Volatile Organic Compounds from Orchids: From Synthesis and Function to Gene Regulation
Abstract
:1. Introduction
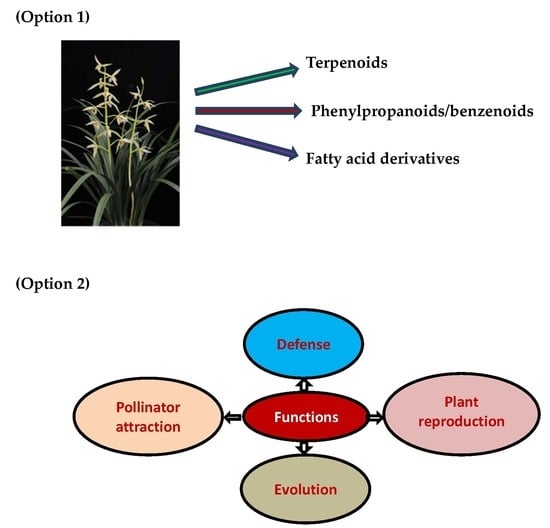
2. Orchid Volatile Compounds and Biosynthetic Pathways
2.1. Terpenoids
2.2. Phenylproponoids and Benzenoids
2.3. Fatty Acid Derivatives
3. Transcriptional Factors in Floral Volatile Regulation
4. Spatial and Temporal Emission of Volatile Organic Compounds
5. Gene Evolution for VOCs
6. Functions of Orchid Volatile Compounds
6.1. Flower Defense
6.2. Pollinator Attraction
6.3. Plant Reproduction
6.4. Evolution
7. Case Studies of Cymbidium Floral Volatiles
7.1. Floral Volatile Research on Cymbidium
7.1.1. Cymbidium goeringii
7.1.2. Cymbidium faberi
7.1.3. Cymbidium ensifolium
7.1.4. Cymbidium Cultivar Sael Bit
7.1.5. Cymbidium Cultivar Sunny Bell
8. Final Remarks and Future Directions for Overcome the Challenges
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Christenhusz, M.J.M.; Byng, J.W. The number of known plant species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef] [Green Version]
- Chase, M.W. Classification of Orchidaceae in the age of DNA data. Curtis’s Bot. Mag. 2005, 22, 2–7. [Google Scholar] [CrossRef]
- Waterman, R.J.; Bidartondo, M.I. Deception above, deception below: Linking pollination and mycorrhizal biology of orchids. J. Exp. Bot. 2008, 59, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, S.; Widmer, A. Orchid diversity: An evolutionary consequence of deception? Trends Ecol. Evol. 2005, 20, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.D.; Brown, A.P.; Dixon, K.W.; Hopper, S.D. Orchid biogeography and factors associated with rarity in a biodiversity hotspot, the Southwest Australian Floristic Region. J. Biogeogr. 2011, 38, 487–501. [Google Scholar] [CrossRef]
- Gravendeel, B.; Smithson, A.; Slik, F.J.W.; Schuiteman, A. Epiphytism and pollinator specialization: Drivers for orchid diversity? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 1523–1535. [Google Scholar] [CrossRef] [Green Version]
- Hew, C.S.; Yong, J.W.H. The Physiology of Tropical Orchids in Relation to the Industry, 2rd ed.; World Scientific Publishing: Singapore, 2004. [Google Scholar]
- Arditti, J. Fundamentals of Orchid Biology; John Wiley Sons: New York, NY, USA, 1992. [Google Scholar]
- Lubinsky, P.; Bory, S.; Hernandez, J.H. Origins and dispersal of cultivated vanilla (Vanilla planifolia Jacks. [Orchidaceae]). Econ. Bot. 2008, 62, 127–138. [Google Scholar] [CrossRef]
- Tsaia, C.F.; Huang, C.L.; Lind, Y.L. The neuroprotective effects of an extract of Gastrodia elata. J. Ethnopharmacol. 2011, 138, 119–125. [Google Scholar] [CrossRef]
- Luo, Y.B.; Jia, J.S.; Wang, C.L. A general review of the conservation status of Chinese orchids. Biodivers. Sci. 2003, 11, 70–77. [Google Scholar]
- Liu, Q.; Chen, J.; Corlett, R.T. Orchid conservation in the biodiversity hotspot of southwestern China. Conserv. Biol. 2015, 29, 1563–1572. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, R.; Fan, Y. Transcriptome profiling provides new insights into the formation of floral scent in Hedychium coronarium. BMC Genom. 2015, 16, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Piechulla, B.; Pott, M.B. Plant scents-mediators of inter-and intraorganismic communication. Planta 2003, 217, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A. Start making scents: The challenge of integrating chemistry into pollination ecology. Entomol. Exp. Appl. 2008, 128, 196–207. [Google Scholar] [CrossRef]
- Borg-Karlson, A.K.; Groth, I.; Agren, L.; Kullenberg, B. Form-specific fragrances from Ophrys insectifera L. (Orchidaceae) attract species of different pollinator genera: Evidence of sympatric speciation? Chemoecology 1993, 4, 39–45. [Google Scholar] [CrossRef]
- Kessler, D.; Gase, K.; Baldwin, I.T. Field experiments with transformed plants reveal the sense of floral scents. Science 2008, 321, 1200–1202. [Google Scholar] [CrossRef] [Green Version]
- Paulus, H.F.; Gack, C. Pollination of Ophrys (Orchidaceae) in Cyprus. Plant Syst. Evol. 1990, 169, 177–207. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Chittka, L.; Raine, N.E. Recognition of flowers by pollinators. Curr. Opin. Plant Biol. 2006, 9, 428–435. [Google Scholar] [CrossRef]
- Delle-Vedove, R.; Juillet, N.; Bessière, J.M.; Dormont, L.; Pailler, T.; Schatz, B. Colour-scent associations in a tropical orchid: Three colours but two odours. Phytochemistry 2011, 72, 735–742. [Google Scholar] [CrossRef]
- Hsiao, Y.; Tsai, W.; Kuoh, C.; Huang, T.; Wang, H.; Wu, T.; Leu, Y.; Chen, W.; Chen, H. Comparison of transcripts in Phalaenopsis bellina and Phalaenopsis equestris (Orchidaceae) flowers to deduce the monoterpene biosynthesis pathway. BMC Plant Biol. 2006, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, Y.; Jeng, M.; Tsai, W.; Chung, Y.; Li, C.; Wu, T.; Kuoh, C.; Chen, W.; Chen, H. A novel homodimeric geranyl diphosphate synthase from the orchid Phalaenopsis bellina lacking a DD(X)2-4D motif. Plant J. 2008, 55, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Leitch, I.J.; Kahandawala, I.; Suda, J.; Hanson, L.; Ingrouille, M.J.; Chase, M.W.; Fay, M.F. Genome size diversity in orchids: Consequences and evolution. Ann. Bot. 2009, 104, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Jersáková, J.; Trávníček, P.; Kubátová, B.; Krejčíková, J.; Urfus, T.; Liu, Z.J.; Lamb, A.; Ponert, J.; Schulte, Z.K.; Čurn, V.; et al. Genome size varia- tion in Orchidaceae subfamily Apostasioideae: Filling the phylogenetic gap. Bot. J. Linn. Soc. 2013, 172, 95–105. [Google Scholar]
- Dudareva, N.; Pichersky, E. Biology of Floral Scent; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ramya, M.; Park, P.H.; Chen, Y.C.; Kwon, O.K.; An, H.R.; Park, P.M.; Baek, Y.S.; Kang, B.C.; Tsai, W.C.; Chen, H.H. RNA sequencing analysis of Cymbidium goeringii identifies floral scent biosynthesis related genes. BMC Plant Biol. 2019, 337, 1–15. [Google Scholar] [CrossRef]
- Ramya, M.; Lee, S.Y.; An, H.R.; Park, P.M.; Kim, N.S.; Park, P.H. MYB1 transcription factor regulation through floral scent in Cymbidium cultivar ‘Sael Bit’. Phytochem. Lett. 2019, 32, 181–187. [Google Scholar] [CrossRef]
- Gupta, A.K.; Schauvinhold, I.; Pichersky, E.; Schiestl, F.P. Eugenol synthase genes in floral scent variation in Gymnadenia species. Funct. Integr. Genom. 2014, 14, 779. [Google Scholar] [CrossRef]
- Huanga, M.; Maa, C.; Yub, R.; Mub, L.; Houa, J.; Yua, Y.; Fana, Y. Concurrent changes in methyl jasmonate emission and the expression of its biosynthesis-related genes in Cymbidium ensifolium flowers. Physiol. Plant. 2015, 153, 503–512. [Google Scholar] [CrossRef]
- Blight, M.M.; Le Metayer, M.; Delegue, M.H.P.; Pickett, J.A.; Marion-Poll, F.; Wadhams, L.J. Identification of floral volatiles involved in recognition of oilseed rape flowers, Brassica napus by honeybees, Apis mellifera. J. Chem. Ecol. 1997, 23, 1715–1727. [Google Scholar] [CrossRef]
- Byers, K.J.; Bradshaw, H.D.; Riffell, J.A. Three floral volatiles contribute to differential pollinator attraction in monkeyflowers (Mimulus). J. Exp. Biol. 2014, 217, 614–623. [Google Scholar] [CrossRef] [Green Version]
- Junker, R.R.; Gershenzon, J.; Unsicker, S.B. Floral odor bouquet loses its ant repellent properties after inhibition of terpene biosynthesis. J. Chem. Ecol. 2011, 37, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Caputi, L.; Aprea, E. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat. Food Nutr. Agric. 2011, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, D.J.; Croteau, R. Terpenoid metabolism. Plant Cell. 1995, 7, 1015–1026. [Google Scholar]
- Baek, Y.S.; Ramya, M.; An, H.R.; Park, P.M.; Lee, S.Y.; Baek, N.I.; Park, P.H. Volatiles Profile of the Floral Organs of a New Hybrid Cymbidium, ‘Sunny Bell’ Using Headspace Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry Analysis. Plants 2019, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Y.; Tian, M.; Zhou, W.W. Analysis of aroma components in different orchid varieties. J. Anal. Sci. 2012, 28, 502–506. [Google Scholar]
- Zhang, Y.; Tian, M.; Wang, C.X.; Chen, S. Component analysis and sensory evaluation of flower aroma of Oncidium Sharry Baby ‘Sweet Fragrance’ under different temperature conditions. J. Plant Resour. Environ. 2015, 24, 112–114. [Google Scholar]
- Mohd-Hairul, A.R.; Parameswari, N.; Gwendoline Ee, C.L.; Janna, O.A. Terpenoid, benzenoid and phenylpropanoid compounds in the floral scent of Vanda Mimi Palmer. J. Plant Biol. 2010, 53, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; An, H.Y.; Park, P.M.; Baek, Y.S.; Kwon, O.K.; Park, S.Y.; Park, P.H. Analysis of floral scent patterns in flowering stages and floral organs of maxillaria using an electronic nose. Flower Res. J. 2016, 24, 171–180. [Google Scholar] [CrossRef]
- Omata, A.; Nakamura, S.; Yomogia, K.; Moriai, K.; Ichikawa, Y.; Watanabe, I. Volatile components of To-Yo-Ran flowers (Cymbidium faberi and Cymbidium virescens). Agric. Biol. Chem. 1990, 54, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Verdonk, J.C.; Haring, M.A.; van Tunen, A.J.; Schuurink, R.C. ODORANT1 regulates fragrance biosynthesis in petunia flowers. Plant Cell 2005, 17, 1612–1624. [Google Scholar] [CrossRef] [Green Version]
- Colquhoun, T.A.; Marciniak, D.M.; Wedde, A.E.; Kim, J.Y.; Schwieterman, M.L.; Levin, L.A.; Moerkercke, A.V.; Schuurink, R.C.; Clark, D.G. A peroxisomally localized acyl-activating enzyme is required for volatile benzenoid formation in a Petunia × hybrida cv. ‘Mitchell Diploid’ flower. J. Exp. Bot. 2012, 63, 4821–4833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlüter, P.M.; Xu, S.; Gagliardini, V.; Whittle, E.; Shanklin, J.; Grossniklaus, U.; Schiestl, F.P. Stearoyl-acyl carrier protein desaturases are associated with floral isolation in sexually deceptive orchids. Proc. Natl. Acad. Sci. USA 2011, 108, 5696–56701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildermuth, M.C. Variations on a theme: Synthesis and modification of plant benzoic acids. Curr. Opin. Plant Biol. 2006, 9, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Klempien, A.; Kaminaga, Y.; Qualley, A.; Nagegowda, D.A.; Widhalm, J.R.; Orlova, I.; Shasany, A.K.; Taguchi, G.; Kish, C.M.; Cooper, B.R.; et al. Contribution of CoA ligases to benzenoid biosynthesis in petunia flowers. Plant Cell 2012, 24, 2015–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qualley, A.V.; Widhalm, J.R.; Adebesin, F.; Kish, C.M.; Dudareva, N. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 16383–16388. [Google Scholar] [CrossRef] [Green Version]
- Huber, F.; Kaiser, R.; Sauter, W.; Schiestl, F. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae). Oecologia 2005, 142, 564–575. [Google Scholar] [CrossRef]
- Suchet, C.; Dormont, L.; Schatz, B.; Giurfa, M.; Simon, V.; Raynaud, C.; Chave, J. Floral scent variation in two Antirrhinum majus subspecies influences the choice of naïve bumblebees. Behav. Ecol. Sociobiol. 2011, 65, 1015–1027. [Google Scholar] [CrossRef]
- Ramya, M.; Kwon, O.K.; An, H.R.; Park, P.M.; Baek, Y.S.; Park, P.H. Floral scent: Regulation and role of MYB transcription factors. Phytochem. Lett. 2017, 19, 114–120. [Google Scholar] [CrossRef]
- Xie, D.Y.; Sharma, S.B.; Wright, E.; Wang, Z.Y.; Dixon, R.A. Metabolic engineering of pro anthocyanidins through co-expression of anthocyanidin reductase and the PAP1 MYB transcription factor. Plant J. 2006, 45, 895–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zvi, M.M.; Shklarman, E.; Masci, T.; Kalev, H.; Debener, T.; Shafir, S. PAP1 transcription factor enhances production of phenylpropanoid and terpenoid scent compounds in rose flowers. New Phytol. 2012, 195, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Ben Zvi, M.M.; Negre-Zakharov, F.; Masci, T.; Ovadis, M.; Shklarman, E. Interlinking showy traits: Co-engineering of scent and colour biosynthesis in flowers. Plant Biotechnol. J. 2008, 6, 403–415. [Google Scholar]
- Yang, C.Q.; Fang, X.; Wu, X.M.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Transcriptional regulation of plant secondary metabolism. J. Integr. Plant Biol. 2012, 54, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, S.; Hong, H.; Jhou, Y. Transcriptomic profiling of the flower scent biosynthesis pathway of Cymbidium faberi Rolfe and functional characterization of its jasmonic acid carboxyl methyltransferase gene. BMC Genom. 2019, 20, 125. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.S.; Abdullah, J.O.; Namasivayam, P. Isolation, cloning, and characterization of fragrance-related transcripts from Vanda Mimi Palmer. Sci. Hortic. 2011, 127, 388–397. [Google Scholar] [CrossRef]
- An, F.M.; Chan, M.T. Transcriptome-wide characterization of miRNA-directed and non-miRNA-directed endonucleolytic cleavage using Degradome analysis under low ambient temperature in Phalaenopsis aphrodite subsp. formosana. Plant Cell Physiol. 2012, 53, 1737–1750. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X.L.; Wang, Y.; Tian, M.; Fan, M.H. Changes of aroma components in Oncidium Sharry Baby in different florescence and flower parts. Sci. Agric. Sin. 2011, 44, 110–117. [Google Scholar]
- Zheng, J.A.; Hu, Z.H.; Guan, X.L.; Dou, D.Q.; Bai, G.; Wang, Y.; Guo, Y.; Li, W.; Leng, P. Transcriptome ssanalysis of Syringa oblata lindl: Inflorescence identifies genes associated with pigment biosynthesis and scent metabolism. PLoS ONE 2015, 10, e142542. [Google Scholar] [CrossRef] [Green Version]
- Teh, S.L.; Chan, W.S.; Janna, O.A.; Parameswari, N. Development of expressed sequence tag resources for Vanda Mimi Palmer and data mining for EST-SSR. Mol. Biol. Rep. 2011, 38, 3903–3909. [Google Scholar] [CrossRef] [Green Version]
- Flach, A.; Donon, R.C.; Singer, R.B.; Koehler, S.; Amaral, M.D.E.; Marsaioli, A.J. The chemistry of pollination in selected brazilian Maxillariinae orchids: Floral rewards and fragrance. J. Chem. Ecol. 2004, 30, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Perraudin, F.; Popovici, J.; Bertrand, C. Analysis of headspace-solid micro extracts from flowers of Maxillaria tenuifolia Lindl. by GC-MS. Electron. J. Nat. Subst. 2006, 1, 1–5. [Google Scholar]
- Baek, Y.S.; Kim, S.K.; Park, P.H.; An, H.R.; Park, P.M.; Baek, N.I.; Kwon, O.K. Analysis of volatile floral scents in maxillaria species and cultivars. Flower Res. J. 2016, 24, 282–289. [Google Scholar] [CrossRef]
- Been, C.G.; Kang, S.B.; Kim, D.G.; Cha, Y.J. Analysis of fragrant compounds and gene expression in fragrant Phalaenopsis. Flower Res. J. 2014, 22, 255–263. [Google Scholar] [CrossRef]
- Colquhoun, T.A.; Verdonk, J.C.; Schimmel, B.C.; Tieman, D.M.; Underwood, B.A.; Clark, D.G. Petunia floral volatile benzenoid/phenylpropanoid genes are regulated in a similar manner. Phytochemistry 2010, 71, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Lewinsohn, E.; Croteau, R. Purification and characterization of Slinalool synthase: An enzyme involved in the production of floral scent in Clarkia breweri. Arch. Biochem. Biophys. 1995, 316, 803–807. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Tsai, W.C.; Dievar, A.; Hsu, C.C.; Hsiao, Y.Y.; Chiou, S.Y.; Huang, H.; Chen, H.H. Post genomics era for orchid research. Bot. Stud. 2017, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Zhang, Y.; Mann, F.M.; Huang, C.; Mukkamala, D.; Hudock, M.P.; Mead, M.E.; Prisic, S.; Wang, K.; Lin, F.Y.; et al. Diterpene cyclases and the nature of the isoprene fold. Proteins 2010, 78, 2417–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, Y.T.; Chao, Y.T.; Chen, W.C.; Shih, M.C.; Chang1, S.B. Segmental and tandem chromosome duplications led to divergent evolution of the chalcone synthase gene family in Phalaenopsis orchids. Ann. Bot. 2019, 123, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.B.; Stockwell, V.O. Management of fire blight: A case study in microbial ecology. Annu. Rev. Phytopathol. 1998, 36, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tholl, D.; Auria, J.C.; Farooq, A.; Pichersky, E.; Gershenzon, J. Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 2003, 15, 481–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-a review. Food Chem. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Huber, F.; Gomez, J. Phenotypic selection on floral scent: Trade-off between attraction and deterrence? Evol. Ecol. 2011, 25, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Pellmyr, O.; Thien, L.B. Insect reproduction and floral fragrances: Keys to the evolution of the angiosperms. Taxon 1986, 35, 76–85. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Ayasse, M. Do changes in floral odor cause speciation in sexually deceptive orchids? Plant Syst. Evol. 2002, 234, 111–119. [Google Scholar] [CrossRef]
- Vereecken, N.J.; Cozzolino, S.; Schiestl, F.P. Hybrid floral scent novelty drives pollinator shift in sexually deceptive orchids. BMC Evol. Biol. 2010, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Dobson, H.E.M.; Danielson, E.M.; Van Wesep, I.D. Pollen odor chemicals as modulators of bumble bee foraging on Rosa rugosa Thunb. (Rosaceae). Plant Species Biol. 1999, 14, 153–166. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Waelti, M.O.; Widmer, A.; Schiestl, F.P. Postpollination changes in floral odor in Silene latifolia: Adaptive mechanisms for seed-predator avoidance? J. Chem. Ecol. 2006, 32, 1855–1860. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Maeda, H.; Chang, C.Y.; San Miguel, P.; Baxter, I.; Cooper, B.; Dudareva, N. Developmental changes in the metabolic network of snapdragon flowers. PLoS ONE 2012, 7, e40381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Fan, Y.P. Analysis of aroma components of Oncidium. Acta Agric. Univ. Jiangxiensis 2012, 34, 692–698. [Google Scholar]
- Hong, G.J.; Xue, X.Y.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, W.C.; Fu, C.H.; Hsiao, Y.Y.; Huang, Y.M.; Chen, L.J.; Wang, M.; Liu, Z.J.; Chen, H.H. OrchidBase 2.0: Comprehensive collection of Orchidaceae floral transcriptomes. Plant Cell Physiol. 2013, 54, 7. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.Y.; Nason, J.D. Spatial demographic and genetic consequences of harvesting within papulations of terrestrial orchid Cymbidium goeringii. Biol. Conserv. 2007, 137, 125–137. [Google Scholar] [CrossRef]
- Chen, X.Q.; Liu, Z.J.; Zhu, G.H.; Lang, K.Y.; Ji, Z.H.; Luo, Y.B.; Jin, X.H.; Cribb, P.J.; Wood, J.J.; Gale, S.W. Orchidaceae. In Flora of China; Science Press & Missouri Botanical Garden Press: Beijing, China, 2009; p. 260. [Google Scholar]
- Du Puy, D.; Cribb, P. The Genus Cymbidium, 2nd ed.; Surrey, Royal Botanic Gardens, Kew Publishing: London, UK, 2007. [Google Scholar]
- Da Silva, J.A.T.; Chan, M.T.; Sanjaya; Chai, M.L.; Tanaka, M. Priming abiotic factors for optimal hybrid Cymbidium (Orchidaceae) callus induction, plantlet formation, and their subsequent cytogenetic stability analysis. Sci. Hortic. 2006, 109, 368–378. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Singh, N.; Tanaka, M. Priming biotic factors for optimal protocorm-like body in hybrid Cymbidium (Orchidaceae), and assessment of cytogenetic stability in regenerated plants. Plant Cell Tissue Organ Cult. 2006, 84, 119–128. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Chin, D.P.; Van, P.T.; Mii, M. Transgenic orchids. Sci. Hortic. 2011, 130, 673–680. [Google Scholar] [CrossRef]
- Huan, L.V.T.; Takamura, T.; Tanaka, M. Callus formation and plant regeneration from callus through somatic embryo structures in Cymbidium orchid. Plant Sci. 2004, 166, 1443–1449. [Google Scholar] [CrossRef]
- Van den Berg, C.; Ryan, A.; Cribb, P.J.; Chase, M.W. Molecular phylogenetics of Cymbidium (Orchidaceae: Maxillarieae): Sequence data from internal transcribed spacers (ITS) of nuclear ribosomal DNA and plastid matK. Lindleyana 2002, 17, 102–111. [Google Scholar]
- Sharma, S.K.; Dkhar, J.; Kumaria, S.; Tandon, P.; Rao, S.R. Assessment of phylogenetic inter-relationships in the genus Cymbidium (Orchidaceae) based on internal transcribed spacer region of rDNA. Gene 2012, 495, 10–15. [Google Scholar] [CrossRef]
- Pornarong, S. DNA barcoding of the Cymbidium species (Orchidaceae) in Thailand. Afr. J. Agric. Res. 2012, 7, 393–404. [Google Scholar] [CrossRef]
- Tao, J.; Yu, L.; Kong, F.; Zhao, D. Effects of plant growth regulators on in vitro propagation of Cymbidium faberi Rolfe. Afr. J. Biotechnol. 2011, 10, 15639–15646. [Google Scholar] [CrossRef]
- Pindel, A. Optimization of isolation conditions of Cymbidium protoplasts. Folia Hortic. 2007, 19, 79–88. [Google Scholar]
- Chen, Y.; Liu, X.; Liu, Y. In vitro plant regeneration from the immature seeds of Cymbidium faberi. Plant Cell Tissue Organ Cult. 2005, 81, 247–251. [Google Scholar] [CrossRef]
- Huang, W.; Fang, Z.; Zeng, S.; Zhang, J.; Wu, K.; Chen, Z.; da Silva, J.A.T.; Duan, J. Molecular cloning and functional analysis of three FLOWERING LOCUS T (FT) homologous genes from Chinese Cymbidium. Int. J. Mol. Sci. 2012, 13, 11385–11398. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Luo, J.; Yan, T.; Xiang, L.; Jin, F.; Qin, D.; Sun, C.; Xie, M. Deep sequencing based analysis of the Cymbidium ensifolium floral transcriptome. PLoS ONE 2013, 8, e85480. [Google Scholar] [CrossRef]
- Ning, H.; Zhang, C.; Fu, J.; Fan, Y. Comparative transcriptome analysis of differentially expressed genes between the curly and normal leaves of Cymbidium goeringii var. longibracteatum. Genes Genom. 2016, 38, 985–998. [Google Scholar] [CrossRef]
- Peng, H. Study on the Volatile, Characteristic Floral Fragrance Components of Chinese Cymbidium. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2009. [Google Scholar]
- Kim, S.M.; Jang, E.J.; Hong, J.W.; Song, S.H.; Pak, C.H. A comparison of functional fragrant components of Cymbidium (Oriental Ochid) species. Korean J. Hortic. Technol. 2016, 34, 331–341. [Google Scholar]
- Christenson, E.A. Phalaenopsis: A Monograph; Timber Press: Portland, OR, USA, 2001; pp. 24–25. [Google Scholar]
- Kaiser, R. The Scent of Orchids-Olfactory and Chemical Investigations; Elsevier: Amsterdam, The Netherlands, 1993; pp. 239–240. [Google Scholar]
- Schlossman, M.L. The Chemistry and Manufacture of Cosmetics; Allured Publishing Corporation: Carol Stream, IL, USA, 2009; Volume 2, p. 851. [Google Scholar]
- Park, P.H.; Ramya, M.; An, H.R.; Park, P.M.; Lee, S.Y. Breeding of Cymbidium ‘Sale Bit’ with Bright Yellow Flowers and Floral Scent. Korean J. Breed. Sci. 2019, 51, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.C.; Wu, K.M.; Chiang, T.Y.; Huang, C.Y.; Chou, C.H.; Li, S.J.; Chiang, Y.C. Comparative transcriptome analysis of Gastrodia elata (Orchidaceae) in response to fungus symbiosis to identify gastrodin biosynthesis-related genes. BMC Genom. 2016, 17, 212. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; Kant, R.; Van, P.T.; Winarto, B.; Zeng, S.; da Silva, J.A.T. The application of biotechnology to orchids. Crit. Rev. Plant Sci. 2013, 32, 69–139. [Google Scholar] [CrossRef]
- Cai, J.; Liu, X.; Vanneste, K.; Proost, S.; Tsai, W.C.; Liu, K.W.; Chen, L.J.; He, Y.; Xu, Q.; Bian, C.; et al. The genome sequence of the orchid Phalaenopsis equestris. Nat. Genet. 2015, 47, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.H.; Kao, Y.K.; Tang, C.Y.; Tsai, C.C.; Lin, T.Y. Estimating nuclear DNA content within 50 species of the genus Phalaenopsis Blume (Orchidaceae). Sci. Hortic. 2013, 161, 70–75. [Google Scholar] [CrossRef]




| Compound | Structure | Species | Reference |
|---|---|---|---|
| Terpenoids | |||
| Linalool |  | P. bellina C. cv. Sunny Bell | [23] [39] |
| Geraniol |  | P. bellina | [24] |
| Ocimene |  | Vanda Mimi Palmer | [42] |
| Farnesol |  | C. goeringii | [28] |
| β-Caryophyllene |  | M. tenufolia | [43] |
| Phenylproponoids/Benzenoids | |||
| Eugenol |  | Gymnadenia Species | [30] |
| 2-methyl butanal |  | C. cv. Sael Bit | [29] |
| Benzyl acetate |  | Vanda Mimi Palmer | [42] |
| Fatty Acid Derivatives | |||
| Methyl jasmonate |  | C. ensifolium C. faberi | [31] [44] |
| Floral Scent Gene | Metabolism Pathway | Species | Reference |
|---|---|---|---|
| Genes | |||
| PbGDS | Terpenoid pathway | Phalaenopsis bellina | [24] |
| VMPAAT | Terpenoid pathway | Vanda species | [59] |
| VMDXS | Terpenoid pathway | Vanda Mimi Palmer | [42] |
| GdEGS | Benzenoid pathway | Gymnadenia species | [30] |
| OsSAD1 | Benzenoid pathway | Ophrys sphegodes | [19] |
| Transcription Factors (TFs) | |||
| CsMYB1 | Phenylprponid/benzenoid | Cymbidium cv. Sael Bit | [29] |
| PbbZIP4 | Monoterpene pathway | Phalaenopsis aphrodite | [60] |
| PbBHLH2 | Monoterpene pathway | Phalaenopsis bellina | [23] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramya, M.; Jang, S.; An, H.-R.; Lee, S.-Y.; Park, P.-M.; Park, P.H. Volatile Organic Compounds from Orchids: From Synthesis and Function to Gene Regulation. Int. J. Mol. Sci. 2020, 21, 1160. https://doi.org/10.3390/ijms21031160
Ramya M, Jang S, An H-R, Lee S-Y, Park P-M, Park PH. Volatile Organic Compounds from Orchids: From Synthesis and Function to Gene Regulation. International Journal of Molecular Sciences. 2020; 21(3):1160. https://doi.org/10.3390/ijms21031160
Chicago/Turabian StyleRamya, Mummadireddy, Seonghoe Jang, Hye-Ryun An, Su-Young Lee, Pil-Man Park, and Pue Hee Park. 2020. "Volatile Organic Compounds from Orchids: From Synthesis and Function to Gene Regulation" International Journal of Molecular Sciences 21, no. 3: 1160. https://doi.org/10.3390/ijms21031160
APA StyleRamya, M., Jang, S., An, H.-R., Lee, S.-Y., Park, P.-M., & Park, P. H. (2020). Volatile Organic Compounds from Orchids: From Synthesis and Function to Gene Regulation. International Journal of Molecular Sciences, 21(3), 1160. https://doi.org/10.3390/ijms21031160