N.; Hadda, T.B.; Hammouti, B. Metal ions as Antitumor Complexes-Review. J. Mater. Environ. Sci. 2013, 4, 542. [Google Scholar]
- Alkorta, I.; Elguero, J.; Grabowski, S.J. How To Determine Whether Intramolecular H···H Interactions Can Be Classified as Dihydrogen Bonds. J. Phys. Chem. A 2008, 112, 2721. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.; De Gala, S.; Crabtree, H.; Siegbahn, M. Unconventional Hydrogen Bonds: Intermolecular B-H···H-N Interactions. J. Am. Chem. Soc. 1995, 117, 12875. [Google Scholar] [CrossRef]
- Cramer, J.; Gladfelter, L. Ab Initio Characterization of [H3N·BH3]2, [H3N·AlH3]2, and [H3N·GaH3]2. Inorg. Chem. 1997, 36, 5358. [Google Scholar] [CrossRef]
- Warad, I.; Alkanad, K.; Suleiman, M.; Kumara, K.; Al-Ali, A.; Mohammed, Y.; Lokanath, N.K.; Zarrouk, A. Design, structural, C–H….H–C supramolecular interactions and computational investigations of Cd(N∩N″)X 2 complexes based on an asymmetrical 1,2-diamine ligand: Physicochemical and thermal analysis. J. Coord. Chem. 2019, 72, 12. [Google Scholar] [CrossRef]
- Thakurta, S.; Pilet, G.; Luneau, D.S. A novel tetra(μ3-phenoxo) bridged copper(II) Schiff base complex containing a Cu4O4 cubane core: Synthesis, structural aspects and magneto-structural correlations. Mitra Polyhedron 2009, 28, 819. [Google Scholar]
- Tauc, J.; Menth, A.; Optical processes in solids. Non-Cryst, J. Solids 1972, 8, 569. [Google Scholar]
- Aziz, B.; Abdullah, M. Crystalline and amorphous phase identification from the tanδ relaxation peaks and impedance plots in polymer blend electrolytes based on [CS:AgNt]x:PEO(x-1) (10 ≤ x ≤ 50). Electrochim. Acta 2018, 285, 30. [Google Scholar] [CrossRef]
- Kaddouri, Y.; Haddari, H.; Titi, A.; Yousfi, E.B.; Chetouani, A.; El Kodadi, M.; Touzani, R. Catecholase catalytic properties of copper (II) complexes prepared in-situ with heterocyclic ligands: Experimental and DFT study. Mor. J. Chem. 2020, 8, 184. [Google Scholar]
- Titi, A.; Al-Noaimi, M.; Kaddouri, Y.; El Ati, R.; Yousfi, E.B.; El Kodadi, M.; Touzani, R. Study of the catecholase catalytic properties of copper (II) complexes prepared in-situ with monodentate ligands. Mater. Today Proc. 2019, 13, 1134. [Google Scholar] [CrossRef]
- Modak, R.; Sikdar, Y.; Mandal, S.; Goswami, S. Synthesis, Crystal Structures, Magnetic Properties and Catecholase Activity of Double Phenoxido-Bridged Penta-Coordinated Dinuclear Nickel(II) Complexes Derived from Reduced Schiff-Base Ligands: Mechanistic Inference of Catecholase Activity. Inorg. Chem. Commun. 2013, 9, 26. [Google Scholar]
- Indira, S.; Vinoth, G.; Bharathi, M.; Bharathi, S.; Rahiman, A.K.; Bharathi, K.S. Catechol oxidase and phenoxazinone synthase mimicking activities of mononuclear Fe(III) and Co(III) complexes of amino-bis(phenolate)-based mixed ligands: Synthesis, spectral and electrochemical studies. Inorg. Chim. Acta 2019, 495, 118988. [Google Scholar] [CrossRef]
- Mandal, L.; Sasmal, S.; Sparkes, H.A.; Howard, J.A.K.; Mohanta, S. Crystal structure, catecholase activity and ESI-MS of a mixed valence cobalt(III)–cobalt(II) complex derived from a macrocyclic ligand: Identification/proposition of hydrogen bonded metal complex⋯solvent aggregates in ESI-MS. Inorg. Chim. Acta 2014, 412, 38. [Google Scholar] [CrossRef]











| No. | Bond Type | Bond Length Å | No. | Angle Type | Angle Value (°) | |||
|---|---|---|---|---|---|---|---|---|
| 1 | Cu2 | N1 | 1.972 (5) | 1 | N2 | Cu1 | N8 | 170.5 (2) |
| 2 | Cu1 | N2 | 1.951 (6) | 2 | N2 | Cu1 | O3 | 94.5 (2) |
| 3 | Cu1 | O3 | 2.030 (4) | 3 | N2 | Cu1 | O4 | 93.7 (2) |
| 4 | Cu1 | O4 | 2.041 (6) | 4 | N2 | Cu1 | O5 | 92.8 (2) |
| 5 | Cu1 | O5 | 2.662 (8) | 5 | N2 | Cu1 | Cl1 | 96.3 (2) |
| 6 | Cu1 | Cl1 | 2.712 (3) | 6 | N8 | Cu1 | O3 | 80.1 (2) |
| 7 | Cu2 | O1 | 2.008 (4) | 7 | N8 | Cu1 | O4 | 89.3 (2) |
| 8 | Cu2 | N6 | 2.010 (5) | 8 | N8 | Cu1 | O5 | 81.9 (2) |
| 2 | Cu1 | N8 | 1.995 (6) | 9 | N8 | Cu1 | Cl1 | 91.5 (2) |
| 10 | Cu2 | O2 | 1.927 (4) | 10 | O3 | Cu1 | O4 | 161.0 (2) |
| 11 | Cu2 | O3 | 2.279 (5) | 11 | O3 | Cu1 | O5 | 110.5 (2) |
| 12 | Cd1 | Cl1 | 2.542 (3) | 12 | O3 | Cu1 | Cl1 | 89.2 (1) |
| 13 | Cd1 | N4 | 2.301 (5) | 13 | O4 | Cu1 | O5 | 51.9 (2) |
| 14 | Cd1 | O1 | 2.472 (4) | 14 | O4 | Cu1 | Cl1 | 106.9 (2) |
| 15 | Cd1 | O2 | 2.235 (5) | 15 | O5 | Cu1 | Cl1 | 157.5 (2) |
| 16 | Cd1 | O1 | 2.299 (5) | 16 | N1 | Cu2 | N6 | 101.6 (2) |
| 17 | Cd1 | O3 | 2.362 (4) | 17 | N1 | Cu2 | O1 | 93.2 (2) |
| 18 | Cl1 | Cu1 | 2.712 (3) | 18 | N1 | Cu2 | O2 | 167.2 (2) |
| 19 | N1 | N2 | 1.395 (9) | 19 | N1 | Cu2 | O3 | 90.6 (2) |
| D-H | d(D-H) | d(H..A) | <DHA | d(D..A) | A |
|---|---|---|---|---|---|
| C6-H6A | 0.970 | 2.620 | 127.17 | 3.298 | O2 i |
| C18-H18A | 0.970 | 2.578 | 141.13 | 3.388 | O6 ii |
| Empirical Formula | C48 H71 Cd2 Cl2 Cu4 N19 O12 |
|---|---|
| Formula weight | 1656.09 |
| CCDC | 1956507 |
| Temperature | 298(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P 21/c |
| Unit cell dimensions | a = 10.860(9) Å |
| b = 20.665(19) Å | |
| c = 17.212(16) Å | |
| Β = 95.96(3)° | |
| Volume | 3842(6) Å3 |
| Z | 2 |
| Density (calculated) | 1.432 g/cm3 |
| Absorption coefficient | 1.759 mm−1 |
| F(000) | 1668 |
| Crystal size | 0.220 × 0.180 × 0.060 mm3 |
| Theta range for data collection | 2.576 to 29.850°. |
| Index ranges | −14 ≤ h ≤ 14, −27 ≤ k ≤ 27, −23 ≤ L ≤ 24 |
| Reflections collected | 107,448 |
| Independent reflections | 9719 [R(int) = 0.0711] |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 9719/2/401 |
| Goodness-of-fit on F2 | 1.111 |
| Final R indices [I>2sigma(I)] | R1 = 0.0719, wR2 = 0.2085 |
| R indices (all data) | R1 = 0.1029, wR2 = 0.2370 |
| Largest diff. peak and hole | 1.825 and −1.000 e.Å−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titi, A.; Messali, M.; Touzani, R.; Fettouhi, M.; Zarrouk, A.; Al-Zaqri, N.; Alsalme, A.; Alharthi, F.A.; Alsyahi, A.; Warad, I. Synthesis of Novel Tetra(µ3-Methoxo) Bridged with [Cu(II)-O-Cd(II)] Double-Open-Cubane Cluster: XRD/HSA-Interactions, Spectral and Oxidizing Properties. Int. J. Mol. Sci. 2020, 21, 8787. https://doi.org/10.3390/ijms21228787
Titi A, Messali M, Touzani R, Fettouhi M, Zarrouk A, Al-Zaqri N, Alsalme A, Alharthi FA, Alsyahi A, Warad I. Synthesis of Novel Tetra(µ3-Methoxo) Bridged with [Cu(II)-O-Cd(II)] Double-Open-Cubane Cluster: XRD/HSA-Interactions, Spectral and Oxidizing Properties. International Journal of Molecular Sciences. 2020; 21(22):8787. https://doi.org/10.3390/ijms21228787
Chicago/Turabian StyleTiti, Abderrahim, Mouslim Messali, Rachid Touzani, Mohammed Fettouhi, Abdelkader Zarrouk, Nabil Al-Zaqri, Ali Alsalme, Fahad A. Alharthi, Amjad Alsyahi, and Ismail Warad. 2020. "Synthesis of Novel Tetra(µ3-Methoxo) Bridged with [Cu(II)-O-Cd(II)] Double-Open-Cubane Cluster: XRD/HSA-Interactions, Spectral and Oxidizing Properties" International Journal of Molecular Sciences 21, no. 22: 8787. https://doi.org/10.3390/ijms21228787
APA StyleTiti, A., Messali, M., Touzani, R., Fettouhi, M., Zarrouk, A., Al-Zaqri, N., Alsalme, A., Alharthi, F. A., Alsyahi, A., & Warad, I. (2020). Synthesis of Novel Tetra(µ3-Methoxo) Bridged with [Cu(II)-O-Cd(II)] Double-Open-Cubane Cluster: XRD/HSA-Interactions, Spectral and Oxidizing Properties. International Journal of Molecular Sciences, 21(22), 8787. https://doi.org/10.3390/ijms21228787