Lingonberry (Vaccinium vitis-idaea L.) Fruit as a Source of Bioactive Compounds with Health-Promoting Effects—A Review
Abstract
:1. Introduction
2. Chemical Composition of Lingonberry Fruit
3. Bioavailability of Lingonberry Polyphenols
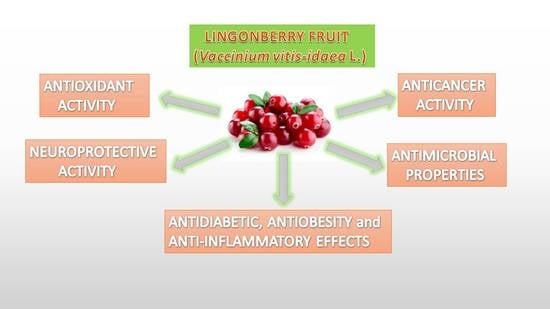
4. Biological Activity and Health-Promoting Effects
4.1. Antioxidant Properties
4.2. Anticancer Activity
4.3. Neuroprotective Activity
4.4. Antidiabetic, Antiobesity and Anti-Inflammatory Effects
4.5. Antimicrobial Properties
4.6. Antioxidant Capacity and Bioactive Compounds Content in Lingonberry Food Products
5. Conclusions
Funding
Conflicts of Interest
References
- Mane, C.; Loonis, M.; Juhel, C.; Dufour, C.; Malien-Aubert, C. Food Grade Lingonberry Extract: Polyphenolic Composition and In Vivo Protective Effect against Oxidative Stress. J. Agric. Food Chem. 2011, 59, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, B.A. Genetic variation in horticulturally important traits of fifteen wild lingonberry Vaccinium vitis-idaea L. populations. Euphytica 2001, 120, 173–182. [Google Scholar] [CrossRef]
- Dróżdż, P.; Šėžienė, V.; Wójcik, J.; Pyrzyńska, K. Evaluation of Bioactive Compounds, Minerals and Antioxidant Activity of Lingonberry (Vaccinium vitis-idaea L.) Fruits. Molecules 2017, 23, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szakiel, A.; Pączkowski, C.; Koivuniemi, H.; Huttunen, S. Comparison of the Triterpenoid Content of Berries and Leaves of Lingonberry Vaccinium vitis-idaea from Finland and Poland. J. Agric. Food Chem. 2012, 60, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, A.S.; Siltari, A.; Ehlers, P.I.; Korpela, R.; Vapaatalo, H. Lingonberry juice negates the effects of a high salt diet on vascular function and low-grade inflammation. J. Funct. Foods 2014, 7, 238–245. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Zielińska-Wasielica, J.; Olkowicz, M. Inhibitory effects of lingonberry (Vaccinium vitis-idaea L.) fruit extract on obesity-induced inflammation in 3T3-L1 adipocytes and RAW 264.7 macrophages. J. Funct. Foods 2019, 54, 371–380. [Google Scholar] [CrossRef]
- McDougall, G.J.; Ross, H.A.; Ikeji, M.; Stewart, D. Berry Extracts Exert Different Antiproliferative Effects against Cervical and Colon Cancer Cells Grown in Vitro. J. Agric. Food Chem. 2008, 56, 3016–3023. [Google Scholar] [CrossRef]
- Olsson, M.E.; Gustavsson, K.E.; Andersson, S.; Nilsson, A.; Duan, R.D. Inhibition of Cancer Cell Proliferation in Vitro by Fruit and Berry Extracts and Correlations with Antioxidant Levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef]
- Esposito, D.; Overall, J.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Alaskan Berry Extracts Promote Dermal Wound Repair Through Modulation of Bioenergetics and Integrin Signaling. Front. Pharmacol. 2019, 10, 1058. [Google Scholar] [CrossRef]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Pilon, G.; Dudonné, S.; Trottier, J.; St-Pierre, P.; Harris, C.S.; Lucas, M.; Lemire, M.; et al. Arctic berry extracts target the gut–liver axis to alleviate metabolic endotoxaemia, insulin resistance and hepatic steatosis in diet-induced obese mice. Diabetologia 2018, 61, 919–931. [Google Scholar] [CrossRef] [Green Version]
- Eid, H.M.; Ouchfoun, M.; Brault, A.; Vallerand, D.; Musallam, L.; Arnason, J.T.; Haddad, P.S. Lingonberry (Vaccinium vitis-idaea L.) Exhibits Antidiabetic Activities in a Mouse Model of Diet-Induced Obesity. Evid. Based Complement. Altern. Med. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Heyman, L.; Axling, U.; Blanco, N.; Sterner, O.; Holm, C.; Berger, K. Evaluation of Beneficial Metabolic Effects of Berries in High-Fat Fed C57BL/6J Mice. J. Nutr. Metab. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Shea, E.; Daneshtalab, M.; Weber, J.T. Chemical Analysis of Extracts from Newfoundland Berries and Potential Neuroprotective Effects. Antioxidants 2016, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Reichert, K.P.; Schetinger, M.R.C.; Gutierres, J.M.; Pelinson, L.P.; Stefanello, N.; Dalenogare, D.P.; Baldissarelli, J.; Lopes, T.F.; Morsch, V.M. Lingonberry Extract Provides Neuroprotection by Regulating the Purinergic System and Reducing Oxidative Stress in Diabetic Rats. Mol. Nutr. Food Res. 2018, 62, e1800050. [Google Scholar] [CrossRef]
- Alam, Z.; Roncal, J.; Peña-Castillo, L. Genetic variation associated with healthy traits and environmental conditions in Vaccinium vitis-idaea. BMC Genom. 2018, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Finn, C.E. Lingonberry (Vaccinium vitis-idaea L.) grown in the Pacific Northwest of North America: Anthocyanin and free amino acid composition. J. Funct. Foods 2012, 4, 213–218. [Google Scholar] [CrossRef]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of Phenolic Compounds from Lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Esposito, D.; Dunlap, K.L.; Lila, M.A. Comparative Analysis of Phenolic Content and Profile, Antioxidant Capacity, and Anti-inflammatory Bioactivity in Wild Alaskan and CommercialVacciniumBerries. J. Agric. Food Chem. 2014, 62, 4007–4017. [Google Scholar] [CrossRef] [Green Version]
- Dróżdż, P.; Šėžienė, V.; Pyrzynska, K. Phytochemical Properties and Antioxidant Activities of Extracts from Wild Blueberries and Lingonberries. Plant Foods Hum. Nutr. 2017, 72, 360–364. [Google Scholar] [CrossRef] [Green Version]
- Bhullar, K.S.; Rupasinghe, H.V. Antioxidant and cytoprotective properties of partridgeberry polyphenols. Food Chem. 2015, 168, 595–605. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of Anthocyanins and Ellagitannins in Selected Foods Consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; Leppänen, T.; Welling, J.; Moilanen, E.; Heinonen, M. Lingonberry (Vaccinium vitis-idaea) and European Cranberry (Vaccinium microcarpon) Proanthocyanidins: Isolation, Identification, and Bioactivities. J. Agric. Food Chem. 2011, 59, 3373–3384. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Peiretti, P.G. Identification of Polyphenolic Compounds in Edible Wild Fruits Grown in the North-West of Italy by Means of HPLC-DAD-ESI HRMS. Plant Foods Hum. Nutr. 2020, 75, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Bujor, O.-C.; Ginies, C.; Popa, V.I.; Dufour, C. Phenolic compounds and antioxidant activity of lingonberry (Vaccinium vitis-idaea L.) leaf, stem and fruit at different harvest periods. Food Chem. 2018, 252, 356–365. [Google Scholar] [CrossRef]
- Brown, E.M.; Nitecki, S.; Pereira-Caro, G.; McDougall, G.J.; Stewart, D.; Rowland, I.; Crozier, A.; Gill, C.I. Comparison ofin vivoandin vitrodigestion on polyphenol composition in lingonberries: Potential impact on colonic health. BioFactors 2014, 40, 611–623. [Google Scholar] [CrossRef]
- Lehtonen, H.-M.; Rantala, M.; Suomela, J.-P.; Viitanen, M.; Kallio, H. Urinary Excretion of the Main Anthocyanin in Lingonberry (Vaccinium vitis-idaea), Cyanidin 3-O-Galactoside, and Its Metabolites. J. Agric. Food Chem. 2009, 57, 4447–4451. [Google Scholar] [CrossRef]
- Lehtonen, H.-M.; Lindstedt, A.; Järvinen, R.; Sinkkonen, J.; Graça, G.; Viitanen, M.; Kallio, H.; Gil, A.M. 1H NMR-based metabolic fingerprinting of urine metabolites after consumption of lingonberries (Vaccinium vitis-idaea) with a high-fat meal. Food Chem. 2013, 138, 982–990. [Google Scholar] [CrossRef]
- Erlund, I.; Marniemi, J.; Hakala, P.; Alfthan, G.; Meririnne, E.; Aro, A. Consumption of black currants, lingonberries and bilberries increases serum quercetin concentrations. Eur. J. Clin. Nutr. 2003, 57, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Lehtonen, H.-M.; Lehtinen, O.; Suomela, J.-P.; Viitanen, M.; Kallio, H. Flavonol Glycosides of Sea Buckthorn (Hippophaë rhamnoides ssp. sinensis) and Lingonberry (Vaccinium vitis-idaea) Are Bioavailable in Humans and Monoglucuronidated for Excretion. J. Agric. Food Chem. 2010, 58, 620–627. [Google Scholar] [CrossRef]
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Koli, R.; Erlund, I.; Jula, A.; Marniemi, J.; Mattila, P.; Alfthan, G. Bioavailability of Various Polyphenols from a Diet Containing Moderate Amounts of Berries. J. Agric. Food Chem. 2010, 58, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, T.; Mursu, J.; Heinonen, M.; Nurmi, A.; Hiltunen, R.; Voutilainen, S. Metabolism of Berry Anthocyanins to Phenolic Acids in Humans. J. Agric. Food Chem. 2009, 57, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Dinstel, R.R.; Cascio, J.; Koukel, S. The antioxidant level of Alaska’s wild berries: High, higher and highest. Int. J. Circumpolar Health 2013, 72, 72. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Feng, R.; Bowman, L.; Penhallegon, R.; Ding, M.; Lu, Y. Antioxidant Activity in Lingonberries (Vaccinium vitis-idaea L.) and Its Inhibitory Effect on Activator Protein-1, Nuclear Factor-κB, and Mitogen-Activated Protein Kinases Activation. J. Agric. Food Chem. 2005, 53, 3156–3166. [Google Scholar] [CrossRef]
- Misikangas, M.; Pajari, A.-M.; Päivärinta, E.; Oikarinen, S.I.; Rajakangas, J.; Marttinen, M.; Tanayama, H.; Törrönen, R.; Mutanen, M. Three Nordic Berries Inhibit Intestinal Tumorigenesis in Multiple Intestinal Neoplasia/+ Mice by Modulating β-Catenin Signaling in the Tumor and Transcription in the Mucosa. J. Nutr. 2007, 137, 2285–2290. [Google Scholar] [CrossRef] [Green Version]
- Hoornstra, D.; Vesterlin, J.; Pärnänen, P.; Al-Samadi, A.; Zlotogorski-Hurvitz, A.; Vered, M.; Salo, T. Fermented Lingonberry Juice Inhibits Oral Tongue Squamous Cell Carcinoma Invasion In Vitro Similarly to Curcumin. In Vivo 2018, 32, 1089–1095. [Google Scholar] [CrossRef] [Green Version]
- Vauzour, D. Dietary Polyphenols as Modulators of Brain Functions: Biological Actions and Molecular Mechanisms Underpinning Their Beneficial Effects. Oxidative Med. Cell. Longev. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bhullar, K.S.; Rupasinghe, H.V. Partridgeberry polyphenols protect primary cortical and hippocampal neurons against β-amyloid toxicity. Food Res. Int. 2015, 74, 237–249. [Google Scholar] [CrossRef]
- Kowalska, K.; Dembczyński, R.; Gołąbek, A.; Olkowicz, M.; Olejnik, A. ROS Modulating Effects of Lingonberry (Vaccinium vitis-idaea L.) Polyphenols on Obese Adipocyte Hypertrophy and Vascular Endothelial Dysfunction. Nutrients 2021, 13, 885. [Google Scholar] [CrossRef]
- Ho, G.T.T.; Nguyen, T.K.Y.; Kase, E.T.; Tadesse, M.; Barsett, H.; Wangensteen, H. Enhanced Glucose Uptake in Human Liver Cells and Inhibition of Carbohydrate Hydrolyzing Enzymes by Nordic Berry Extracts. Molecules 2017, 22, 1806. [Google Scholar] [CrossRef] [Green Version]
- Podsędek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In Vitro Inhibitory Effect on Digestive Enzymes and Antioxidant Potential of Commonly Consumed Fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef]
- Ryyti, R.; Hämäläinen, M.; Peltola, R.; Moilanen, E. Beneficial effects of lingonberry (Vaccinium vitis-idaea L.) supplementation on metabolic and inflammatory adverse effects induced by high-fat diet in a mouse model of obesity. PLoS ONE 2020, 15, e0232605. [Google Scholar] [CrossRef]
- Marungruang, N.; Kovalenko, T.; Osadchenko, I.; Voss, U.; Huang, F.; Burleigh, S.; Ushakova, G.; Skibo, G.; Nyman, M.; Prykhodko, O.; et al. Lingonberries and their two separated fractions differently alter the gut microbiota, improve metabolic functions, reduce gut inflammatory properties, and improve brain function in ApoE−/− mice fed high-fat diet. Nutr. Neurosci. 2018, 23, 600–612. [Google Scholar] [CrossRef] [Green Version]
- Al Hamimi, S.; Heyman-Lindén, L.; Plaza, M.; Turner, C.; Berger, K.; Spégel, P. Alterations in the plasma metabolite profile associated with improved hepatic function and glycemia in mice fed lingonberry supplemented high-fat diets. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Kivimäki, A.S.; Ehlers, P.I.; Siltari, A.; Turpeinen, A.M.; Vapaatalo, H.; Korpela, R. Lingonberry, cranberry and blackcurrant juices affect mRNA expressions of inflammatory and atherothrombotic markers of SHR in a long-term treatment. J. Funct. Foods 2012, 4, 496–503. [Google Scholar] [CrossRef]
- Matziouridou, C.; Marungruang, N.; Nguyen, T.D.; Nyman, M.; Fåk, F. Lingonberries reduce atherosclerosis inApoe-/-mice in association with altered gut microbiota composition and improved lipid profile. Mol. Nutr. Food Res. 2016, 60, 1150–1160. [Google Scholar] [CrossRef]
- Heyman-Lindén, L.; Kotowska, D.; Sand, E.; Bjursell, M.; Plaza, M.; Turner, C.; Holm, C.; Fåk, F.; Berger, K. Lingonberries alter the gut microbiota and prevent low-grade inflammation in high-fat diet fed mice. Food Nutr. Res. 2016, 60, 29993. [Google Scholar] [CrossRef] [Green Version]
- Anhê, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with dietary polyphenols: A new weapon to combat the metabolic syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Cioch, M.; Satora, P.; Skotniczny, M.; Semik-Szczurak, D.; Tarko, T. Characterisation of Antimicrobial Properties of Extracts of Selected Medicinal Plants. Pol. J. Microbiol. 2017, 66, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.Y.; Tsai, C.C.; Huang, J.S.; Chen, C.P.; Lin, T.C.; Lin, C.C. Antimicrobial activity of tannin components from Vaccinium vitis-idaea L. J. Pharm. Pharmacol. 2001, 53, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Kokubu, E.; Kinoshita, E.; Ishihara, K. Inhibitory Effects of Lingonberry Extract on Oral Streptococcal Biofilm Formation and Bioactivity. Bull. Tokyo Dent. Coll. 2019, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pärnänen, P.; Nikula-Ijäs, P.; Sorsa, T. Antimicrobial and Anti-inflammatory Lingonberry Mouthwash—A Clinical Pilot Study in the Oral Cavity. Microorganisms 2019, 7, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pärnänen, P.; Nawaz, A.; Sorsa, T.; Meurman, J.; Nikula-Ijäs, P. The Effect of Fermented Lingonberry Juice on Candida glabrata Intracellular Protein Expression. Int. J. Dent. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Ermis, E.; Hertel, C.; Schneider, C.; Carle, R.; Stintzing, F.; Schmidt, H. Characterization of in vitro antifungal activities of small and American cranberry (Vaccinium oxycoccos L. and V. macrocarpon Aiton) and lingonberry (Vaccinium vitis-idaea L.) concentrates in sugar reduced fruit spreads. Int. J. Food Microbiol. 2015, 204, 111–117. [Google Scholar] [CrossRef]
- Nikolaeva-Glomb, L.; Mukova, L.; Nikolova, N.; Badjakov, I.; Dincheva, I.; Kondakova, V.; Doumanova, L.; Galabov, A.S. In Vitro Antiviral Activity of a Series of Wild Berry Fruit Extracts against Representatives of Picorna-, Orthomyxo- and Paramyxoviridae. Nat. Prod. Commun. 2014, 9, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Häkkinen, S.H.; Kärenlampi, S.O.; Mykkänen, H.M.; Törrönen, A.R. Influence of Domestic Processing and Storage on Flavonol Contents in Berries. J. Agric. Food Chem. 2000, 48, 2960–2965. [Google Scholar] [CrossRef]
| Type and Locality | Total Phenolics (mg/100g FW) | Total Anthocyanins (mg/100g FW) | Ref. |
|---|---|---|---|
| Poland (wild) | 598 | 40.5 | [3,19] |
| Alaska US (wild) | 624 | 194 | [18] |
| Oregon US (cultivated) | 566 | 40 | [16] |
| Source and Treatment | Type of Model | Effects | Reference |
|---|---|---|---|
| Antioxidant Activity | |||
| Freeze-dried lingonberry extract (1–5 mg/mL) | 3T3-L1 adipocytes | ↓ROS production, ↓NOX4, ↑SOD2, ↑GPx, ↑catalase | [6] |
| Lingonberry extract (23 mg/kg of body weight) for 42 days | Rats fed HFD | ↓Total oxidant status, ↑hepatic and erythrocyte SOD, ↑hepatic glutathione reductase, ↓uric acid plasma concentration | [1] |
| Anticancer Activity | |||
| Lingonberry extract (proanthocyanidins) | HeLa and Caco-2 cells | ↓Cancer cell proliferation | [7] |
| Lingonberry extract (quercetin, quercetin glycosides, benzoic acid, ellagic acid) and anthocyanin fraction | HT-29 and MCF-7 cells | ↓Cancer cell proliferation | [8] |
| Fermented lingonberry juice (2.5–5.0 mg/mL) | HSC-3 and SCC-25 cells | Anti-proliferative and anti- invasive effect | [37] |
| Lingonberry extract (0.28 mg/g anthocyanins, 0.95 mg/g phenolics) | JB6 P+ mouse epidermal cells | ↓AP-1 and NF-kB activity, ↓MAPK phosphorylation, ↓ERK1, ↓ERK2, ↓p38, and ↓MEK1/2 kinase | [35] |
| Lingonberry extract | HL-60 cells | Induced cell apoptosis | [35] |
| Freeze-dried lingonberry (10% w/w; 472 mg/kg total anthocyanins, 97 mg/kg total flavonols) | Mice fed HFD | ↓Intestinal adenomas formation, ↓ tumor number and size, ↓ADA and 5-NT expression, ↓cyclin D1, ↓PGE2 | [36] |
| Neuroprotective Activity | |||
| Lingonberry extract (1 µL) | Cortical cell cultures from neonatal rat pups | Protected from cells injury | [13] |
| Lingonberry polyphenol fraction | Primary cortical and hippocampal neurons | ↓β-Amyloid levels, ↓ AChE activity, ↓ Apoptotic caspases | [39] |
| Lingonberry extract for 30 days | Diabetic rats | ↑NTPDase activity, restored density of purinergic receptors, ↓RS, ↓ TBARS | [14] |
| Antidiabetic, antiobesity and anti-inflammatory activity | |||
| Freeze-dried lingonberry extract (1–5 mg/mL) | 3T3-L1 adipocytes | ↓IL-6, ↓MCP-1, ↓IL-1β, ↓leptin, ↑IL-10, ↑adiponectin | [6] |
| Lingonberry anthocyanin and non-anthocyanin polyphenol fractions (5–20 µg/mL) | 3T3-L1 adipocytes | ↓Lipid accumulation, ↓TG content, ↓aP2, ↓FAS, ↓DGAT1 | [40] |
| Lingonberry non-anthocyanin polyphenol fraction (0.1–10 µg/mL) | HUVECs | ↓IL-6, ↓IL-1), ↓VCAM-1, ↓ICAM-1, ↓SELE | [40] |
| Freeze-dried lingonberry extract (0.05–1 mg/mL) | RAW 264.7 macrophages | ↓IL-6, ↓TNF-α, ↓IL-1β, ↓MCP-1, ↓COX-2, ↓iNOS, ↓NO generation | [6] |
| Lingonberry crude extract and polyphenol-rich fraction | RAW 264.7 macrophages | ↓IL-6, ↓IL-1β, ↓COX-2, ↓iNOS, | [18] |
| Lingonberry extract (12.5–50 µg/mL, benzoic acids) | HepG2 cells | ↑Glucose uptake, ↓ α-glucosidase and ↓α-amylase activity | [41] |
| Freeze-dried lingonberry extract (20% w/w) for 13 weeks | C57BL/6J mice fed HFD | ↓Body fat and hepatic lipid, ↓fasting insulin, ↓PAI-1 and ↓ALT plasma levels, ↓total cholesterol | [12] |
| Lingonberry (freeze-dried) ethanol extract (125, 250, 500 mg/kg) for 8 weeks | Mice with diet-induced obesity (DIO) | ↓Blood glucose levels, ↓hepatic steatosis, ↓hyperlipidemia, ↓liver triglyceride, ↓total plasma cholesterol, ↓LDL level, ↑GLUT4 expression, ↑AMPK phosphorylation | [11] |
| Air-dried lingonberry powder (20% w/w) for 6 weeks | C57BL/6J mice fed HFD | ↓Weight gain, ↓epididymal fat, ↓blood cholesterol, ↓glucose level, ↓leptin, ↑adiponectin, ↓inflammatory markers (SAA) | [43] |
| Lingonberry fruit and insoluble fraction of lingonberries for 8 weeks | ApoE−/− mice fed HFD | ↓Weight gain and fat deposition, ↑HDL cholesterol, changed the cecal microbiota composition (↓Mucispillirum, ↑Akkermansia) | [44] |
| Lingonberry extract (200 mg extract/kg body weight) for 8 weeks | C57BL/6J mice fed HFHS | ↓Fasting and postprandial hyperinsulinaemia, ↑insulin sensitivity, ↓metabolic endotoxaemia, ↓intestinal inflammation (↑Akkermansia, ↑Turicibacter, ↑ Oscillibacter) | [10] |
| Freeze-dried lingonberry (20% w/w) | Mice fed HFD | ↑Glycemia and liver function, ↑PC, ↑LPC, ↓serine, ↓SPH | [45] |
| Lingonberry juice for 10 weeks | Rats fed high salt diet | ↓Biomarkers of low-grade inflammation, ↓COX-2 expression | [5] |
| Lingonberry juice for 8 weeks | SHR rats | ↓ACE1, ↓COX-2, ↓P-selectin, ↓MCP-1, ↓VCAM-1, ↓angiotensin II | [46] |
| Dried lingonberry fruit (44% in diet) for 8 weeks | Apoe-/- mice fed HFD | ↓Body weight gain, ↓liver and epididymal fat weights, ↓atherosclerotic plaques, ↓total cholesterol, ↓LDL-VLDL, ↑HDLcholesterol, change in cecal microbiota (↑Bacteroidetes, ↓Firmicutes) | [47] |
| Freeze-dried lingonberry (20% w/w) for 11 weeks | C57BL/6J mice fed HFD | ↓Low-grade inflammation, ↓endotoxemia, change in gut microbiota (↑Bacteroidetes, ↓Firmicutes) | [48] |
| Source | Microorganism | Effects | Reference |
|---|---|---|---|
| Tannins from lingonberries | Porphyromonas gingivalis Prevotella intermedia | Inhibited bacteria growth with MIC 25 µg/mL | [51] |
| Lingonberry polyphenol rich fraction (586.8 mg/g total polyphenols and 374.6 mg/g total flavanols) | Streptococcus mutans, Streptococcus sobrinus, Streptococcus sanguinis | Reduced biofilm formation | [52] |
| Fermented lingonberry juice (mouthwasch) | Streptococcus mutans Candida | Reduced visible plaque index and bleeding | [53] |
| Lingonberry extracts (methanol, ethanol, water) | E. coli, M. luteus, P. putida, P. myxofaciens, Clostridium sp., Bifidobacterium sp., A. niger | Inhibited bacteria growth (MIC 2–4 mg/mL) | [50] |
| Fermented lingonberry juice | Candida glabrata | Reduced biofilm formation | [54] |
| Comercial lingonberry concentrate (benzoic acid, p-hydroxybenzoic acid) | Absidia glauca, Penicillium brevicompactum, Saccharomyces cerevisiae Zygosaccharomyces bailii, Penicillium and Eurotium | Inhibited the growth of fungi (3–24% lingonberry conc.) | [55] |
| Methanol extract from freeze-dried lingonberries (flavonoid and phenolic fractions) | Coxsackievirus B1 (CV-B1) and influenza virus A/H3N2 | Inhibited viruses replication (IC50 100–800 µg/mL) | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, K. Lingonberry (Vaccinium vitis-idaea L.) Fruit as a Source of Bioactive Compounds with Health-Promoting Effects—A Review. Int. J. Mol. Sci. 2021, 22, 5126. https://doi.org/10.3390/ijms22105126
Kowalska K. Lingonberry (Vaccinium vitis-idaea L.) Fruit as a Source of Bioactive Compounds with Health-Promoting Effects—A Review. International Journal of Molecular Sciences. 2021; 22(10):5126. https://doi.org/10.3390/ijms22105126
Chicago/Turabian StyleKowalska, Katarzyna. 2021. "Lingonberry (Vaccinium vitis-idaea L.) Fruit as a Source of Bioactive Compounds with Health-Promoting Effects—A Review" International Journal of Molecular Sciences 22, no. 10: 5126. https://doi.org/10.3390/ijms22105126
APA StyleKowalska, K. (2021). Lingonberry (Vaccinium vitis-idaea L.) Fruit as a Source of Bioactive Compounds with Health-Promoting Effects—A Review. International Journal of Molecular Sciences, 22(10), 5126. https://doi.org/10.3390/ijms22105126