Airway Ciliary Beating Affected by the Pcp4 Dose-Dependent [Ca2+]i Increase in Down Syndrome Mice, Ts1Rhr
Abstract
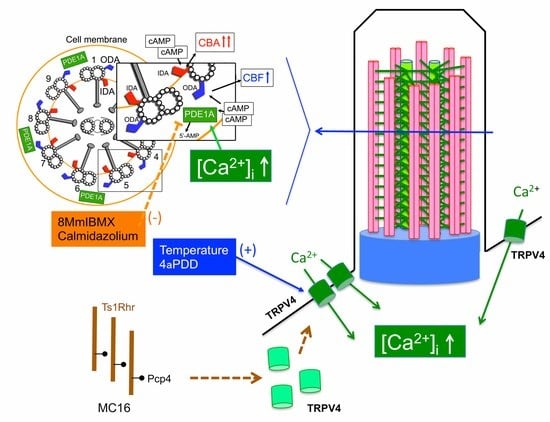
:1. Introduction
2. Results
2.1. CBF and CBA Under Unstimulated Conditions
2.2. Effects of Procaterol on CBF and CBA
2.2.1. Effects of 1 nM Procaterol on CBF and CBA Increase in Wt, Ts1Rhr and Ts1Rhr:Pcp4+/+/-
2.2.2. Effects of 10 nM Procaterol on CBF and CBA Increase in Wt, Ts1Rhr and Ts1Rhr:Pcp4+/+/-
2.3. Effects of PDE1 Inhibition on Procaterol-Stimulated CBF and CBA
2.3.1. Effects of 8MmIBMX on the CBF and CBA Increases Stimulated by 1 nM Procaterol
2.3.2. Effects of Calmidazolium on the CBF and CBA Increases Stimulated by 1 nM Procaterol
2.4. Cyclic AMP Contents in Isolated Lung Cells
2.5. [Ca2+]i of Airway Ciliary Cells Stimulated by Temperature and 4αPDD
3. Discussion
4. Materials and Methods
4.1. Mouse Lines and Genotyping
4.2. Generation of Pcp4 Knockout Mice
4.3. Solution and Chemicals
4.4. Cell Preparation
4.5. Ethical Approval
4.6. CBA and CBF Measurements
4.7. Monitoring of the Intracellular Ca2+ Concentration ([Ca2+]i)
4.8. Measurement of cAMP Contents
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antonarakis, S.E.; Lyle, R.; Dermitzakis, E.T.; Reymond, A.; Deutsch, S. Chromosome 21 and Down syndrome: From genomics to pathophysiology. Nat. Rev. Genet. 2004, 5, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.E.; Mai, C.T.; Canfield, M.A.; Rickard, R.; Wang, Y.; Meyer, R.E.; Anderson, P.; Mason, C.A.; Collins, J.S.; Kirby, R.S.; et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth. Defects Res. A Clin. Mol. Teratol. 2010, 88, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Alsubie, H.S.; Rosen, D. The evaluation and management of respiratory disease in children with Down syndrome (DS). Pediat. Resp. Rev. 2018, 26, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, P.; Navarro, H.; Caussade, S.; Holmgren, N.; Sánchez, I. Airway anomalies in children with Down syndrome: Endoscopic findings. Pediatr. Pulmonol. 2003, 36, 137–141. [Google Scholar] [CrossRef] [PubMed]
- So, S.A.; Urbano, R.C.; Hodapp, R.M. Hospitalizations of infants and young children with Down syndrome: Evidence from inpatient person-records from a statewide administrative database. J. Intellect. Disabil. Res. 2007, 51, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Rasmussen, S.A.; Friedman, J.M. Mortality associated with Down’s syndrome in the USA from 1983 to 1997: A population-based study. Lancet (London) 2002, 359, 1019–1025. [Google Scholar] [CrossRef]
- Cooney, T.P.; Thurlbeck, W.M. Pulmonary hypoplasia in Down’s syndrome. N. Engl. J. Med. 1982, 307, 1170–1173. [Google Scholar] [CrossRef]
- Piatti, G.; Allegra, L.; Ambrosetti, U.; De Santi, M.M. Nasal ciliary function and ultrastructure in Down syndrome. Laryngoscope 2001, 111, 1227–1230. [Google Scholar] [CrossRef]
- Wanner, A.; Salathe, M.; O’Riordan, T.G. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 1996, 154, 1868–1902. [Google Scholar] [CrossRef]
- Lana-Elola, E.; Watson-Scales, S.D.; Fisher, E.M.; Tybulewicz, V.L. Down syndrome: Searching for the genetic culprits. Dis. Model. Mech. 2011, 4, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Das, I.; Reeves, R.H. The use of mouse models to understand and improve cognitive deficits in Down syndrome. Dis. Model. Mech. 2011, 4, 596–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herault, Y.; Delabar, J.M.; Fisher, E.M.C.; Tybulewicz, V.L.; Yu, E.; Brault, V. Rodent models in Down syndrome research: Impact and future opportunities. Dis. Model. Mech 2017, 10, 1165–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakawa, K. Towards the understanding of Down syndrome using mouse models. Congenit. Anom. 2012, 52, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Raveau, M.; Nakahari, T.; Asada, S.; Ishihara, K.; Amano, K.; Shimohata, A.; Sago, H.; Yamakawa, K. Brain ventriculomegaly in Down syndrome mice is caused by Pcp4 dose-dependent cilia dysfunction. Hum. Mol. Genet. 2017, 26, 923–931. [Google Scholar] [CrossRef]
- Ishihara, K.; Kanai, S.; Sago, H.; Yamakawa, K.; Akiba, S. Comparative proteomic profiling reveals aberrant cell proliferation in the brain of embryonic Ts1Cje, a mouse model of Down syndrome. Neuroscience 2014, 281, 1–15. [Google Scholar] [CrossRef]
- Takaki, E.; Fujimoto, M.; Nakahari, T.; Yonemura, S.; Miyata, Y.; Hayashida, N.; Yamamoto, K.; Vallee, R.B.; Mikuriya, T.; Sugahara, K.; et al. Heat shock transcription factor 1 is required for maintenance of ciliary beating in mice. J. Biol. Chem. 2007, 282, 37285–37292. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Miyamoto, H.; Nakahari, T.; Inoue, I.; Suemoto, T.; Jiang, B.; Hirota, Y.; Itohara, S.; Saido, T.C.; Tsumot, T.; et al. Efhc1 deficiency causes spontaneous myoclonus and increased seizure susceptibility. Hum. Mol. Genet. 2009, 18, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Komatani-Tamiya, N.; Daikoku, E.; Takemura, Y.; Shimamoto, C.; Nakano, T.; Iwasaki, Y.; Kohda, Y.; Matsumura, H.; Marunaka, Y.; Nakahari, T. Procaterol-stimulated increases in ciliary bend amplitude and ciliary beat frequency in mouse bronchioles. Cell. Physiol. Biochem. 2012, 29, 511–522. [Google Scholar] [CrossRef]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Shimamoto, C.; Matsumura, H.; Nakano, T.; Sano, K.I.; Inui, T.; Marunaka, Y.; et al. A low [Ca2+]i-induced enhancement of cAMP-activated ciliary beating by PDE1A inhibition in mouse airway cilia. Pflügers Arch. 2017, 469, 1215–1227. [Google Scholar] [CrossRef]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Inui, T.; Marunaka, Y.; Nakahari, T. [Ca(2+) ]i modulation of cAMP-stimulated ciliary beat frequency via PDE1 in airway ciliary cells of mice. Exp. Physiol. 2018, 103, 381–390. [Google Scholar] [CrossRef]
- Kogiso, H.; Ikeuchi, Y.; Sumiya, M.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Inui, T.; Marunaka, Y.; Nakahari, T. Seihai-to (TJ-90)-Induced Activation of Airway Ciliary Beatings of Mice: Ca(2+) Modulation of cAMP-Stimulated Ciliary Beatings via PDE1. Int. J. Mol. Sci. 2018, 19, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brokaw, C.J.; Kamiya, R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskeleton 1987, 8, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Hard, R.; Blaustein, K.; Scarcello, L. Reactivation of outer arm-depleted lung axonemes: Evidence for functional differences between inner and outer dynein arms in situ. Cell Motil. Cytoskeleton 1992, 21, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Chilvers, M.A.; Rutman, A.; O’Callaghan, C. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J. Allergy Clin. Immunol. 2003, 112, 518–524. [Google Scholar] [CrossRef]
- De Iongh, R.U.; Rutland, J. Ciliary defects in healthy subjects, bronchiectasis, and primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 1995, 151, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, I.M.; Liedtke, W.; Sanderson, M.J.; Valverde, A. TRPV4 channel participates in receptor operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Pro. Natl. Acad. Sci. USA 2008, 105, 12611–12616. [Google Scholar] [CrossRef] [Green Version]
- Alenmyr, L.; Uller, L.; Greiff, L.; Hogestatt, E.D.; Zygmunt, P.M. TRPV4-mediated calcium influx and ciliary activity in human native airway epithelial cells. Basic. Clin. Pharmcol. Toxicol. 2014, 114, 210–216. [Google Scholar] [CrossRef]
- White, J.P.M.; Cibelli, M.; Urban, L.; Nilius, B.; McGeown, J.G.; Nagy, I. TRPV4: Molecu;ar conductor of a diverse orchestra. Physiol. Rev. 2016, 96, 911–973. [Google Scholar] [CrossRef] [Green Version]
- Mouton-Liger, F.; Thomas, S.; Rattenbach, R.; Mabnol, L.; Larigaldie, V.; Ledru, A.; Hérault, Y.; Verney, C.; Créau, N. PCP4 (PEP19) overexpression induces premature neuronal differentiation associated with Ca2+/calmodulin-dependent kinase II-δ activation in mouse model of down syndrome. J. Comp. Neurol. 2011, 519, 2779–2802. [Google Scholar] [CrossRef]
- Mouton-Liger, F.; Sahún, I.; Collin, T.; Pereira, P.L.; Masini, D.; Thomas, S.; Paly, E.; Luilier, S.; Même, S.; Jouhault, Q.; et al. Developmental molecular and functional cerebellar alterations inducedby PCP4/PEP19 overexpression: Implications for Down syndrome. Neurobiol. Dis. 2014, 63, 92–106. [Google Scholar] [CrossRef]
- Cabin, D.E.; Gsrdiner, K.; Reeves, R.H. Molecular genetic characterization and comparative mapping of the human PCP4 gene. Somat. Cell. Mol. Genet. 1996, 22, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Putkey, J.A.; Kleerekoper, Q.K.; Gaertner, T.R.; Waxham, M.N. A new role for IQ motif proteins in regulating calmodulin function. J. Biol. Chem. 2003, 278, 49667–49670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putkey, J.A.; Waxham, M.N.; Gaertner, T.R.; Brewer, K.J.; Goldsmith, M.; Kubota, Y.; Kleerekoper, Q.K. Acidic/IQ motif regulator of calmodulin. J. Biol. Chem. 2008, 283, 1401–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L.D.; Willoughby, C.A.; Burke, S.H.; Paik, D.S.; Jenkins, K.J.; Tombes, R.M. delta Ca2+/calmodulin-dependent protein kinase II isozyme-specific induction of neurite out-growth in P19 embryonal cartinoma cells. J Neurochem. 2000, 75, 2380–2391. [Google Scholar] [CrossRef] [PubMed]
- Salathe, M. Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 2007, 69, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Afzelius, B.A. Cilia-related diseases. J. Pathol. 2004, 204, 470–477. [Google Scholar] [CrossRef]
- Horani, A.; Ferkol, T.W.; Dutcher, S.K.; Brody, S.L. Genetics and biology of primary ciliary dyskinesia. Peadiat. Resp. Rev. 2016, 18, 18–24. [Google Scholar]
- Lucas, J.S.; Adam, E.C.; Goggin, P.A.; Jackson, C.L.; Powles-Glover, N.; Patel, S.H.; Humphreys, J.; Fray, M.D.; Falconnet, E.; Blouin, J.-L.; et al. Static respiratory cilia associated with mutations in Dnahc/DNAH11: A mouse model of PCD. Num. Mut. 2012, 33, 495–503. [Google Scholar]
- Lakso, M.; Pichel, J.G.; Gorman, J.R.; Sauer, B.; Okamoto, Y.; Lee, E.; Alt, F.W.; Westphal, H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA 1996, 93, 5860–5865. [Google Scholar] [CrossRef] [Green Version]
- Shiima-Kinoshita, C.; Min, K.-Y.; Hanafusa, T.; Mori, H.; Nakahari, T. β2-adrenergic regulation of ciliary beat frequency in rat bronchiolar epithelium: Potentiation by isosmotic cell shrinkage. J. Physiol. 2004, 554, 403–416. [Google Scholar] [CrossRef]
- Delmotte, P.; Sanderson, M.J. Ciliary beat frequency is main- tained at maximal rate in the small airways of mouse lung slices. Am. J. Respir. Cell Mol. Biol. 2006, 35, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeuchi, Y.; Kogiso, H.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Matsumura, H.; Inui, T.; Marunaka, Y.; Nakahari, T. Calbocisteine stimulated an increase in ciliary bend angle via decrease in [Cl-]i in mouse airway cilia. Pflügers Arch. 2019, 471, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Murakami, K.; Yasuda, M.; Hirano, S.; Ikeuchi, Y.; Kogiso, H.; Hosogi, S.; Inui, T.; Marunaka, Y.; Nakahari, T. Ciliary beating amplitude controlled by intracellular Cl- and a high rate of CO2 production in ciliated human nasal epithelial cells. Pflügers Arch. 2019, 471, 1127–1142. [Google Scholar] [CrossRef] [PubMed]








| CBF (Hz) | CBA (Degree) | |
|---|---|---|
| Wt | 11.1 ± 0.1 (n = 4) | 93.1 ± 1.1 (n = 4) |
| Ts1Rhr | 9.6 ± 0.5 (n = 4) | 90.6 ± 0.6 (n = 4) |
| Ts1Rhr:Pcp4+/+/- | 10.8 ± 0.2 (n = 3) | 92.7 ± 0.1 (n = 3) |
| Proc (1 nM) | CBA Ratio | CBF Ratio (5 min) | CBF ratio (10 min) |
| Wt | 1.64 ± 0.01 (n = 5) | 1.62 ± 0.01 (n = 5) | 1.67 ± 0.02 |
| Ts1Rhr | 1.49 ± 0.04 (n = 5) | 1.33 ± 0.04 (n = 6) | 1.45 ± 0.04 |
| Ts1Rhr:Pcp4+/+/- | 1.44 ± 0.04 (n = 4) | 1.42 ± 0.03 (n = 6) | 1.48 ± 0.03 |
| Proc (10 nM) | CBA ratio | CBF ratio (5 min) | CBF ratio (10 min) |
| Wt | 1.73 ± 0.02 (n = 5) | 2.04 ± 0.05 (n = 4) | 1.96 ± 0.07 |
| Ts1Rhr | 1.59 ± 0.01 (n = 4) | 2.07 ± 0.05 (n = 6) | 2.00 ± 0.05 |
| Proc (1 nM) | τCBA (min) | τCBF (min) |
| Wt | 1.16 ± 0.04 (n = 5) | 4.33 ± 0.43 (n = 5) |
| Ts1Rhr | 1.16 ± 0.06 (n = 5) | * 6.05 ± 0.40 (n = 6) |
| Ts1Rhr:Pcp4+/+/- | 1.16 ± 0.08 (n = 4) | † 4.39 ± 0.56 (n = 6) |
| Proc (10 nM) | τCBA (min) | τCBF (min) |
| Wt | 1.46 ± 0.22 (n = 4) | 2.63 ± 0.17 (n = 5) |
| Ts1Rhr | 1.53 ± 0.07 (n = 4) | 2.52 ± 0.12 (n = 6) |
| 8MmIBMX + Proc (1nM) | τCBA (min) | τCBF (min) |
| Wt | 2.73 ± 0.14 (n = 5) | 2.85 ± 0.20 (n = 4) |
| Ts1Rhr | 2.70 ± 0.09 (n = 5) | 2.83 ± 0.21 (n = 5) |
| Calmidaz + Proc (1 nM) | τCBA (min) | τCBF (min) |
| Wt | 3.21 ± 0.28 (n = 4) | 3.23 ± 0.39 (n = 5) |
| Ts1Rhr | 3.27 ± 0.39 (n = 4) | 3.33 ± 0.55 (n = 5) |
| CBA ratio | CBF Ratio | |||
|---|---|---|---|---|
| 8MmIBMX (40 µM) | Before Proc | Proc (5 min) | Before Proc | Proc (5 min) |
| Wt | 1.21 ± 0.02 (n = 5) | 1.67 ± 0.02 | 1.26 ± 0.03 (n = 4) | 1.71 ± 0.02 |
| Ts1Rhr | 1.20 ± 0.02 (n = 5) | 1.65 ± 0.02 | 1.20 ± 0.02 (n = 5) | 1.65 ± 0.04 |
| Calmidaz (25 µM) | Before Proc | Proc (5 min) | Before Proc | Proc (5 min) |
| Wt | 1.13 ± 0.01 (n = 4) | 1.45 ± 0.02 | 1.13 ± 0.02 (n = 5) | 1.45 ± 0.02 |
| Ts1Rhr | 1.10 ± 0.01 (n = 4) | 1.39 ± 0.02 | 1.40 ± 0.04 (n = 5) | 1.65 ± 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogiso, H.; Raveau, M.; Yamakawa, K.; Saito, D.; Ikeuchi, Y.; Okazaki, T.; Asano, S.; Inui, T.; Marunaka, Y.; Nakahari, T. Airway Ciliary Beating Affected by the Pcp4 Dose-Dependent [Ca2+]i Increase in Down Syndrome Mice, Ts1Rhr. Int. J. Mol. Sci. 2020, 21, 1947. https://doi.org/10.3390/ijms21061947
Kogiso H, Raveau M, Yamakawa K, Saito D, Ikeuchi Y, Okazaki T, Asano S, Inui T, Marunaka Y, Nakahari T. Airway Ciliary Beating Affected by the Pcp4 Dose-Dependent [Ca2+]i Increase in Down Syndrome Mice, Ts1Rhr. International Journal of Molecular Sciences. 2020; 21(6):1947. https://doi.org/10.3390/ijms21061947
Chicago/Turabian StyleKogiso, Haruka, Matthieu Raveau, Kazuhiro Yamakawa, Daichi Saito, Yukiko Ikeuchi, Tomonori Okazaki, Shinji Asano, Toshio Inui, Yoshinori Marunaka, and Takashi Nakahari. 2020. "Airway Ciliary Beating Affected by the Pcp4 Dose-Dependent [Ca2+]i Increase in Down Syndrome Mice, Ts1Rhr" International Journal of Molecular Sciences 21, no. 6: 1947. https://doi.org/10.3390/ijms21061947
APA StyleKogiso, H., Raveau, M., Yamakawa, K., Saito, D., Ikeuchi, Y., Okazaki, T., Asano, S., Inui, T., Marunaka, Y., & Nakahari, T. (2020). Airway Ciliary Beating Affected by the Pcp4 Dose-Dependent [Ca2+]i Increase in Down Syndrome Mice, Ts1Rhr. International Journal of Molecular Sciences, 21(6), 1947. https://doi.org/10.3390/ijms21061947