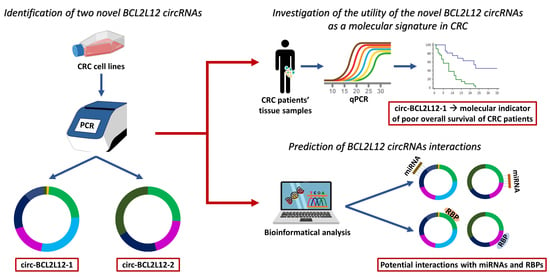
Identification of Two Novel Circular RNAs Deriving from BCL2L12 and Investigation of Their Potential Value as a Molecular Signature in Colorectal Cancer
Abstract
:1. Introduction
2. Results
2.1. Identification of Two Novel circRNAs Deriving from BCL2L12
2.2. Putative Interactions of BCL2L12 circRNAs with miRNAs and RBPs
2.3. Standardization of Real-Time qPCR Assays for BCL2L12 circRNA Quantification
2.4. Expression Analysis of BCL2L12 circRNAs in CRC Cell Lines
2.5. Expression of BCL2L12 circRNAs in Malignant Tumors and Non-Cancerous Tissues
2.6. Association of Circ-BCL2L12-2 Expression with TNM II and III Stages
2.7. Circ-BCL2L12-1 as a Potential Molecular Indicator of Poor Prognosis in CRC
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Tissue Sample Collection
4.3. RNA Extraction and Reverse Transcription
4.4. Primer Designing and PCR
4.5. Agarose Gel Electrophoresis and Sanger Sequencing
4.6. Bioinformatical Analysis for Prediction of circRNA Interactions with miRNAs and RBPs
4.7. Pre-Amplification and qPCR
4.8. Biostatistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| aa | Amino acid |
| BCa | Bias-corrected and accelerated |
| BH | BCL2 homology |
| CELF1 | CUGBP Elav-like family member 1 |
| CI | Confidence interval |
| circRNA | Circular RNA |
| ciRNA | Circular intronic RNA |
| CRC | Colorectal cancer |
| CT | Threshold cycle |
| DAPK1 | Death-associated protein kinase 1 |
| DFS | Disease-free survival |
| EAF1 | ELL-associated factor 1 |
| EcircRNA | Exonic circRNA |
| EIcircRNA | Exonic-intronic circRNA |
| FUS | FUS RNA-binding protein |
| HDAC2 | Histone deacetylase 2 |
| HNRNPH1 | Heterogeneous nuclear ribonucleoprotein H1 |
| HPRT1 | Hypoxanthine phosphoribosyltransferase 1 |
| HR | Hazard ratio |
| IGF2BP1 | Insulin-like growth factor 2 mRNA-binding protein 1 |
| IRES | Internal ribosomal entry sites |
| MBNL1 | Muscleblind-like splicing regulator 1 |
| miRNA | microRNA |
| MMR | Mismatch repair |
| nt | Nucleotide |
| ORF | Open reading frame |
| OS | Overall survival |
| PI3K | Phosphoinositide 3-kinase |
| PRDX3 | Peroxiredoxin 3 |
| PTBP1 | Polypyrimidine tract binding protein 1 |
| RNA-seq | RNA sequencing |
| RQU | Relative quantification unit |
| SAMD4A | Sterile alpha motif domain-containing 4A |
| SE | Standard error |
| SRSF1 | Serine and arginine-rich splicing factor 1 |
| SRSF2 | Serine and arginine-rich splicing factor 2 |
| SRSF3 | Serine and arginine-rich splicing factor 3 |
| SRSF5 | Serine and arginine-rich splicing factor 5 |
| TACSTD2 | Tumor-associated calcium signal transducer 2 |
| TBE | Tris/Borate/EDTA |
| TGF | Transforming growth factor-β |
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Artemaki, P.I.; Scorilas, A.; Kontos, C.K. Circular RNAs: A New Piece in the Colorectal Cancer Puzzle. Cancers 2020, 12, 2464. [Google Scholar] [CrossRef]
- De Rosa, M.; Pace, U.; Rega, D.; Costabile, V.; Duraturo, F.; Izzo, P.; Delrio, P. Genetics, diagnosis and management of colorectal cancer (Review). Oncol. Rep. 2015, 34, 1087–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooqi, A.A.; de la Roche, M.; Djamgoz, M.B.A.; Siddik, Z.H. Overview of the oncogenic signaling pathways in colorectal cancer: Mechanistic insights. Semin. Cancer Biol. 2019, 58, 65–79. [Google Scholar] [CrossRef]
- Kaczanowski, S. Apoptosis: Its origin, history, maintenance and the medical implications for cancer and aging. Phys. Biol. 2016, 13, 031001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, J.C. Apoptosis and cell death. Foreword. Oncogene 2008, 27, 6192–6193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Thomadaki, H.; Scorilas, A. BCL2 family of apoptosis-related genes: Functions and clinical implications in cancer. Crit. Rev. Clin. Lab. Sci. 2006, 43, 1–67. [Google Scholar] [CrossRef]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef]
- Scorilas, A.; Kyriakopoulou, L.; Yousef, G.M.; Ashworth, L.K.; Kwamie, A.; Diamandis, E.P. Molecular cloning, physical mapping, and expression analysis of a novel gene, BCL2L12, encoding a proline-rich protein with a highly conserved BH2 domain of the Bcl-2 family. Genomics 2001, 72, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Adamopoulos, P.G.; Kontos, C.K.; Tsiakanikas, P.; Scorilas, A. Identification of novel alternative splice variants of the BCL2L12 gene in human cancer cells using next-generation sequencing methodology. Cancer Lett. 2016, 373, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Kontos, C.K.; Scorilas, A. Molecular cloning of novel alternatively spliced variants of BCL2L12, a new member of the BCL2 gene family, and their expression analysis in cancer cells. Gene 2012, 505, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, A.; Saraste, M.; Wilmanns, M. High-resolution crystal structures of tyrosine kinase SH3 domains complexed with proline-rich peptides. Nat. Struct. Biol. 1994, 1, 546–551. [Google Scholar] [CrossRef]
- Nikcevic, G.; Drazilov, S.S.; Djurasevic, T.K.; Tosic, N.; Kontos, C.K.; Scorilas, A.; Pavlovic, S. Complex transcriptional regulation of the BCL2L12 gene: Novel, active promoter in K562 cells. Gene 2020, 750, 144723. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Stegh, A.H.; DePinho, R.A. Beyond effector caspase inhibition. Cell Cycle 2011, 10, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Avgeris, M.; Stamati, L.; Kontos, C.K.; Piatopoulou, D.; Marmarinos, A.; Xagorari, M.; Baka, M.; Doganis, D.; Anastasiou, T.; Kosmidis, H.; et al. BCL2L12 improves risk stratification and prediction of BFM-chemotherapy response in childhood acute lymphoblastic leukemia. Clin. Chem. Lab. Med. 2018, 56, 2104–2118. [Google Scholar] [CrossRef]
- Fendri, A.; Kontos, C.K.; Khabir, A.; Mokdad-Gargouri, R.; Scorilas, A. BCL2L12 is a novel biomarker for the prediction of short-term relapse in nasopharyngeal carcinoma. Mol. Med. (Camb. Mass.) 2011, 17, 163–171. [Google Scholar] [CrossRef]
- Giotakis, A.I.; Lazaris, A.C.; Kataki, A.; Kontos, C.K.; Giotakis, E.I. Positive BCL2L12 expression predicts favorable prognosis in patients with laryngeal squamous cell carcinoma. Cancer Biomark. Sect. A Dis Markers 2019, 25, 141–149. [Google Scholar] [CrossRef]
- Kontos, C.K.; Fendri, A.; Khabir, A.; Mokdad-Gargouri, R.; Scorilas, A. Quantitative expression analysis and prognostic significance of the BCL2-associated X gene in nasopharyngeal carcinoma: A retrospective cohort study. BMC Cancer 2013, 13, 293. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, S.G.; Kontos, C.K.; Foukas, P.G.; Panopoulou, E.; Vasilatou, D.; Rapti, S.M.; Gkontopoulos, K.; Bazani, E.; Panayiotides, I.G.; Dimitriadis, G.; et al. BCL2L12 protein overexpression is associated with favorable outcome in diffuse large B-cell lymphoma patients in the rituximab era. Leuk. Lymphoma 2016, 57, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.G.; Kontos, C.K.; Pappa, V.; Thomadaki, H.; Kontsioti, F.; Dervenoulas, J.; Papageorgiou, E.; Economopoulos, T.; Scorilas, A. The novel member of the BCL2 gene family, BCL2L12, is substantially elevated in chronic lymphocytic leukemia patients, supporting its value as a significant biomarker. Oncologist 2011, 16, 1280–1291. [Google Scholar] [CrossRef] [Green Version]
- Kontos, C.K.; Avgeris, M.; Vassilacopoulou, D.; Ardavanis, A.; Scorilas, A. Molecular Effects of Treatment of Human Colorectal Cancer Cells with Natural and Classical Chemotherapeutic Drugs: Alterations in the Expression of Apoptosis-related BCL2 Family Members, Including BCL2L12. Curr. Pharm. Biotechnol. 2018, 19, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Kontos, C.K.; Papadopoulos, I.N.; Scorilas, A. Quantitative expression analysis and prognostic significance of the novel apoptosis-related gene BCL2L12 in colon cancer. Biol. Chem. 2008, 389, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Cocquerelle, C.; Mascrez, B.; Hetuin, D.; Bailleul, B. Mis-splicing yields circular RNA molecules. FASEB J. 1993, 7, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.B.; Jaffrey, S.R. 5’ UTR m(6)A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef] [Green Version]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef]
- Du, W.W.; Zhang, C.; Yang, W.; Yong, T.; Awan, F.M.; Yang, B.B. Identifying and Characterizing circRNA-Protein Interaction. Theranostics 2017, 7, 4183–4191. [Google Scholar] [CrossRef]
- Guo, J.U.; Agarwal, V.; Guo, H.; Bartel, D.P. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014, 15, 409. [Google Scholar] [CrossRef]
- Papatsirou, M.; Artemaki, P.I.; Scorilas, A.; Kontos, C.K. The role of circular RNAs in therapy resistance of patients with solid tumors. Per. Med. 2020, 17, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Yan, M.; Li, H. Emerging Role of Circular RNAs in Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.; Su, Y.; Zhang, X. Screening potential biomarkers for colorectal cancer based on circular RNA chips. Oncol. Rep. 2018, 39, 2499–2512. [Google Scholar] [CrossRef] [PubMed]
- Glažar, P.; Papavasileiou, P.; Rajewsky, N. circBase: A database for circular RNAs. RNA 2014, 20, 1666–1670. [Google Scholar] [CrossRef] [Green Version]
- Ragan, C.; Goodall, G.J.; Shirokikh, N.E.; Preiss, T. Insights into the biogenesis and potential functions of exonic circular RNA. Sci. Rep. 2019, 9, 2048. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.R.; Blazquez, L.; Ule, J. Lessons from non-canonical splicing. Nat. Rev. Genet. 2016, 17, 407–421. [Google Scholar] [CrossRef]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 2014, 56, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Barbagallo, D.; Caponnetto, A.; Cirnigliaro, M.; Brex, D.; Barbagallo, C.; D’Angeli, F.; Morrone, A.; Caltabiano, R.; Barbagallo, G.M.; Ragusa, M.; et al. CircSMARCA5 Inhibits Migration of Glioblastoma Multiforme Cells by Regulating a Molecular Axis Involving Splicing Factors SRSF1/SRSF3/PTB. Int. J. Mol. Sci. 2018, 19, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Han, F.; Liu, W.; Shi, X. PTBP1 promotes tumorigenesis by regulating apoptosis and cell cycle in colon cancer. Bull. Du Cancer 2018, 105, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, R.; Guo, W.; Qin, X.; Yang, Z.; Yuan, Z.; Wei, Y.; Mo, C.; Zeng, Z.; Luo, J.; et al. RNA-binding protein CELF1 enhances cell migration, invasion, and chemoresistance by targeting ETS2 in colorectal cancer. Clin. Sci. 2020, 134, 1973–1990. [Google Scholar] [CrossRef]
- Song, I.S.; Jeong, Y.J.; Jeong, S.H.; Heo, H.J.; Kim, H.K.; Bae, K.B.; Park, Y.H.; Kim, S.U.; Kim, J.M.; Kim, N.; et al. FOXM1-Induced PRX3 Regulates Stemness and Survival of Colon Cancer Cells via Maintenance of Mitochondrial Function. Gastroenterology 2015, 149, 1006–1016.e9. [Google Scholar] [CrossRef] [PubMed]
- Stypula-Cyrus, Y.; Damania, D.; Kunte, D.P.; Cruz, M.D.; Subramanian, H.; Roy, H.K.; Backman, V. HDAC up-regulation in early colon field carcinogenesis is involved in cell tumorigenicity through regulation of chromatin structure. PLoS ONE 2013, 8, e64600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer 2015, 6, 84–105. [Google Scholar] [CrossRef] [Green Version]
- Steinmann, S.; Kunze, P.; Hampel, C.; Eckstein, M.; Bertram Bramsen, J.; Muenzner, J.K.; Carlé, B.; Ndreshkjana, B.; Kemenes, S.; Gasparini, P.; et al. DAPK1 loss triggers tumor invasion in colorectal tumor cells. Cell Death Dis. 2019, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Zhang, D.; Xie, X.; Ouyang, G.; Liu, X.; Sun, Y.; Xiao, W. Eaf1 and Eaf2 negatively regulate canonical Wnt/β-catenin signaling. Development 2013, 140, 1067–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Zhang, H.; Guo, X.; Zhu, Z.; Cai, H.; Kong, X. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) in cancer. J. Hematol. Oncol. 2018, 11, 88. [Google Scholar] [CrossRef] [Green Version]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Purcell, R.V.; Schmeier, S.; Lau, Y.C.; Pearson, J.F.; Frizelle, F.A. Molecular subtyping improves prognostication of Stage 2 colorectal cancer. BMC Cancer 2019, 19, 1155. [Google Scholar] [CrossRef] [Green Version]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-omics of 34 colorectal cancer cell lines—A resource for biomedical studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef]
- Panda, A.C.; Gorospe, M. Detection and Analysis of Circular RNAs by RT-PCR. Bio-Protocol 2018, 8, e2775. [Google Scholar] [CrossRef] [Green Version]
- Papatsirou, M.; Adamopoulos, P.G.; Artemaki, P.I.; Georganti, V.P.; Scorilas, A.; Vassilacopoulou, D.; Kontos, C.K. Next-generation sequencing reveals alternative L-DOPA decarboxylase (DDC) splice variants bearing novel exons, in human hepatocellular and lung cancer cells. Gene 2020, 145262. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2019, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G.; et al. DIANA-TarBase v8: A decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz, I.; Kosti, I.; Ares, M., Jr.; Cline, M.; Mandel-Gutfreund, Y. RBPmap: A web server for mapping binding sites of RNA-binding proteins. Nucleic Acids Res. 2014, 42, W361–W367. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Sheng, Q.; Liu, Q.; Shyr, Y. beRBP: Binding estimation for human RNA-binding proteins. Nucleic Acids Res. 2019, 47, e26. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gribskov, M. IRESpy: An XGBoost model for prediction of internal ribosome entry sites. BMC Bioinform. 2019, 20, 409. [Google Scholar] [CrossRef]
- Kalioraki, M.A.; Artemaki, P.I.; Sklirou, A.D.; Kontos, C.K.; Adamopoulos, P.G.; Papadopoulos, I.N.; Trougakos, I.P.; Scorilas, A. Heat shock protein beta 3 (HSPB3) is an unfavorable molecular biomarker in colorectal adenocarcinoma. Mol. Carcinog. 2020, 59, 116–125. [Google Scholar] [CrossRef]
- Artemaki, P.I.; Sklirou, A.D.; Kontos, C.K.; Liosi, A.A.; Gianniou, D.D.; Papadopoulos, I.N.; Trougakos, I.P.; Scorilas, A. High clusterin (CLU) mRNA expression levels in tumors of colorectal cancer patients predict a poor prognostic outcome. Clin. Biochem. 2020, 75, 62–69. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]




| BCL2L12 circRNA | miRNAs Binding to circRNA | Prediction Score 1 | Binding Motifs |
|---|---|---|---|
| circ-BCL2L12-1 | miR-1915-5p | 86 | GGCAAGGA |
| miR-6721-5p | 62 | CCTGCCCA | |
| miR-6822-3p | 59 | CCCTGCTA/CCCTGCT | |
| miR-6510-5p | 57 | GAAGCCA/AGAAGCC | |
| miR-1237-3p | 54 | AGAAGGA/CAGAAGG | |
| miR-6815-3p | 52 | AGAGCCA/TAGAGCC | |
| miR-6849-3p | 50 | GGCTGGA/AGGCTGG | |
| circ-BCL2L12-2 | miR-4767 | 59 | CGCCCG |
| miR-4763-5p | 51 | GGCAGG | |
| miR-6729-3p | 51 | CTCGCCCA | |
| miR-4649-5p | 51 | CTCGCCCA | |
| miR-6849-3p | 50 | GGCTGGA/AGGCTGG |
| RBP | circ-BCL2L12-1 | circ-BCL2L12-2 | ||||
|---|---|---|---|---|---|---|
| Number of Binding Sites | Z-Score 2 | p-Value 3 | Number of Binding Sites | Z-Score 2 | p-Value 3 | |
| CUGBP Elav-like family member 1 (CELF1) | 24 | 2.54 | 0.006 | 22 | 3.70 | 0.001 |
| FUS RNA-binding protein (FUS) | 0 | – | – | 5 | 3.60 | <0.001 |
| Muscleblind-like splicing regulator 1 (MBNL1) | 31 | 2.40 | 0.008 | 25 | 2.40 | 0.008 |
| Sterile alpha motif domain-containing 4A (SAMD4A) | 6 | 3.56 | <0.001 | 6 | 3.56 | <0.001 |
| Serine and arginine-rich splicing factor 1 (SRSF1) | 21 | 3.69 | <0.001 | 22 | 3.81 | <0.001 |
| Serine and arginine-rich splicing factor 2 (SRSF2) | 27 | 3.31 | <0.001 | 21 | 3.31 | <0.001 |
| Serine and arginine-rich splicing factor 3 (SRSF3) | 54 | 2.92 | 0.001 | 55 | 3.29 | <0.001 |
| Serine and arginine-rich splicing factor 5 (SRSF5) | 9 | 2.61 | 0.004 | 6 | 2.22 | 0.013 |
| Polypyrimidine tract binding protein 1 (PTBP1) | 20 | 3.23 | <0.001 | 11 | 2.58 | 0.005 |
| Heterogeneous nuclear ribonucleoprotein H1 (HNRNPH1) | 6 | 3.70 | 0.003 | 6 | 2.74 | 0.003 |
| Variable. | Mean ± SE 1 | Range | Percentiles | ||
|---|---|---|---|---|---|
| 25th | 50th (Median) | 75th | |||
| circ-BCL2L12-1 expression (RQU 2) | |||||
| in malignant tumors (n = 168) | 6.03 ± 2.05 | 0.001–246.8 | 0.096 | 0.52 | 2.25 |
| in non-cancerous tissues (n = 63) | 8.46 ± 3.39 | 0.001–167.3 | 0.10 | 0.48 | 2.93 |
| circ-BCL2L12-2 expression (RQU 2) | |||||
| in malignant tumors (n = 60) | 2.16 ± 0.76 | 0.001–40.82 | 0.035 | 0.52 | 1.51 |
| in non-cancerous tissues (n = 27) | 1.30 ± 0.44 | 0.004–9.49 | 0.034 | 0.15 | 1.16 |
| Covariate | Univariate Analysis (n = 168) | Multivariate Analysis 1 (n = 168) | ||||
|---|---|---|---|---|---|---|
| HR 2 | BCa 95% Bootstrap CI 3 | Bootstrap p-Value 4 | HR 2 | BCa 95% Bootstrap CI 3 | Bootstrap p-Value 4 | |
| circ-BCL2L12-1 expression status | ||||||
| Low (n = 88) | 1.00 | 1.00 | ||||
| High (n = 80) | 1.92 | 1.01–3.76 | 0.035 | 1.74 | 0.80–3.90 | 0.14 |
| circ-BCL2L12-2 expression status | ||||||
| Low (n = 108) | 1.00 | |||||
| High (n = 60) | 0.81 | 0.38–1.55 | 0.56 | |||
| Tumor site | ||||||
| Colon (n = 111) | 1.00 | 1.00 | ||||
| Rectum (n = 57) | 1.99 | 0.98–4.05 | 0.028 | 1.63 | 0.71–3.53 | 0.16 |
| Tumor sidedness | ||||||
| Left (n = 115) | 1.00 | |||||
| Right (n = 53) | 0.79 | 0.36–1.46 | 0.49 | |||
| Histological grade | ||||||
| I (n = 15) | 1.00 | 1.00 | ||||
| II (n = 129) | 0.61 | 0.17–3.67 | 0.29 | 0.44 | 0.12–1.61 | 0.11 |
| III (n = 24) | 1.60 | 0.39–12.96 | 0.40 | 1.03 | 0.22–5.66 | 0.97 |
| Venous invasion | ||||||
| Absent (n = 118) | 1.00 | |||||
| Present (n = 19) | 1.58 | 0.43–3.80 | 0.35 | |||
| Lymphatic invasion | ||||||
| Absent (n = 121) | 1.00 | |||||
| Present (n = 16) | 1.61 | 0.65–3.20 | 0.25 | |||
| TNM stage | ||||||
| I (n = 20) | 1.00 | 1.00 | ||||
| II (n = 72) | 1.33 | 0.34–4.0 × 104 | 0.62 | 1.69 | 0.39–4.7 × 104 | 0.37 |
| III (n = 59) | 2.02 | 0.53–5.1 × 104 | 0.25 | 2.79 | 0.70–7.2 × 104 | 0.086 |
| IV (n = 17) | 12.58 | 2.89–4.0 × 105 | <0.001 | 14.58 | 3.05–6.1 × 105 | <0.001 |
| Variables | Number of Patients (%) |
|---|---|
| Gender | |
| Male | 89 (53.0%) |
| Female | 79 (47.0%) |
| Tumor site | |
| Colon | 111 (66.1%) |
| Rectum | 57 (33.9%) |
| Tumor sidedness | |
| Left | 115 (68.5%) |
| Right | 53 (31.5%) |
| Histological grade | |
| I | 15 (8.9%) |
| II | 129 (76.8%) |
| III | 24 (14.3%) |
| Venous invasion (137 of 168 patients) | |
| Absent | 118 (86.1%) |
| Present | 19 (13.9%) |
| Lymphatic invasion (137 of 168 patients) | |
| Absent | 121 (88.3%) |
| Present | 16 (11.7%) |
| T (tumor invasion) | |
| T1 | 4 (2.4%) |
| T2 | 20 (11.9%) |
| T3 | 107 (63.7%) |
| T4 | 37 (22.0%) |
| N (nodal status) | |
| N0 | 95 (56.5%) |
| N1 | 42 (25.0%) |
| N2 | 31 (18.5%) |
| TNM stage | |
| I | 20 (11.9%) |
| II | 72 (42.9%) |
| III | 59 (35.1%) |
| IV | 17 (10.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karousi, P.; Artemaki, P.I.; Sotiropoulou, C.D.; Christodoulou, S.; Scorilas, A.; Kontos, C.K. Identification of Two Novel Circular RNAs Deriving from BCL2L12 and Investigation of Their Potential Value as a Molecular Signature in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 8867. https://doi.org/10.3390/ijms21228867
Karousi P, Artemaki PI, Sotiropoulou CD, Christodoulou S, Scorilas A, Kontos CK. Identification of Two Novel Circular RNAs Deriving from BCL2L12 and Investigation of Their Potential Value as a Molecular Signature in Colorectal Cancer. International Journal of Molecular Sciences. 2020; 21(22):8867. https://doi.org/10.3390/ijms21228867
Chicago/Turabian StyleKarousi, Paraskevi, Pinelopi I. Artemaki, Christina D. Sotiropoulou, Spyridon Christodoulou, Andreas Scorilas, and Christos K. Kontos. 2020. "Identification of Two Novel Circular RNAs Deriving from BCL2L12 and Investigation of Their Potential Value as a Molecular Signature in Colorectal Cancer" International Journal of Molecular Sciences 21, no. 22: 8867. https://doi.org/10.3390/ijms21228867
APA StyleKarousi, P., Artemaki, P. I., Sotiropoulou, C. D., Christodoulou, S., Scorilas, A., & Kontos, C. K. (2020). Identification of Two Novel Circular RNAs Deriving from BCL2L12 and Investigation of Their Potential Value as a Molecular Signature in Colorectal Cancer. International Journal of Molecular Sciences, 21(22), 8867. https://doi.org/10.3390/ijms21228867