Novel ACE Inhibitory Peptides Derived from Simulated Gastrointestinal Digestion in Vitro of Sesame (Sesamum indicum L.) Protein and Molecular Docking Study
Abstract
:1. Introduction
2. Results
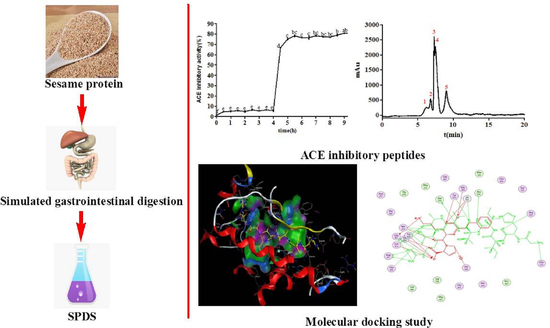
2.1. Changes of ACE Inhibitory Activity during Simulated Gastrointestinal Digestion
2.2. Separation of ACE Inhibitory Peptide from SPDS by Ultrafiltration
2.3. Purification of ACE Inhibitory Peptide by NGC Quest™ 10 Plus Chromatography System
2.4. Characterization of ACE Inhibitory Peptide by Nano UHPLC-ESI-MS/MS from Peak 4
2.5. Molecular Docking Simulation between Peptide and ACE
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction of Sesame Protein
4.3. Preparation of in Vitro Simulated Gastrointestinal Digestion of Sesame Protein
4.4. Separation of ACE Inhibitory Peptide from SPDS by Ultrafiltration
4.5. Purification of ACE Inhibitory Peptide by NGC Quest™ 10 Plus Chromatography System
4.6. Identification of the Purified ACE Inhibitory Peptide by Nano UHPLC-ESI-MS/MS
4.7. Peptide Match and Peptide Synthesis
4.8. Analysis of Degree of Hydrolysis
4.9. Determination of Protein and Peptide Content
4.10. Assay of the ACE Inhibitory Activity
4.11. Molecular Docking Simulation between Peptide and ACE
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin I-converting enzyme |
| Nano UHPLC-ESI-MS/MS | Nano ultra-high performance liquid chromatography-electrospray ionization mass spectrometry/mass spectrometry |
| SPD | Sesame protein digestive solution |
| DH | Degree of hydrolysis |
| MWCO | Molecular weight cut-off |
| RAS | Renin-angiotensin system |
| KKS | Kallikrein kinin system |
| MOE | Molecular Operating Environments |
References
- Vokonas, P.S.; Kannel, W.B.; Cupples, L.A. Epidemiology and risk of hypertension in the elderly: The Framingham-Study. J. Hypertens Suppl. 1988, 6, S3–S9. [Google Scholar]
- Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: A comparative risk assessment. Lancet Diabetes Endocrinol. 2014, 2, 634–647. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [PubMed]
- Asoodeh, A.; Haghighi, L.; Chamani, J.; Ansari-Ogholbeyk, M.A.; Mojallal-Tabatabaei, Z.; Lagzian, M. Potential angiotensin I converting enzyme inhibitory peptides from gluten hydrolysate: Biochemical characterization and molecular docking study. J. Cereal Sci. 2014, 60, 92–98. [Google Scholar] [CrossRef]
- López-Barrios, L.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bioactive peptides and hydrolysates from pulses and their potential use as functional ingredients. J. Food Sci. 2014, 79, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.W.; Nakayama, S.; Hsu, M.N.; Samaranayaka, A.G.; Li-Chan, E.C. Angiotensin-I converting enzyme inhibitory activity of hydrolysates from oat (Avena Sativa) proteins by in silico and in vitro analyses. J Agric Food Chem. 2009, 57, 9234–9242. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wu, J. LC-MS/MS coupled with QSAR modeling in characterising of angiotensin I-converting enzyme inhibitory peptides from soybean proteins. Food Chem. 2013, 141, 2682–2690. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Moller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and protein from foods: Indication for health effects. Eur J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Badii, F.; Howell, N.K. Purification and characterization of antioxidative peptides derived from chicken skin gelatin hydrolysate. Food Hydrocoll. 2018, 85, 311–320. [Google Scholar] [CrossRef]
- Sun, Y.; Chang, R.; Li, Q.; Li, B. Isolation and characterization of an antibacterial peptide from protein hydrolysates of Spirulina platensis. Eur Food Res Technol. 2016, 242, 685–692. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by proline containing casein-derived peptides. J. Funct Foods. 2013, 5, 1909–1917. [Google Scholar]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Isolation and characterization of angiotensin I-converting enzyme (ACE) inhibitory peptides from Ulva rigida C. Agardh protein hydrolysate. J. Funct Foods. 2016, 26, 65–76. [Google Scholar] [CrossRef]
- Rani, S.; Pooja, K.; Pal, G.K. Exploration of potential angiotensin converting enzyme inhibitory peptides generated from enzymatic hydrolysis of goat milk proteins. Biocatal. Agric. Biotechnol. 2017, 11, 83–88. [Google Scholar] [CrossRef]
- Tanzadehpanah, H.; Asoodeh, A.; Saberi, M.R.; Chamani, J. Identification of a novel angiotensin-I converting enzyme inhibitory peptide from ostrich egg white and studying its interactions with the enzyme. Innov. Food Sci. Emerg. Technol. 2013, 18, 212–219. [Google Scholar] [CrossRef]
- He, H.L.; Liu, D.; Ma, C.B. Review on the angiotensin-I-converting enzyme (ACE) inhibitor peptides from marine proteins. Appl. Biochem. Biotechnol. 2013, 169, 738–749. [Google Scholar] [CrossRef]
- Anilakumar, K.R.; Pal, A.; Khanum, F.; Bawa, A.S. Nutritional, medicinal and industrial uses of sesame (Sesamum indicum L.) seeds—An overview. Agric. Conspec. Sci. 2010, 75, 159–168. [Google Scholar]
- Al-Bachir, M. Some microbial, chemical and sensorial properties of gamma irradiated sesame (Sesamum indicum L.) seeds. Food Chem. 2016, 197, 191–197. [Google Scholar] [CrossRef]
- Nakano, D.; Ogura, K.; Miyakoshi, M.; Ishii, F.; Kawanishi, H.; Kurumazuka, D.; Kwak, C.J.; Ikemura, K.; Takaoka, M.; Moriguchi, S.; et al. Antihypertensive effect of angiotensin I-converting enzyme inhibitory peptides from a sesame protein hydrolysate in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006, 70, 1118–1126. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.P.; Cheng, G.Y.; Liu, H.M.; Kong, Y. Purification and identification of a novel angiotensin I-converting enzyme inhibitory peptide from sesame meal. Int. J. Pept. Res. Ther. 2015, 21, 433–442. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, D.; Zhang, D.; Chen, G.; Liu, Y. Analysis of protein profiles and peptides during in vitro gastrointestinal digestion of four Chinese dry-cured hams. LWT-Food Sci. Technol. 2020, 120, 108881. [Google Scholar] [CrossRef]
- Fan, J.; He, J.T.; Zhuang, Y.L.; Sun, L.P. Purification and identification of antioxidant peptides from enzymatic hydrolysates of Tilapia (Oreochromis niloticus) frame protein. Molecules 2012, 17, 12836–12850. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M. Production of antioxidant and ACE-inhibitory peptides from Kluyveromyces marxianus protein hydrolysates: Purification and molecular docking. J. Food Drug Anal. 2018, 26, 696–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Yuan, F.; Xu, Z.; Wang, W.; Di, X.; Barba, F.J.; Shen, W.; Koubaa, M. Stirring-assisted dead-end ultrafiltration for protein and polyphenol recovery from purple sweet potato juices: Filtration behavior investigation and HPLC-DAD-ESI-MS2 profiling. Sep. Purif. Technol. 2016, 169, 25–32. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-inhibitory activity of enzymatic protein hydrolysates from Lupin and other Legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef]
- Fu, W.; Chen, C.; Zeng, H.; Lin, J.; Zhang, Y.; Hu, J.; Zheng, B. Novel angiotensin-converting enzyme inhibitory peptides derived from Trichiurus lepturus myosin: Molecular docking and surface plasmon resonance study. LWT-Food Sci. Technol. 2019, 110, 54–63. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, L.; Han, X.; Cheng, D. Novel angiotensin I-converting enzyme inhibitory peptides from protease hydrolysates of Qula casein: Quantitative structure-activity relationship modeling and molecular docking study. J. Funct. Foods. 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Achouri, A.; Nail, V.; Boye, J.I. Sesame protein isolate: Fractionation; secondary structure and functional properties. Food Res. Int. 2012, 46, 360–369. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Sun, Q.; Song, G. Extraction; identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2019, 116, 707–716. [Google Scholar] [CrossRef]
- Salampessy, J.; Reddy, N.; Phillips, M.; Kailasapathy, K. Isolation and characterization of nutraceutically potential ACE-Inhibitory peptides from leatherjacket (Meuchenia sp.) protein hydrolysates. LWT-Food Sci. Technol. 2017, 80, 430–436. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Jeon, Y.-J.; Kim, Y.-T.; Je, J.-Y. Angiotensin I converting enzyme (ACE) inhibitory peptides from salmon byproduct protein hydrolysate by alcalase hydrolysis. Process Biochem. 2012, 47, 2240–2245. [Google Scholar] [CrossRef]
- Toopcham, T.; Mes, J.J.; Wichers, H.J.; Roytrakul, S.; Yongsawatdigul, J. Bioavailability of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from Virgibacillus halodenitrificans SK1–3-7 proteinases hydrolyzed tilapia muscle proteins. Food Chem. 2017, 220, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Jiang, X.; Xiong, B.; Zhang, T.; Zeng, X.; Wu, Z.; Sun, Y.; Pan, D. Production and transepithelial transportation of angiotensin-I-converting enzyme (ACE)-inhibitory peptides from whey protein hydrolyzed by immobilized Lactobacillus helveticus proteinase. J. Dairy Sci. 2019, 102, 961–975. [Google Scholar] [CrossRef] [Green Version]
- Forghani, B.; Zarei, M.; Ebrahimpour, A.; Philip, R.; Bakar, J.; Hamid, AA.; Saari, N. Purification and characterization of angiotensin converting enzyme-inhibitory peptides derived from Stichopus horrens: Stability study against the ACE and inhibition kinetics. J. Funct. Foods 2016, 20, 276–290. [Google Scholar] [CrossRef]
- Ko, S.-C.; Jang, J.; Ye, B.-R.; Kim, M.-S.; Choi, I.-W.; Park, W.-S.; Heo, S.-J.; Jung, W.-K. Purification and molecular docking study of angiotensin I-converting enzyme (ACE) inhibitory peptides from hydrolysates of marine sponge Stylotella aurantium. Process Biochem. 2017, 54, 180–187. [Google Scholar] [CrossRef]
- Salampessy, J.; Reddy, N.; Kailasapathy, K.; Phillips, M. Functional and potential therapeutic ACE-inhibitory peptides derived from bromelain hydrolysis of trevally proteins. J. Funct. Foods. 2015, 14, 716–725. [Google Scholar] [CrossRef]
- Xie, C.L.; Choung, S.-Y.; Cao, G.-P.; Lee, K.W.; Choi, Y.J. In silico investigation of action mechanism of four novel angiotensin-I converting enzyme inhibitory peptides modified with Trp. J. Funct. Foods 2015, 17, 632–639. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT-Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Safavi, M.; Hadizadeh, M. In vitro and in silico studies of novel synthetic ACE-inhibitory peptides derived from Saccharomyces cerevisiae protein hydrolysate. Bioorg. Chem. 2019, 87, 647–654. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, R.; Jridi, M.; Mora, L.; Oseguera-Toledo, M.E.; Aristoy, M.-C.; Amara, I.B.; Toldrá, F.; Nasri, M. In silico analysis and antihypertensive effect of ACE-inhibitory peptides from smooth-hound viscera protein hydrolysate: Enzyme-peptide interaction study using molecular docking simulation. Process Biochem. 2017, 58, 145–159. [Google Scholar] [CrossRef]
- Wang, C.; Tu, M.; Wu, D.; Chen, H.; Chen, C.; Wang, Z.; Jiang, L. Identification of an ACE-Inhibitory peptide from walnut protein and its evaluation of the inhibitory mechanism. Int. J. Mol. Sci. 2018, 19, 1156. [Google Scholar] [CrossRef] [Green Version]
- Priyanto, A.D.; Doerksen, R.J.; Chang, C.I.; Sung, W.C.; Widjanarko, S.B.; Kusnadi, J.; Lin, Y.C.; Wang, T.C.; Hsu, J.L. Screening, discovery, and characterization of angiotensin-I converting enzyme inhibitory peptides derived from proteolytic hydrolysate of bitter melon seed proteins. J. Proteomics. 2015, 128, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Gomez, R.; Martinez-Villaluenga, C. High-pressure improves enzymatic proteolysis and the release of peptides with angiotensin I converting enzyme inhibitory and antioxidant activities from lentil proteins. Food Chem. 2015, 171, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Wang, J.; Liao, W.; Jiang, X.; Wu, J. Identification and characterization of gastrointestinal-resistant angiotensin-converting enzyme inhibitory peptides from egg white proteins. J. Agric. Food Chem. 2019, 67, 7147–7156. [Google Scholar] [CrossRef]
- García-Mora, P.; Martín-Martínez, M.; Bonache, M.A.; González-Múniz, R.; Peñas, E.; Frias, J.; Martinez-Villaluenga, C. Identification, functional gastrointestinal stability and molecular docking studies of lentil peptides with dual antioxidant and Angiotensin I converting enzyme inhibitory activities. Food chem. 2017, 221, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Saatchi, A.; Kiani, H.; Labbafi, M. A new functional protein-polysaccharide conjugate based on protein concentrate from sesame processing by-products: Functional and physico-chemical properties. Int. J. Biol Macromol. 2019, 122, 659–666. [Google Scholar] [CrossRef]
- Sangsawad, P.; Roytrakul, S.; Yongsawatdigul, J. Angiotensin converting enzyme (ACE) inhibitory peptides derived from the simulated in vitro gastrointestinal digestion of cooked chicken breast. J. Funct. Foods 2017, 29, 77–83. [Google Scholar] [CrossRef]
- Chen, C.; Li, Z.; Huang, H.; Suzek, B.E.; Wu, C.H.; UniProt Consortium. A fast Peptide Match service for UniProt Knowledgebase. Bioinformatics 2013, 29, 2808–2809. [Google Scholar] [CrossRef] [Green Version]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzene sulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Chatterjee, R.; Dey, T.K.; Ghosh, M.; Dhar, P. Enzymatic modification of sesame seed protein, sourced from waste resource for nutraceutical application. Food Bioprod. Process. 2015, 94, 70–81. [Google Scholar] [CrossRef]
- Marcó, A.; Rubio, R.; Compañó, R.; Casals, I. Comparison of the Kjeldahl method and a combustion method for total nitrogen determination in animal feed. Talanta 2002, 57, 1019–1026. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation; purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016, 204, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yin, L.J.; Lu, X.; Li, L.T. Changes in angiotensin I-converting enzyme inhibitory activities during the ripening of douchi (a Chinese traditional soybean product) fermented by various starter cultures. Int. J. Food Prop. 2010, 13, 512–524. [Google Scholar] [CrossRef]
- Jain, A.N. Surflex: Fully automatic flexible molecular docking using a molecular similarity-based search engine. J. Med. Chem. 2003, 46, 499–511. [Google Scholar] [CrossRef]
- Jain, A.N. Scoring noncovalent protein-ligand interactions: A continuous differentiable function tuned to compute binding affinities. J. Comput. Aided Mol. Design 1996, 10, 427–440. [Google Scholar] [CrossRef]
- Vaijanathappa, J.; Puttaswamygowda, J.; Bevanhalli, R.; Dixit, S.; Prabhakaran, P. Molecular docking, antiproliferative and anticonvulsant activities of swertiamarin isolated from Enicostemma axillare. Bioorg. Chem. 2020, 94, 103428. [Google Scholar] [CrossRef]





| Fraction | IC50 Values (μg/mL) |
|---|---|
| SPDS-I (>100 kDa) | 35.143 ± 1.122 a |
| SPDS-II (50–100 kDa) | 15.066 ± 0.042 b |
| SPDS-III (30–50 kDa) | 9.146 ± 0.005 c |
| SPDS-IV (10–30 kDa) | 6.108 ± 0.001 cd |
| SPDS-V (5–10 kDa) | 5.106 ± 0.003 ce |
| SPDS-VI (3–5 kDa) | 4.583 ± 0.003 cf |
| SPDS-VII (<3 kDa) | 2.720 ± 0.003 cg |
| Fraction | Retention Time (min) | IC50 Values (μg/mL) |
|---|---|---|
| peak 1 | 5.11–6.52 | 2.847 ± 0.045 a |
| peak 2 | 6.52–7.12 | 1.421 ± 0.035 c |
| peak 3 | 7.12–7.42 | 1.838 ± 0.026 b |
| peak 4 | 7.49–8.27 | 0.558 ± 0.003 e |
| peak 5 | 8.27–10.80 | 0.757 ± 0.014 d |
| Peptide | Protein Name | Molecular Weight | IC50 Value (μM) |
|---|---|---|---|
| GHIITVAR | 11S globulin isoform 4 (Q2XSW6_SESIN) | 866.0 | 3.60 ± 0.10 k |
| IGGIGTVPVGR | Elongation factor 1-alpha-like | 1025.2 | 6.97 ± 0.18 j |
| HIGNILSL | TBCC domain-containing protein 1 | 866.0 | 36.69 ± 0.33 f |
| FMPGVPGPIQR | Oil body-associated protein 1A | 1198.4 | 11.08 ± 0.15 i |
| PNYHPSPR | 11S globulin seed storage protein 2 precursor (Q9XHP0_SESIN) | 967.0 | 18.98 ± 0.26 h |
| AFPAGAAHW | 11S globulin isoform 4 (Q2XSW6_SESIN) | 927.0 | 29.00 ± 0.20 g |
| HIITLGR | Protein NDH-DEPENDENT CYCLIC ELECTRON FLOW 5 | 808.9 | 74.65 ± 0.13 d |
| LAGNPAGR | 11S globulin isoform 4 (Q2XSW6_SESIN) | 754.8 | 148.41 ± 0.35 b |
| MPGVPGPIQR | Oil body-associated protein 1A | 1051.2 | 54.79 ± 0.37 e |
| AGALGDSVTVTR | 60S ribosomal protein L22-2-like | 1146.2 | 68.49 ± 0.14 c |
| INTLSGR | 11S globulin isoform 4 (Q2XSW6_SESIN) | 759.8 | 149.63 ± 0.33 a |
| Peptide | Total_Score 1 | Crash 2 | Polar 3 | D_score 4 | PMF_Score 5 | G_Score 6 | Chem Score 7 | C Score 8 |
|---|---|---|---|---|---|---|---|---|
| AGALGDSVTVTR | 13.66 | −7.05 | 11.00 | −395.98 | −306.63 | −707.54 | −41.93 | 4 |
| HIITLGR | 12.35 | −6.48 | 8.63 | −288.74 | −264.56 | −519.33 | −26.18 | 4 |
| GHIITVAR | 10.03 | −9.60 | 6.79 | −357.58 | −308.79 | −685.80 | −40.87 | 4 |
| Lisinopril | 11.24 | −2.48 | 7.27 | −160.13 | −179.32 | −284.82 | −23.95 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Lu, X.; Sun, Q.; Gao, J.; Ma, L.; Huang, J. Novel ACE Inhibitory Peptides Derived from Simulated Gastrointestinal Digestion in Vitro of Sesame (Sesamum indicum L.) Protein and Molecular Docking Study. Int. J. Mol. Sci. 2020, 21, 1059. https://doi.org/10.3390/ijms21031059
Wang R, Lu X, Sun Q, Gao J, Ma L, Huang J. Novel ACE Inhibitory Peptides Derived from Simulated Gastrointestinal Digestion in Vitro of Sesame (Sesamum indicum L.) Protein and Molecular Docking Study. International Journal of Molecular Sciences. 2020; 21(3):1059. https://doi.org/10.3390/ijms21031059
Chicago/Turabian StyleWang, Ruidan, Xin Lu, Qiang Sun, Jinhong Gao, Lin Ma, and Jinian Huang. 2020. "Novel ACE Inhibitory Peptides Derived from Simulated Gastrointestinal Digestion in Vitro of Sesame (Sesamum indicum L.) Protein and Molecular Docking Study" International Journal of Molecular Sciences 21, no. 3: 1059. https://doi.org/10.3390/ijms21031059
APA StyleWang, R., Lu, X., Sun, Q., Gao, J., Ma, L., & Huang, J. (2020). Novel ACE Inhibitory Peptides Derived from Simulated Gastrointestinal Digestion in Vitro of Sesame (Sesamum indicum L.) Protein and Molecular Docking Study. International Journal of Molecular Sciences, 21(3), 1059. https://doi.org/10.3390/ijms21031059