Primary Human Chondrocytes Affected by Cigarette Smoke—Therapeutic Challenges
Abstract
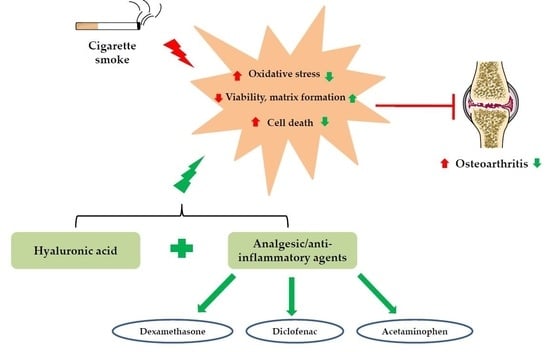
:1. Introduction
2. Results
2.1. CSE Exposure Inhibited the Viability, Proliferation, and Matrix Formation of Primary Human Chondrocytes in a Dose-Dependent Manner
2.2. CSE Exposure Induced Cell Death by Increasing ROS Production
2.3. Clinical Dose of Dex Reduced Viability of Primary Human Chondrocytes
2.4. Effects of HA and Decreased Concentrations of Dex on the CSE-Impaired Primary Human Chondrocytes
2.5. Ace and Dic Did not Augment Adverse the Effects of CSE on Primary Human Chondrocytes
2.6. Analgesic/Anti-Inflammatory Agents in Combination with HA Rescued the CSE-Impaired Primary Human Chondrocytes
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Generation of Cigarette Smoke Extract (CSE)
4.3. Isolation and Culture of Human Primary Chondrocytes
4.4. Resazurin Conversion Assay
4.5. Sulforhodamine B (SRB) Staining
4.6. Live/Dead Staining with Calcein-AM and Ethidium Homodimer
4.7. Histochemical Analysis of ECM Production
4.8. Reactive Oxygen Species (ROS) Production Analysis with DCFH-DA Assay
4.9. Alkaline Phosphatase (AP) Activity Assay
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ace | Acetaminophen |
| CSE | Cigarette smoke extract |
| OA | Osteoarthritis |
| ROS | Reactive oxygen species |
| ECM | Extracellular matrix |
| IA | Intra-articular |
| HA | Hyaluronic acid |
| CSs | Corticosteroids |
| NSAID | Non-steroidal anti-inflammatory drug |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| RT | Room temperature |
| SRB | Sulforhodamine B |
| GAG | Glycosaminoglycan |
| DCFH-DA | Dichlorfluorescein-diacetate |
| AP | Alkaline Phosphatase |
| H2O2 | Hydrogen peroxide |
| TGF-β | Transforming growth factor β |
| PAHs | Polycyclic aromatic hydrocarbons |
| Dic | Diclofenac |
| Dex | Dexamethasone |
| EC 50 | Half maximal effective concentration |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FCS | Fetal Calf Serum |
References
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Env. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef]
- Kamceva, G.; Arsova-Sarafinovska, Z.; Ruskovska, T.; Zdravkovska, M.; Kamceva-Panova, L.; Stikova, E. Cigarette Smoking and Oxidative Stress in Patients with Coronary Artery Disease. Open Access Maced. J. Med. Sci. 2016, 4, 636–640. [Google Scholar] [CrossRef] [Green Version]
- Sreekumar, V.; Aspera-Werz, R.; Ehnert, S.; Strobel, J.; Tendulkar, G.; Heid, D.; Schreiner, A.; Arnscheidt, C.; Nussler, A.K. Resveratrol protects primary cilia integrity of human mesenchymal stem cells from cigarette smoke to improve osteogenic differentiation in vitro. Arch. Toxicol. 2017, 92. [Google Scholar] [CrossRef]
- Skott, M.; Andreassen, T.T.; Ulrich-Vinther, M.; Chen, X.; Keyler, D.E.; LeSage, M.G.; Pentel, P.R.; Bechtold, J.E.; Soballe, K. Tobacco extract but not nicotine impairs the mechanical strength of fracture healing in rats. J. Orthop. Res. 2006, 24, 1472–1479. [Google Scholar] [CrossRef]
- Ehnert, S.; Aspera-Werz, R.H.; Ihle, C.; Trost, M.; Zirn, B.; Flesch, I.; Schroter, S.; Relja, B.; Nussler, A.K. Smoking Dependent Alterations in Bone Formation and Inflammation Represent Major Risk Factors for Complications Following Total Joint Arthroplasty. J. Clin. Med. 2019, 8, 406. [Google Scholar] [CrossRef] [Green Version]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Racunica, T.L.; Szramka, M.; Wluka, A.E.; Wang, Y.; English, D.R.; Giles, G.G.; O’Sullivan, R.; Cicuttini, F.M. A positive association of smoking and articular knee joint cartilage in healthy people. Osteoarthr. Cartil. 2007, 15, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Davies-Tuck, M.L.; Wluka, A.E.; Forbes, A.; Wang, Y.; English, D.R.; Giles, G.G.; Cicuttini, F. Smoking is associated with increased cartilage loss and persistence of bone marrow lesions over 2 years in community-based individuals. Rheumatology 2009, 48, 1227–1231. [Google Scholar] [CrossRef] [Green Version]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Ying, X.; Cheng, S.; Shen, Y.; Cheng, X.; An Rompis, F.; Wang, W.; Lin, Z.; Chen, Q.; Zhang, W.; Kou, D.; et al. Nicotine promotes proliferation and collagen synthesis of chondrocytes isolated from normal human and osteoarthritis patients. Mol. Cell Biochem. 2012, 359, 263–269. [Google Scholar] [CrossRef]
- Gullahorn, L.; Lippiello, L.; Karpman, R. Smoking and osteoarthritis: Differential effect of nicotine on human chondrocyte glycosaminoglycan and collagen synthesis. Osteoarthr. Cartil. 2005, 13, 942–943. [Google Scholar] [CrossRef] [Green Version]
- Starek, A.; Podolak, I. Carcinogenic effect of tobacco smoke. Rocz. Panstw. Zakl. Hig. 2009, 60, 299–310. [Google Scholar]
- Charlier, E.; Relic, B.; Deroyer, C.; Malaise, O.; Neuville, S.; Collee, J.; Malaise, M.G.; De Seny, D. Insights on Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2146. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.M.; Fuller, C.J.; Whittles, C.E.; Sharif, M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthr. Cartil. 2007, 15, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco Smoke: Involvement of Reactive Oxygen Species and Stable Free Radicals in Mechanisms of Oxidative Damage, Carcinogenesis and Synergistic Effects with Other Respirable Particles. Int. J. Env. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef]
- Collins, J.A.; Wood, S.T.; Nelson, K.J.; Rowe, M.A.; Carlson, C.S.; Chubinskaya, S.; Poole, L.B.; Furdui, C.M.; Loeser, R.F. Oxidative Stress Promotes Peroxiredoxin Hyperoxidation and Attenuates Pro-survival Signaling in Aging Chondrocytes. J. Biol. Chem. 2016, 291, 6641–6654. [Google Scholar] [CrossRef] [Green Version]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Zhang, W.; Nuki, G.; Moskowitz, R.W.; Abramson, S.; Altman, R.D.; Arden, N.K.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis: Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr. Cartil. 2010, 18, 476–499. [Google Scholar] [CrossRef] [Green Version]
- Temple-Wong, M.M.; Ren, S.; Quach, P.; Hansen, B.C.; Chen, A.C.; Hasegawa, A.; D’Lima, D.D.; Koziol, J.; Masuda, K.; Lotz, M.K.; et al. Hyaluronan concentration and size distribution in human knee synovial fluid: Variations with age and cartilage degeneration. Arthritis Res. 2016, 18, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef] [Green Version]
- Jordan, K.M.; Arden, N.K.; Doherty, M.; Bannwarth, B.; Bijlsma, J.W.; Dieppe, P.; Gunther, K.; Hauselmann, H.; Herrero-Beaumont, G.; Kaklamanis, P.; et al. EULAR Recommendations 2003: An evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann. Rheum. Dis. 2003, 62, 1145–1155. [Google Scholar] [CrossRef]
- Akmal, M.; Singh, A.; Anand, A.; Kesani, A.; Aslam, N.; Goodship, A.; Bentley, G. The effects of hyaluronic acid on articular chondrocytes. J. Bone Jt. Surg. Br. 2005, 87, 1143–1149. [Google Scholar] [CrossRef]
- Yu, C.J.; Ko, C.J.; Hsieh, C.H.; Chien, C.T.; Huang, L.H.; Lee, C.W.; Jiang, C.C. Proteomic analysis of osteoarthritic chondrocyte reveals the hyaluronic acid-regulated proteins involved in chondroprotective effect under oxidative stress. J. Proteom. 2014, 99, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Fioravanti, A.; Cantarini, L.; Chellini, F.; Manca, D.; Paccagnini, E.; Marcolongo, R.; Collodel, G. Effect of hyaluronic acid (MW 500-730 kDa) on proteoglycan and nitric oxide production in human osteoarthritic chondrocyte cultures exposed to hydrostatic pressure. Osteoarthr. Cartil. 2005, 13, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Wernecke, C.; Braun, H.J.; Dragoo, J.L. The Effect of Intra-articular Corticosteroids on Articular Cartilage: A Systematic Review. Orthop. J. Sports Med. 2015, 3, 2325967115581163. [Google Scholar] [CrossRef]
- Huebner, K.D.; Shrive, N.G.; Frank, C.B. Dexamethasone inhibits inflammation and cartilage damage in a new model of post-traumatic osteoarthritis. J. Orthop. Res. 2014, 32, 566–572. [Google Scholar] [CrossRef]
- Tu, Y.; Xue, H.; Francis, W.; Davies, A.P.; Pallister, I.; Kanamarlapudi, V.; Xia, Z. Lactoferrin inhibits dexamethasone-induced chondrocyte impairment from osteoarthritic cartilage through up-regulation of extracellular signal-regulated kinase 1/2 and suppression of FASL, FAS, and Caspase 3. Biochem. Biophys. Res. Commun. 2013, 441, 249–255. [Google Scholar] [CrossRef]
- Song, Y.W.; Zhang, T.; Wang, W.B. Gluococorticoid could influence extracellular matrix synthesis through Sox9 via p38 MAPK pathway. Rheumatol. Int. 2012, 32, 3669–3673. [Google Scholar] [CrossRef]
- Arun, O.; Canbay, O.; Celebi, N.; Sahin, A.; Konan, A.; Atilla, P.; Aypar, U. The analgesic efficacy of intra-articular acetaminophen in an experimental model of carrageenan-induced arthritis. Pain Res. Manag. 2013, 18, e63–e67. [Google Scholar] [CrossRef]
- Cannava, C.; Tommasini, S.; Stancanelli, R.; Cardile, V.; Cilurzo, F.; Giannone, I.; Puglisi, G.; Ventura, C.A. Celecoxib-loaded PLGA/cyclodextrin microspheres: Characterization and evaluation of anti-inflammatory activity on human chondrocyte cultures. Colloids Surf. B Biointerfaces 2013, 111, 289–296. [Google Scholar] [CrossRef]
- Dandona, P.; Mohanty, P.; Hamouda, W.; Aljada, A.; Kumbkarni, Y.; Garg, R. Effect of dexamethasone on reactive oxygen species generation by leukocytes and plasma interleukin-10 concentrations: A pharmacodynamic study. Clin. Pharmacol. Ther. 1999, 66, 58–65. [Google Scholar] [CrossRef]
- Zhao, Y.; Zuo, Y.; Huo, H.; Xiao, Y.; Yang, X.; Xin, D. Dexamethasone reduces ATDC5 chondrocyte cell viability by inducing autophagy. Mol. Med. Rep. 2014, 9, 923–927. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, J.R. Intra-articular corticosteroids. Guide to selection and indications for use. Drugs 1996, 52, 507–514. [Google Scholar] [CrossRef]
- Grodzinsky, A.J.; Wang, Y.; Kakar, S.; Vrahas, M.S.; Evans, C.H. Intra-articular dexamethasone to inhibit the development of post-traumatic osteoarthritis. J. Orthop. Res. 2017, 35, 406–411. [Google Scholar] [CrossRef] [Green Version]
- Amin, S.; Niu, J.; Guermazi, A.; Grigoryan, M.; Hunter, D.J.; Clancy, M.; LaValley, M.P.; Genant, H.K.; Felson, D.T. Cigarette smoking and the risk for cartilage loss and knee pain in men with knee osteoarthritis. Ann. Rheum. Dis. 2007, 66, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P., 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharm. 2009, 192, 29–60. [Google Scholar] [CrossRef] [Green Version]
- Barua, R.S.; Sharma, M.; Dileepan, K.N. Cigarette Smoke Amplifies Inflammatory Response and Atherosclerosis Progression Through Activation of the H1R-TLR2/4-COX2 Axis. Front. Immunol. 2015, 6, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fricker, M.; Goggins, B.J.; Mateer, S.; Jones, B.; Kim, R.Y.; Gellatly, S.L.; Jarnicki, A.G.; Powell, N.; Oliver, B.G.; Radford-Smith, G.; et al. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. Jci. Insight 2018, 3, e94040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspera-Werz, R.H.; Chen, T.; Ehnert, S.; Zhu, S.; Frohlich, T.; Nussler, A.K. Cigarette Smoke Induces the Risk of Metabolic Bone Diseases: Transforming Growth Factor Beta Signaling Impairment via Dysfunctional Primary Cilia Affects Migration, Proliferation, and Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 2915. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.M.; Herlofsen, S.R.; Karlsen, T.A.; Kuchler, A.M.; Floisand, Y.; Brinchmann, J.E. Similar properties of chondrocytes from osteoarthritis joints and mesenchymal stem cells from healthy donors for tissue engineering of articular cartilage. PLoS ONE 2013, 8, e62994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehne, T.; Karlsson, C.; Ringe, J.; Sittinger, M.; Lindahl, A. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation. Arthritis Res. 2009, 11, R133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoop, R.; Albrecht, D.; Gaissmaier, C.; Fritz, J.; Felka, T.; Rudert, M.; Aicher, W.K. Comparison of marker gene expression in chondrocytes from patients receiving autologous chondrocyte transplantation versus osteoarthritis patients. Arthritis Res. 2007, 9, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspera-Werz, R.H.; Ehnert, S.; Heid, D.; Zhu, S.; Chen, T.; Braun, B.; Sreekumar, V.; Arnscheidt, C.; Nussler, A.K. Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke. Oxid. Med. Cell Longev. 2018, 2018, 3172480. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef]
- Chen, C.T.; Burton-Wurster, N.; Borden, C.; Hueffer, K.; Bloom, S.E.; Lust, G. Chondrocyte necrosis and apoptosis in impact damaged articular cartilage. J. Orthop. Res. 2001, 19, 703–711. [Google Scholar] [CrossRef]
- Chang, J.; Wang, W.; Zhang, H.; Hu, Y.; Wang, M.; Yin, Z. The dual role of autophagy in chondrocyte responses in the pathogenesis of articular cartilage degeneration in osteoarthritis. Int. J. Mol. Med. 2013, 32, 1311–1318. [Google Scholar] [CrossRef] [Green Version]
- Almonte-Becerril, M.; Navarro-Garcia, F.; Gonzalez-Robles, A.; Vega-Lopez, M.A.; Lavalle, C.; Kouri, J.B. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of Osteoarthritis within an experimental model. Apoptosis 2010, 15, 631–638. [Google Scholar] [CrossRef]
- Grillet, B.; Dequeker, J. Intra-articular steroid injection. A risk-benefit assessment. Drug Saf. 1990, 5, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Dehnade, F.; Zafarullah, M. Regulation of tissue inhibitor of metalloproteinases-3 gene expression by transforming growth factor-beta and dexamethasone in bovine and human articular chondrocytes. DNA Cell Biol. 1996, 15, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Busse, P.; Vater, C.; Stiehler, M.; Nowotny, J.; Kasten, P.; Bretschneider, H.; Goodman, S.B.; Gelinsky, M.; Zwingenberger, S. Cytotoxicity of drugs injected into joints in orthopaedics. Bone Jt. Res. 2019, 8, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.A.; Byron, C.R.; Pondenis, H.C.; Stewart, M.C. Effect of dexamethasone supplementation on chondrogenesis of equine mesenchymal stem cells. Am. J. Vet. Res. 2008, 69, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Sirin, D.Y.; Kaplan, N.; Yilmaz, I.; Karaarslan, N.; Ozbek, H.; Akyuva, Y.; Kaya, Y.E.; Oznam, K.; Akkaya, N.; Guler, O.; et al. The association between different molecular weights of hyaluronic acid and CHAD, HIF-1alpha, COL2A1 expression in chondrocyte cultures. Exp. Med. 2018, 15, 4205–4212. [Google Scholar]
- Concoff, A.; Sancheti, P.; Niazi, F.; Shaw, P.; Rosen, J. The efficacy of multiple versus single hyaluronic acid injections: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2017, 18, 542. [Google Scholar] [CrossRef] [Green Version]
- Kraus, V.B.; Stabler, T.V.; Kong, S.Y.; Varju, G.; McDaniel, G. Measurement of synovial fluid volume using urea. Osteoarthr. Cartil. 2007, 15, 1217–1220. [Google Scholar] [CrossRef] [Green Version]
- Responte, D.J.; Natoli, R.M.; Athanasiou, K.A. Identification of potential biophysical and molecular signalling mechanisms underlying hyaluronic acid enhancement of cartilage formation. J. R Soc. Interface 2012, 9, 3564–3573. [Google Scholar] [CrossRef] [Green Version]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Mechanistic biomarkers in acetaminophen-induced hepatotoxicity and acute liver failure: From preclinical models to patients. Expert. Opin. Drug Metab. Toxicol. 2014, 10, 1005–1017. [Google Scholar] [CrossRef] [Green Version]
- Mertz, N.; Larsen, S.W.; Kristensen, J.; Ostergaard, J.; Larsen, C. Long-Acting Diclofenac Ester Prodrugs for Joint Injection: Kinetics, Mechanism of Degradation, and In Vitro Release From Prodrug Suspension. J. Pharm. Sci. 2016, 105, 3079–3087. [Google Scholar] [CrossRef]
- Winek, C.L.; Wahba, W.W.; Winek, C.L., Jr.; Balzer, T.W. Drug and chemical blood-level data 2001. Forensic Sci. Int. 2001, 122, 107–123. [Google Scholar] [CrossRef]
- Hodgman, M.J.; Garrard, A.R. A review of acetaminophen poisoning. Crit. Care Clin. 2012, 28, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, S.; Ichiyama, H.; Kondo, E.; Yasuda, K. Randomized clinical comparisons of diclofenac concentration in the soft tissues and blood plasma between topical and oral applications. Br. J. Clin. Pharm. 2009, 67, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Blot, L.; Marcelis, A.; Devogelaer, J.P.; Manicourt, D.H. Effects of diclofenac, aceclofenac and meloxicam on the metabolism of proteoglycans and hyaluronan in osteoarthritic human cartilage. Br. J. Pharmacol. 2000, 131, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Qin, X.; Yang, R.; Qin, J.; Li, W.; Luan, K.; Wu, Z.; Song, L. Intra-articular Administration of Chitosan Thermosensitive In Situ Hydrogels Combined With Diclofenac Sodium-Loaded Alginate Microspheres. J. Pharm. Sci. 2016, 105, 122–130. [Google Scholar] [CrossRef]
- Euppayo, T.; Punyapornwithaya, V.; Chomdej, S.; Ongchai, S.; Nganvongpanit, K. Effects of hyaluronic acid combined with anti-inflammatory drugs compared with hyaluronic acid alone, in clinical trials and experiments in osteoarthritis: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2017, 18, 387. [Google Scholar] [CrossRef] [Green Version]
- Siengdee, P.; Radeerom, T.; Kuanoon, S.; Euppayo, T.; Pradit, W.; Chomdej, S.; Ongchai, S.; Nganvongpanit, K. Effects of corticosteroids and their combinations with hyaluronanon on the biochemical properties of porcine cartilage explants. BMC Vet. Res. 2015, 11, 298. [Google Scholar] [CrossRef] [Green Version]
- Tendulkar, G.; Ehnert, S.; Sreekumar, V.; Chen, T.; Kaps, H.P.; Golombek, S.; Wendel, H.P.; Nussler, A.K.; Avci-Adali, M. Exogenous Delivery of Link N mRNA into Chondrocytes and MSCs-The Potential Role in Increasing Anabolic Response. Int. J. Mol. Sci. 2019, 20, 1716. [Google Scholar] [CrossRef] [Green Version]
- Ehnert, S.; van Griensven, M.; Unger, M.; Scheffler, H.; Falldorf, K.; Fentz, A.K.; Seeliger, C.; Schroter, S.; Nussler, A.K.; Balmayor, E.R. Co-Culture with Human Osteoblasts and Exposure to Extremely Low Frequency Pulsed Electromagnetic Fields Improve Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 994. [Google Scholar] [CrossRef] [Green Version]
- Mi, S.; Du, Z.; Xu, Y.; Wu, Z.; Qian, X.; Zhang, M.; Sun, W. Microfluidic co-culture system for cancer migratory analysis and anti-metastatic drugs screening. Sci. Rep. 2016, 6, 35544. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Luyten, F.P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001, 44, 85–95. [Google Scholar] [CrossRef]
- Nadzir, M.M.; Kino-oka, M.; Maruyama, N.; Sato, Y.; Kim, M.H.; Sugawara, K.; Taya, M. Comprehension of terminal differentiation and dedifferentiation of chondrocytes during passage cultures. J. Biosci. Bioeng. 2011, 112, 395–401. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Ehnert, S.; Tendulkar, G.; Zhu, S.; Arnscheidt, C.; Aspera-Werz, R.H.; Nussler, A.K. Primary Human Chondrocytes Affected by Cigarette Smoke—Therapeutic Challenges. Int. J. Mol. Sci. 2020, 21, 1901. https://doi.org/10.3390/ijms21051901
Chen T, Ehnert S, Tendulkar G, Zhu S, Arnscheidt C, Aspera-Werz RH, Nussler AK. Primary Human Chondrocytes Affected by Cigarette Smoke—Therapeutic Challenges. International Journal of Molecular Sciences. 2020; 21(5):1901. https://doi.org/10.3390/ijms21051901
Chicago/Turabian StyleChen, Tao, Sabrina Ehnert, Gauri Tendulkar, Sheng Zhu, Christian Arnscheidt, Romina H. Aspera-Werz, and Andreas K. Nussler. 2020. "Primary Human Chondrocytes Affected by Cigarette Smoke—Therapeutic Challenges" International Journal of Molecular Sciences 21, no. 5: 1901. https://doi.org/10.3390/ijms21051901
APA StyleChen, T., Ehnert, S., Tendulkar, G., Zhu, S., Arnscheidt, C., Aspera-Werz, R. H., & Nussler, A. K. (2020). Primary Human Chondrocytes Affected by Cigarette Smoke—Therapeutic Challenges. International Journal of Molecular Sciences, 21(5), 1901. https://doi.org/10.3390/ijms21051901