Cardiovascular Disorders Triggered by Obstructive Sleep Apnea—A Focus on Endothelium and Blood Components
Abstract
:1. Introduction
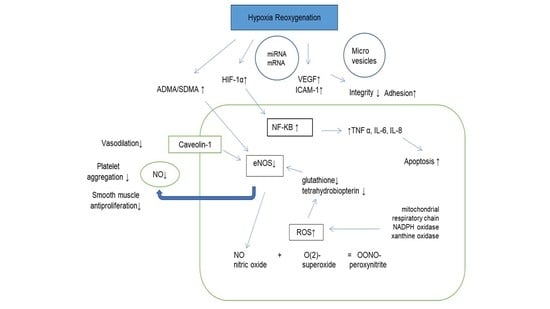
2. Molecular Consequences of Hypoxia/Reoxygenation (H/R) on Endothelial Function in OSA before CPAP Treatment
3. Endothelial Function after Treatment with CPAP
4. Endothelial Function after Treatment with CPAP in Specific Subgroups
4.1. Atherosclerosis
4.2. Myocardial Infarction
4.3. Heart Function
4.4. Diabetes
4.5. Hypertension
4.6. Pulmonary Hypertension
4.7. Atrial Fibrillation and Other Arrhythmias
4.8. Pediatric Population—Effect of Tonsillectomy
| OSA Subpopulation | Demonstrated Molecular Pathomechanism | Effect of OSA Treatment |
|---|---|---|
| Atherosclerosis | endothelial dysfunction | No effect on endothelium, did not reduced PWV [44] |
| (2019, clinical trial, 101 patients) | ||
| Myocardial Infarction | Increased peroxynitrite formation [53], nitration of cardiac contractile proteins (MLC1 and MLC2) and their subsequent degradation (by MMP-2) protective role of TIMP-4 in ischemic preconditioning [54,55,56] | The ISAACC—among non-sleepy patients with acute coronary syndrome, treatment with CPAP did not significantly reduce the prevalence of acute coronary syndromes [58]. |
| (2020, randomized controlled trial, 1264 subjects) | ||
| On the contrary, RICCADSA trial confirmed that CPAP treatment may reduce this risk, if the device is used at least 4 h/day [59] | ||
| (2016, randomized controlled trial, 244 subjects) | ||
| Heart Failure | Increased peroxynitrite formation [53] | No effect on endothelium, lowering the left ventricle end-diastolic volume [61] |
| (2020, randomized controlled trial, 141 patients) | ||
| Diabetes | impairment of the NO bioavailability, ROS | improved HOMA index, no effect on adipokine level [67] |
| (2015, meta-analysis) | ||
| Hypertension | activation of RAAS | SBP—2.32 mm Hg [74] |
| (2015, meta-analysis, 794 patients) | ||
| Pulmonary Hypertension | Increased inflammatory cytokines [81] | decrease in pulmonary artery pressure [83] |
| (2010, metanalysis, 222patients) | ||
| Atrial Fibrillation | down-regulation connexin-43 [86] | (HR 0.41) the probability of AF recurrences [91] |
| (2012, prospective study, 153 patients) | ||
| Children | decreased eNOS expression [96] | FMD improvement after tonsillectomy [95] |
| (2015, clinical trial, 144 patients) |
5. Platelet Function
Hypercoagulative State in OSA
| Demonstrated Platelet-Derived Molecular Pathomechanism | Thromboembolic Disorders | Clinical Effect of OSA Treatment-Based on Clinical Studies |
|---|---|---|
| -platelet hyperaggregability for both ADP and collagen | Stroke | Reduction in risk of stroke in elderly patients [110] (2021, retrospective cohort study, 5757 patients) |
| -lower inhibitory rate of the ADP-dependent aggregation | Myocardial Infarction | No significant effect in the MI incidence reduction in prospective observation of the CPAP treatment patients [58] (2020, randomized controlled trial, 2551 patients), [111] (2016, randomized controlled trial, 2717 patients) |
| -high residual platelet reactivity after acetylsalicylic acid or clopidogrel therapy [98] |
6. Erythrocytes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Sethi, R.; Li, R.; Ho, H.H.; Hein, T.; Jim, M.H.; Loo, G.; Koo, C.Y.; Gao, X.F.; Chandra, S.; et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation 2016, 133, 2008–2017. [Google Scholar] [CrossRef] [Green Version]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Kooy, N.W.; Lewis, S.J.; Royall, J.A.; Ye, Y.Z.; Kelly, D.R.; Beckman, J.S. Extensive tyrosine nitration in human myocardial inflammation: Evidence for the presence of peroxynitrite. Crit. Care Med. 1997, 25, 812–819. [Google Scholar] [CrossRef]
- Roggensack, A.M.; Zhang, Y.; Davidge, S.T. Evidence for Peroxynitrite Formation in the Vasculature of Women with Preeclampsia. Hypertension 1999, 33, 83–89. [Google Scholar] [CrossRef]
- Ohmori, H.; Kanayama, N. Immunogenicity of an inflammation-associated product, tyrosine nitrated self-proteins. Autoimmun. Rev. 2005, 4, 224–229. [Google Scholar] [CrossRef]
- De Pascali, F.; Hemann, C.; Samons, K.; Chen, C.A.; Zweier, J.L. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation. Biochemistry 2014, 53, 3679–3688. [Google Scholar] [CrossRef]
- Alonso-Fernandez, A.; Garcia-Rio, F.; Arias, M.A.; Hernanz, A.; de la Pena, M.; Pierola, J.; Barcelo, A.; Lopez-Collazo, E.; Agusti, A. Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: A randomised trial. Thorax 2009, 64, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Tuleta, I.; França, C.N.; Wenzel, D.; Fleischmann, B.; Nickenig, G.; Werner, N.; Skowasch, D. Intermittent Hypoxia Impairs Endothelial Function in Early Preatherosclerosis. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2015; Volume 858, pp. 1–7. [Google Scholar]
- Wang, J.; Chen, S.; Ma, X.; Cheng, C.; Xiao, X.; Chen, J.; Liu, S.; Zhao, B.; Chen, Y. Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis. Oxid. Med. Cell. Longev. 2013, 2013, 572729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shang, M.; Zhang, M.; Wang, Y.; Chen, Y.; Wu, Y.; Liu, M.; Song, J.; Liu, Y. Microvesicles derived from hypoxia/reoxygenation-treated human umbilical vein endothelial cells promote apoptosis and oxidative stress in H9c2 cardiomyocytes. BMC Cell Biol. 2016, 17, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, R.; Khalyfa, A.; Khalyfa, A.A.; Mokhlesi, B.; Kheirandish-Gozal, L.; Almendros, I.; Peris, E.; Malhotra, A.; Gozal, D. Exosomal cargo properties, endothelial function and treatment of obesity hypoventilation syndrome: A proof of concept study. J. Clin. Sleep Med. 2018, 14, 797–807. [Google Scholar] [CrossRef] [Green Version]
- Priou, P.; Gagnadoux, F.; Tesse, A.; Mastronardi, M.L.; Agouni, A.; Meslier, N.; Racineux, J.L.; Martinez, M.C.; Trzepizur, W.; Andriantsitohaina, R. Endothelial dysfunction and circulating microparticles from patients with obstructive sleep apnea. Am. J. Pathol. 2010, 177, 974–983. [Google Scholar] [CrossRef]
- Sharma, P.; Dong, Y.; Somers, V.K.; Peterson, T.E.; Zhang, Y.; Wang, S.; Li, G.; Singh, P. Intermittent hypoxia regulates vasoactive molecules and alters insulin-signaling in vascular endothelial cells. Sci. Rep. 2018, 8, 14110. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, E.; Bakker, J.P.; Clarke, D.N.; Csizmadia, E.; Kocher, O.; Veves, A.; Tecilazich, F.; O’Donnell, C.P.; Ferran, C.; Malhotra, A. Molecular Biomarkers of Vascular Dysfunction in Obstructive Sleep Apnea. PLoS ONE 2013, 8, e70559. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Zhang, H.; Li, K.; Wu, H.; Zhan, X.; Fang, F.; Qin, Y.; Wei, Y. ESM-1 promotes adhesion between monocytes and endothelial cells under intermittent hypoxia. J. Cell. Physiol. 2019, 234, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Bai, W.; Liu, Q.; Cui, J.; Zhang, W. Intermittent Hypoxia Enhances THP-1 Monocyte Adhesion and Chemotaxis and Promotes M1 Macrophage Polarization via RAGE. Biomed Res. Int. 2018, 2018, 1650456. [Google Scholar] [CrossRef]
- Chuang, L.P.; Chen, N.H.; Lin, Y.; Ko, W.S.; Pang, J.H.S. Increased MCP-1 gene expression in monocytes of severe OSA patients and under intermittent hypoxia. Sleep Breath. 2016, 20, 425–433. [Google Scholar] [CrossRef]
- Gileles-Hillel, A.; Almendros, I.; Khalyfa, A.; Zhang, S.X.; Wang, Y.; Gozal, D. Early intermittent hypoxia induces proatherogenic changes in aortic wall macrophages in a murine model of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 190, 958–961. [Google Scholar] [CrossRef] [Green Version]
- Gautier-Veyret, E.; Bäck, M.; Arnaud, C.; Belaïdi, E.; Tamisier, R.; Lévy, P.; Arnol, N.; Perrin, M.; Pépin, J.L.; Stanke-Labesque, F. Cysteinyl-leukotriene pathway as a new therapeutic target for the treatment of atherosclerosis related to obstructive sleep apnea syndrome. Pharmacol. Res. 2018, 134, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Głuszko, A.; Cyran, A.; Bednarek-Rajewska, K.; Proczka, R.; Smith, D.F.; Ishman, S.L.; Migacz, E.; Kukwa, W. TLRs and RAGE are elevated in carotid plaques from patients with moderate-to-severe obstructive sleep apnea syndrome. Sleep Breath. 2020, 24, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.; Scharf, S.M. Obstructive sleep apnea, cardiovascular disease, and inflammation—Is NF-κB the key? Sleep Breath. 2007, 11, 69–76. [Google Scholar] [CrossRef]
- Kyotani, Y.; Takasawa, S.; Yoshizumi, M. Proliferative pathways of vascular smooth muscle cells in response to intermittent hypoxia. Int. J. Mol. Sci. 2019, 20, 2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Q.; Zhang, B.; Suo, Y.; Liu, C.; Yang, Q.; Zhang, K.; Yuan, M.; Yuan, M.; Zhang, Y.; Li, G. Intermittent hypoxia mediated by TSP1 dependent on STAT3 induces cardiac fibroblast activation and cardiac fibrosis. eLife 2020, 9, e49923. [Google Scholar] [CrossRef]
- Panoutsopoulos, A.; Kallianos, A.; Kostopoulos, K.; Seretis, C.; Koufogiorga, E.; Protogerou, A.; Trakada, G.; Kostopoulos, C.; Zakopoulos, N.; Nikolopoulos, I. Effect of CPAP treatment on endothelial function and plasma CRP levels in patients with sleep apnea. Med. Sci. Monit. 2012, 18, CR747–CR751. [Google Scholar] [CrossRef] [Green Version]
- Ning, Y.; Zhang, T.S.; Wen, W.W.; Li, K.; Yang, Y.X.; Qin, Y.W.; Zhang, H.N.; Du, Y.H.; Li, L.Y.; Yang, S.; et al. Effects of continuous positive airway pressure on cardiovascular biomarkers in patients with obstructive sleep apnea: A meta-analysis of randomized controlled trials. Sleep Breath. 2019, 23, 77–86. [Google Scholar] [CrossRef]
- Schwarz, E.I.; Puhan, M.A.; Schlatzer, C.; Stradling, J.R.; Kohler, M. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: A systematic review and meta-analysis. Respirology 2015, 20, 889–895. [Google Scholar] [CrossRef]
- Thunström, E.; Glantz, H.; Yucel-Lindberg, T.; Lindberg, K.; Saygin, M.; Peker, Y. Cpap does not reduce inflammatory biomarkers in patients with coronary artery disease and nonsleepy obstructive sleep apnea: A randomized controlled trial. Sleep 2017, 40, zsx157. [Google Scholar] [CrossRef] [Green Version]
- Campos-Rodriguez, F.; Asensio-Cruz, M.I.; Cordero-Guevara, J.; Jurado-Gamez, B.; Carmona-Bernal, C.; Gonzalez-Martinez, M.; Troncoso, M.F.; Sanchez-Lopez, V.; Arellano-Orden, E.; Garcia-Sanchez, M.I.; et al. Effect of continuous positive airway pressure on inflammatory, antioxidant, and depression biomarkers in women with obstructive sleep apnea: A randomized controlled trial. Sleep 2019, 42, zsz145. [Google Scholar] [CrossRef]
- Arias, M.A.; García-Río, F.; Alonso-Fernández, A.; Hernanz, Á.; Hidalgo, R.; Martínez-Mateo, V.; Bartolomé, S.; Rodríguez-Padial, L. CPAP decreases plasma levels of soluble tumour necrosis factor-α receptor 1 in obstructive sleep apnoea. Eur. Respir. J. 2008, 32, 1009–1015. [Google Scholar] [CrossRef] [Green Version]
- Khayat, R.N.; Varadharaj, S.; Porter, K.; Sow, A.; Jarjoura, D.; Gavrilin, M.A.; Zweier, J.L. Angiotensin Receptor Expression and Vascular Endothelial Dysfunction in Obstructive Sleep Apnea. Am. J. Hypertens. 2018, 31, 355–361. [Google Scholar] [CrossRef]
- Chen, W.J.; Liaw, S.F.; Lin, C.C.; Chiu, C.H.; Lin, M.W.; Chang, F.T. Effect of Nasal CPAP on SIRT1 and Endothelial Function in Obstructive Sleep Apnea Syndrome. Lung 2015, 193, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- López-Cano, C.; Rius, F.; Sánchez, E.; Gaeta, A.M.; Betriu, À.; Fernández, E.; Yeramian, A.; Hernández, M.; Bueno, M.; Gutiérrez-Carrasquilla, L.; et al. The influence of sleep apnea syndrome and intermittent hypoxia in carotid adventitial vasa vasorum. PLoS ONE 2019, 14, e0211742. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.C.; Zhang, L.J.; Li, H.; Zeng, H.; Ye, Y.; Wang, T.; Wu, Q.; Chen, L.; Xu, Q.; Zheng, Y.; et al. Impact of continuous positive airway pressure on vascular endothelial growth factor in patients with obstructive sleep apnea: A meta-analysis. Sleep Breath. 2019, 23, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, C.D.; Rossi, V.A.; Santer, P.; Schwarz, E.I.; Stradling, J.R.; Petousi, N.; Kohler, M. Effect of OSA on hypoxic and inflammatory markers during CPAP withdrawal: Further evidence from three randomized control trials. Respirology 2017, 22, 793–799. [Google Scholar] [CrossRef]
- Schulz, R.; Flötotto, C.; Jahn, A.; Eisele, H.J.; Weissmann, N.; Seeger, W.; Rose, F. Circulating adrenomedullin in obstructive sleep apnoea. J. Sleep Res. 2006, 15, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Paz, Y.; Mar, H.L.; Hazen, S.L.; Tracy, R.P.; Strohl, K.P.; Auckley, D.; Bena, J.; Wang, L.; Walia, H.K.; Patel, S.R.; et al. Effect of Continuous Positive Airway Pressure on Cardiovascular Biomarkers: The Sleep Apnea Stress Randomized Controlled Trial. Chest 2016, 150, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Borges, Y.G.; Cipriano, L.H.C.; Aires, R.; Zovico, P.V.C.; Campos, F.V.; de Araújo, M.T.M.; Gouvea, S.A. Oxidative stress and inflammatory profiles in obstructive sleep apnea: Are short-term CPAP or aerobic exercise therapies effective? Sleep Breath. 2020, 24, 541–549. [Google Scholar] [CrossRef]
- Lee, M.Y.K.; Ge, G.; Fung, M.L.; Vanhoutte, P.M.; Mak, J.C.W.; Ip, M.S.M. Low but not high frequency of intermittent hypoxia suppresses endothelium-dependent, oxidative stress-mediated contractions in carotid arteries of obese mice. J. Appl. Physiol. 2018, 125, 1384–1395. [Google Scholar] [CrossRef]
- Bozkurt, H.; Neyal, A.; Geyik, S.; Taysi, S.; Anarat, R.; Bulut, M.; Neyal, A.M. Investigation of the plasma nitrite levels and oxidant-antioxidant status in obstructive sleep apnea syndrome. Noropsikiyatri Ars. 2015, 52, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Cereda, C.W.; Tamisier, R.; Manconi, M.; Andreotti, J.; Frangi, J.; Pifferini, V.; Bassetti, C.L. Endothelial dysfunction and arterial stiffness in ischemic stroke: The role of sleep-disordered breathing. Stroke 2013, 44, 1175–1178. [Google Scholar] [CrossRef] [Green Version]
- Sforza, E.; Millasseau, S.; Hupin, D.; Barthélémy, J.C.; Roche, F. Arterial stiffness alteration and obstructive sleep apnea in an elderly cohort free of cardiovascular event history: The PROOF cohort study. Sleep Breath. 2019, 23, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Joyeux-Faure, M.; Tamisier, R.; Borel, J.C.; Millasseau, S.; Galerneau, L.M.; Destors, M.; Bailly, S.; Pepin, J.L. Contribution of obstructive sleep apnoea to arterial stiffness: A meta-analysis using individual patient data. Thorax 2018, 73, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Gupta, V.; Modi, R.; Munnur, K.; Cameron, J.D.; Seneviratne, S.; Edwards, B.A.; Landry, S.A.; Joosten, S.A.; Hamilton, G.S.; et al. Severe obstructive sleep apnea is associated with significant coronary artery plaque burden independent of traditional cardiovascular risk factors. Int. J. Cardiovasc. Imaging 2019. [Google Scholar] [CrossRef] [PubMed]
- Shpilsky, D.; Erqou, S.; Patel, S.R.; Kip, K.E.; Ajala, O.; Aiyer, A.; Strollo, P.J.; Reis, S.E.; Olafiranye, O. Association of obstructive sleep apnea with microvascular endothelial dysfunction and subclinical coronary artery disease in a community-based population. Vasc. Med. (UK) 2018, 23, 331–339. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.Y.; Kim, N.H.; Abbott, R.D.; Kim, C.; Lee, S.K.; Kim, S.H.; Shin, C. Relationship of obstructive sleep apnoea severity and subclinical systemic atherosclerosis. Eur. Respir. J. 2020, 55, 1900959. [Google Scholar] [CrossRef] [PubMed]
- Somuncu, M.U.; Karakurt, S.T.; Karakurt, H.; Serbest, N.G.; Cetin, M.S.; Bulut, U. The additive effects of OSA and nondipping status on early markers of subclinical atherosclerosis in normotensive patients: A cross-sectional study. Hypertens. Res. 2019, 42, 195–203. [Google Scholar] [CrossRef]
- Kim, J.; Mohler, E.R.; Keenan, B.T.; Maislin, D.; Arnardottir, E.S.; Gislason, T.; Benediktsdottir, B.; Gudmundsdottir, S.; Sifferman, A.; Staley, B.; et al. Carotid artery wall thickness in obese and nonobese adults with obstructive sleep apnea before and following positive airway pressure treatment. Sleep 2017, 40, zsx126. [Google Scholar] [CrossRef]
- Català, R.; Ferré, R.; Cabré, A.; Girona, J.; Porto, M.; Texidó, A.; Masana, L. Efecto a largo plazo del tratamiento con presión positiva continua de la vía aérea sobre la arteriosclerosis subclínica en el síndrome de apnea-hipopnea durante el sueño. Med. Clin. (Barc.) 2016, 147, 1–6. [Google Scholar] [CrossRef]
- Chen, L.D.; Lin, L.; Lin, X.J.; Ou, Y.W.; Wu, Z.; Ye, Y.M.; Xu, Q.Z.; Huang, Y.P.; Cai, Z.M. Effect of continuous positive airway pressure on carotid intima-media thickness in patients with obstructive sleep apnea: A meta-analysis. PLoS ONE 2017, 12, e0184293. [Google Scholar] [CrossRef] [Green Version]
- Polewicz, D.; Cadete, V.J.J.; Doroszko, A.; Hunter, B.E.; Sawicka, J.; Szczesna-Cordary, D.; Light, P.E.; Sawicki, G. Ischemia induced peroxynitrite dependent modifications of cardiomyocyte MLC1 increases its degradation by MMP-2 leading to contractile dysfunction. J. Cell. Mol. Med. 2011, 15, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Doroszko, A.; Polewicz, D.; Sawicka, J.; Richardson, J.S.; Sawicki, G.; Cheung, P.Y. Cardiac dysfunction in an animal model of neonatal asphyxia is associated with increased degradation of MLC1 by MMP-2. Basic Res. Cardiol. 2009, 104, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Doroszko, A.; Polewicz, D.; Cadete, V.J.J.; Sawicka, J.; Jones, M.; Szczesna-Cordary, D.; Cheung, P.Y.; Sawicki, G. Neonatal asphyxia induces the nitration of cardiac myosin light chain 2 that is associated with cardiac systolic dysfunction. Shock 2010, 34, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Cadete, V.J.J.; Arcand, S.A.; Chaharyn, B.M.; Doroszko, A.; Sawicka, J.; Mousseau, D.D.; Sawicki, G. Matrix metalloproteinase-2 is activated during ischemia/reperfusion in a model of myocardial infarction. Can. J. Cardiol. 2013, 29, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Du, Y.; Jia, L.; Fan, J.; Guo, R.; Ma, X.; Wang, X.; Nie, S.; Wei, Y. Association of C1q/TNF-Related Protein-9 (CTRP9) Level with Obstructive Sleep Apnea in Patients with Coronary Artery Disease. Mediators Inflamm. 2020, 2020, 7281391. [Google Scholar] [CrossRef]
- Sánchez-de-la-Torre, M.; Sánchez-de-la-Torre, A.; Bertran, S.; Abad, J.; Duran-Cantolla, J.; Cabriada, V.; Mediano, O.; Masdeu, M.J.; Alonso, M.L.; Masa, J.F.; et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): A randomised controlled trial. Lancet Respir. Med. 2020, 8, 359–367. [Google Scholar] [CrossRef]
- Peker, Y.; Thunström, E.; Glantz, H.; Eulenburg, C. Effect of Obstructive Sleep Apnea and CPAP Treatment on Cardiovascular Outcomes in Acute Coronary Syndrome in the RICCADSA Trial. J. Clin. Med. 2020, 9, 4051. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Bakker, J.P.; Baltzis, D.; Tecilazich, F.; Chan, R.H.; Manning, W.J.; Neilan, T.G.; Wallace, M.L.; Hudson, M.; Malhotra, A.; Patel, S.R.; et al. The effect of continuous positive airway pressure on vascular function and cardiac structure in diabetes and sleep apnea: A randomized controlled trial0. Ann. Am. Thorac. Soc. 2020, 17, 474–483. [Google Scholar] [CrossRef]
- Maripov, A.; Mamazhakypov, A.; Sartmyrzaeva, M.; Akunov, A.; Muratali Uulu, K.; Duishobaev, M.; Cholponbaeva, M.; Sydykov, A.; Sarybaev, A. Right Ventricular Remodeling and Dysfunction in Obstructive Sleep Apnea: A Systematic Review of the Literature and Meta-Analysis. Can. Respir. J. 2017, 2017, 1587865. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Katikireddy, C.K.; McConnell, M.V.; Kushida, C.; Yang, P.C. Nasal continuous positive airway pressure improves myocardial perfusion reserve and endothelial-dependent vasodilation in patients with obstructive sleep apnea. J. Cardiovasc. Magn. Reson. 2010, 12, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomster, H.; Laitinen, T.; Lyyra-Laitinen, T.; Vanninen, E.; Gylling, H.; Peltonen, M.; Martikainen, T.; Sahlman, J.; Kokkarinen, J.; Randell, J.; et al. Endothelial function is well preserved in obese patients with mild obstructive sleep apnea. Sleep Breath. 2014, 18, 177–186. [Google Scholar] [CrossRef]
- Labarca, G.; Reyes, T.; Jorquera, J.; Dreyse, J.; Drake, L. CPAP in patients with obstructive sleep apnea and type 2 diabetes mellitus: Systematic review and meta-analysis. Clin. Respir. J. 2018, 12, 2361–2368. [Google Scholar] [CrossRef]
- De Lima, A.M.J.; Franco, C.M.R.; de Castro, C.M.M.B.; de Andrade Bezerra, A.; Ataíde, L., Jr.; Halpern, A. Effects of Nasal Continuous Positive Airway Pressure Treatment on Oxidative Stress and Adiponectin Levels in Obese Patients with Obstructive Sleep Apnea. Respiration 2010, 79, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, I.H.; Hoyos, C.M.; Phillips, C.L.; Magalang, U.J. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J. Clin. Sleep Med. 2015, 11, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.H.; Hui, C.K.M.; Lui, M.M.S.; Lam, D.C.L.; Fong, D.Y.T.; Ip, M.S.M. Incident Type 2 Diabetes in OSA and Effect of CPAP Treatment: A Retrospective Clinic Cohort Study. Chest 2019, 156, 743–753. [Google Scholar] [CrossRef]
- Janssen, C.; Pathak, A.; Grassi, G.; Van De Borne, P. Endothelin contributes to the blood pressure rise triggered by hypoxia in severe obstructive sleep apnea. J. Hypertens. 2017, 35, 118–124. [Google Scholar] [CrossRef]
- Hou, H.; Zhao, Y.; Yu, W.; Dong, H.; Xue, X.; Ding, J.; Xing, W.; Wang, W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 010405. [Google Scholar] [CrossRef] [PubMed]
- Daniels, F.; De Freitas, S.; Smyth, A.; Garvey, J.; Judge, C.; Gilmartin, J.J.; Sharif, F. Effects of renal sympathetic denervation on blood pressure, sleep apnoea severity and metabolic indices: A prospective cohort study. Sleep Med. 2017, 30, 180–184. [Google Scholar] [CrossRef] [PubMed]
- De Souza, F.; Muxfeldt, E.S.; Margallo, V.; Cortez, A.F.; Cavalcanti, A.H.; Salles, G.F. Effects of continuous positive airway pressure treatment on aldosterone excretion in patients with obstructive sleep apnoea and resistant hypertension: A randomized controlled trial. J. Hypertens. 2017, 35, 837–844. [Google Scholar] [CrossRef]
- Muxfeldt, E.S.; Margallo, V.; Costa, L.M.S.; Guimarães, G.; Cavalcante, A.H.; Azevedo, J.C.M.; De Souza, F.; Cardoso, C.R.L.; Salles, G.F. Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: A randomized controlled trial. Hypertension 2015, 65, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Fan, J.; Chen, S.; Yin, Y.; Zrenner, B. The Role of Continuous Positive Airway Pressure in Blood Pressure Control for Patients with Obstructive Sleep Apnea and Hypertension: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Hypertens. 2015, 17, 215–222. [Google Scholar] [CrossRef]
- Lei, Q.; Lv, Y.; Li, K.; Ma, L.; Du, G.; Xiang, Y.; Li, X. Efeitos da pressão positiva contínua nas vias aéreas na pressão arterial em pacientes com hipertensão resistente e apneia obstrutiva do sono: Revisão sistemática e meta-análise de seis ensaios clínicos controlados aleatórios. J. Bras. Pneumol. 2017, 43, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Pengo, M.F.; Soranna, D.; Giontella, A.; Perger, E.; Mattaliano, P.; Schwarz, E.I.; Lombardi, C.; Bilo, G.; Zambon, A.; Steier, J.; et al. Obstructive sleep apnoea treatment and blood pressure: Which phenotypes predict a response? A systematic review and meta-analysis. Eur. Respir. J. 2020, 55, 1901945. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.I.; Schlatzer, C.; Stehli, J.; Kaufmann, P.A.; Bloch, K.E.; Stradling, J.R.; Kohler, M. Effect of CPAP Withdrawal on myocardial perfusion in OSA: A randomized controlled trial. Respirology 2016, 21, 1126–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbull, C.D.; Sen, D.; Kohler, M.; Petousi, N.; Stradling, J.R. Effect of supplemental oxygen on blood pressure in obstructive sleep apnea (SOX) a randomized continuous positive airway pressure withdrawal trial. Am. J. Respir. Crit. Care Med. 2019, 199, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.S.; Williams, A.J.; Mok, Y. The relationship between pulmonary hypertension and obstructive sleep apnea. Curr. Opin. Pulm. Med. 2017, 23, 517–521. [Google Scholar] [CrossRef]
- Sommer, N.; Strielkov, I.; Pak, O.; Weissmann, N. Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction. Eur. Respir. J. 2016, 47, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chai, Y.; He, X.; Ai, L.; Sun, X.; Huang, Y.; Li, Y. Intermittent hypoxia simulating obstructive sleep apnea causes pulmonary inflammation and activates the Nrf2/HO-1 pathway. Exp. Ther. Med. 2017, 14, 3463–3470. [Google Scholar] [CrossRef] [Green Version]
- Norton, C.E.; Jernigan, N.L.; Kanagy, N.L.; Walker, B.R.; Resta, T.C. Intermittent hypoxia augments pulmonary vascular smooth muscle reactivity to NO: Regulation by reactive oxygen species. J. Appl. Physiol. 2011, 111, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Imran, T.F.; Ghazipura, M.; Liu, S.; Hossain, T.; Ashtyani, H.; Kim, B.; Michael Gaziano, J.; Djoussé, L. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: A meta-analysis. Heart Fail. Rev. 2016, 21, 591–598. [Google Scholar] [CrossRef]
- Huang, B.; Liu, H.; Scherlag, B.J.; Sun, L.; Xing, S.; Xu, J.; Luo, M.; Guo, Y.; Cao, G.; Jiang, H. Atrial fibrillation in obstructive sleep apnea: Neural mechanisms and emerging therapies. Trends Cardiovasc. Med. 2020, 31, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, J.; Yuan, J.; Hu, F.; Yang, W.; Guo, C.; Luo, X.; Liu, R.; Cui, J.; Gao, X.; et al. Implication of Apnea-Hypopnea Index, a Measure of Obstructive Sleep Apnea Severity, for Atrial Fibrillation in Patients With Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2020, 9, e015013. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, L.; Liu, H.; Shao, Y.; Zhang, S. Cardiac sympathetic denervation suppresses atrial fibrillation and blood pressure in a chronic intermittent hypoxia rat model of obstructive sleep apnea. J. Am. Heart Assoc. 2019, 8, e010254. [Google Scholar] [CrossRef] [PubMed]
- Varga, P.; Rosianu, H.; Vesa, S.; Hancu, B.; Beyer, R.; Pop, C. The impact of continuous positive airway pressure on cardiac arrhythmias in patients with sleep apnea. J. Res. Med. Sci. 2020, 25. [Google Scholar] [CrossRef]
- Abumuamar, A.M.; Newman, D.; Dorian, P.; Shapiro, C.M. Cardiac effects of CPAP treatment in patients with obstructive sleep apnea and atrial fibrillation. J. Interv. Card. Electrophysiol. 2019, 54, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Grabowski, C.; Schiedat, F.; Shin, D.I.; Dietrich, J.W.; Mügge, A.; Deneke, T.; Walther, J.W.; Kara, K. Reverse Remodelling of the Atria After Treatment of Obstructive Sleep Apnoea with Continuous Positive Airway Pressure: Evidence from Electro-mechanical and Endocrine Markers. Hear. Lung Circ. 2016, 25, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Fein, A.S.; Shvilkin, A.; Shah, D.; Haffajee, C.I.; Das, S.; Kumar, K.; Kramer, D.B.; Zimetbaum, P.J.; Buxton, A.E.; Josephson, M.E.; et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J. Am. Coll. Cardiol. 2013, 62, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Naruse, Y.; Tada, H.; Satoh, M.; Yanagihara, M.; Tsuneoka, H.; Hirata, Y.; Ito, Y.; Kuroki, K.; Machino, T.; Yamasaki, H.; et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: Clinical impact of continuous positive airway pressure therapy. Hear. Rhythm 2013, 10, 331–337. [Google Scholar] [CrossRef]
- Caples, S.M.; Mansukhani, M.P.; Friedman, P.A.; Somers, V.K. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: A randomized controlled trial. Int. J. Cardiol. 2019, 278, 133–136. [Google Scholar] [CrossRef]
- Korostovtseva, L.S.; Zvartau, N.E.; Rotar, O.P.; Sviryaev, Y.V.; Konradi, A.O. Predictors of heart rhythm disturbances in hypertensive obese patients with obstructive sleep apnea. J. Geriatr. Cardiol. 2017, 14, 553–562. [Google Scholar] [CrossRef]
- Brunetti, L.; Francavilla, R.; Scicchitano, P.; Tranchino, V.; Loscialpo, M.; Gesualdo, M.; Zito, A.; Fornarelli, F.; Sassara, M.; Giordano, P.; et al. Impact of sleep respiratory disorders on endothelial function in children. Sci. World J. 2013, 2013, 719456. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Zicari, A.M.; Occasi, F.; Perri, L.; Carnevale, R.; Angelico, F.; Del Ben, M.; Martino, F.; Nocella, C.; Savastano, V.; et al. Endothelial dysfunction and oxidative stress in children with sleep disordered breathing: Role of NADPH oxidase. Atherosclerosis 2015, 240, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Perikleous, E.; Steiropoulos, P.; Tzouvelekis, A.; Nena, E.; Koffa, M.; Paraskakis, E. DNA methylation in pediatric obstructive sleep apnea: An overview of preliminary findings. Front. Pediatr. 2018, 6, 154. [Google Scholar] [CrossRef] [Green Version]
- Patinkin, Z.W.; Feinn, R.; Santos, M. Metabolic consequences of obstructive sleep apnea in adolescents with obesity: A systematic literature review and meta-analysis. Child. Obes. 2017, 13, 102–110. [Google Scholar] [CrossRef]
- Gong, W.; Wang, X.; Fan, J.; Nie, S.; Wei, Y. Impact of obstructive sleep apnea on platelet function profiles in patients with acute coronary syndrome taking dual antiplatelet therapy. J. Am. Heart Assoc. 2018, 7, e008808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.N.; Yun, H.C.; Yoo, J.H.; Lee, S.H. Association between hypercoagulability and severe obstructive sleep apnea. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 996–1002. [Google Scholar] [CrossRef]
- Jiang, X.M.; Qian, X.S.; Gao, X.F.; Ge, Z.; Tian, N.L.; Kan, J.; Zhang, J.J. Obstructive Sleep Apnea Affecting Platelet Reactivity in Patients Undergoing Percutaneous Coronary Intervention. Chin. Med. J. (Engl.) 2018, 131, 1023–1029. [Google Scholar] [CrossRef]
- Basili, S.; Pignatelli, P.; Tanzilli, G.; Mangieri, E.; Carnevale, R.; Nocella, C.; Di Santo, S.; Pastori, D.; Ferroni, P.; Violi, F. Anoxia-reoxygenation enhances platelet thromboxane A2 production via reactive oxygen species-generated NOX2: Effect in patients undergoing elective percutaneous coronary intervention. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1766–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, P.Y.; Stevens, J.P.; Haase, E.; Stang, L.; Bigam, D.L.; Etches, W.; Radomski, M.W. Platelet dysfunction in asphyxiated newborn piglets resuscitated with 21% and 100% oxygen. Pediatr. Res. 2006, 59, 636–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oga, T.; Chin, K.; Tabuchi, A.; Kawato, M.; Morimoto, T.; Takahashi, K.; Handa, T.; Takahashi, K.; Taniguchi, R.; Kondo, H.; et al. Effects of obstructive sleep apnea with intermittent hypoxia on platelet aggregability. J. Atheroscler. Thromb. 2009, 16, 862–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahangdale, S.; Yeh, S.Y.; Novack, V.; Stevenson, K.; Barnard, M.R.; Furman, M.I.; Frelinger, A.L.; Michelson, A.D.; Malhotra, A. The influence of intermittent hypoxemia on platelet activation in obese patients with obstructive sleep apnea. J. Clin. Sleep Med. 2011, 7, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Cofta, S.; Wysocka, E.; Dziegielewska-Gesiak, S.; Michalak, S.; Piorunek, T.; Batura-Gabryel, H.; Torlinski, L. Plasma Selectins in Patients with Obstructive Sleep Apnea. In Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2013; Volume 756, pp. 113–119. [Google Scholar]
- Winiarska, H.M.; Cofta, S.; Bielawska, L.; Płóciniczak, A.; Piorunek, T.; Wysocka, E. Circulating P-Selectin and Its Glycoprotein Ligand in Nondiabetic Obstructive Sleep Apnea Patients. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; Volume 1279, pp. 61–69. [Google Scholar]
- Minoguchi, K.; Yokoe, T.; Tazaki, T.; Minoguchi, H.; Oda, N.; Tanaka, A.; Yamamoto, M.; Ohta, S.; O’Donnell, C.P.; Adachi, M. Silent brain infarction and platelet activation in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2007, 175, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Krieger, A.C.; Anand, R.; Hernandez-Rosa, E.; Maidman, A.; Milrad, S.; DeGrazia, M.Q.; Choi, A.J.; Oromendia, C.; Marcus, A.J.; Drosopoulos, J.H.F. Increased platelet activation in sleep apnea subjects with intermittent hypoxemia. Sleep Breath. 2020, 24, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Jóźwik-Plebanek, K.; Prejbisz, A.; Wypasek, E.; Prȩgowska-Chwała, B.; Hanus, K.; Kaszuba, A.M.; Januszewicz, M.; Bieleń, P.; Kabat, M.; Kruk, M.; et al. Altered plasma fibrin clot properties in hypertensive patients with obstructive sleep apnoea are improved by continuous positive airway pressure treatment. J. Hypertens. 2017, 35, 1035–1043. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Bailey, M.D.; Somers, V.K.; Srivastava, M.C.; Scharf, S.M.; Johnson, A.M.; Albrecht, J.S. CPAP adherence is associated with reduced risk for stroke among older adult Medicare beneficiaries with obstructive sleep apnea. J. Clin. Sleep Med. 2021. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Peled, N.; Kassirer, M.; Kramer, M.R.; Rogowski, O.; Shlomi, D.; Fox, B.; Berliner, A.S.; Shitrit, D. Increased erythrocyte adhesiveness and aggregation in obstructive sleep apnea syndrome. Thromb. Res. 2008, 121, 631–636. [Google Scholar] [CrossRef]
- Tsuda, K.; Nishio, I. An association between plasma asymmetric dimethylarginine and membrane fluidity of erythrocytes in hypertensive and normotensive men: An electron paramagnetic resonance investigation. Am. J. Hypertens. 2005, 18, 1243–1248. [Google Scholar] [CrossRef] [Green Version]
- Kuwai, T.; Hayashi, J. Nitric oxide pathway activation and impaired red blood cell deformability with hypercholesterolemia. J. Atheroscler. Thromb. 2006, 13, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Grau, M.; Pauly, S.; Ali, J.; Walpurgis, K.; Thevis, M.; Bloch, W.; Suhr, F. RBC-NOS-Dependent S-Nitrosylation of Cytoskeletal Proteins Improves RBC Deformability. PLoS ONE 2013, 8, e56759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premont, R.T.; Reynolds, J.D.; Zhang, R.; Stamler, J.S. Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle. Circ. Res. 2020, 126, 129–158. [Google Scholar] [CrossRef]
- Bollenbach, A.; Gambaryan, S.; Mindukshev, I.; Pich, A.; Tsikas, D. GC-MS and LC-MS/MS pilot studies on the guanidine (NG)-dimethylation in native, asymmetrically and symmetrically NG-dimethylated arginine-vasopressin peptides and proteins in human red blood cells. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1141, 122024. [Google Scholar] [CrossRef] [PubMed]
- Zwemer, C.F.; Davenport, R.D.; Gomez-Espina, J.; Blanco-Gonzalez, E.; Whitesall, S.E.; D’Alecy, L.G. Packed red blood cells are an abundant and proximate potential source of Nitric oxide synthase inhibition. PLoS ONE 2015, 10, e0119991. [Google Scholar] [CrossRef] [Green Version]
- Tsikas, D.; Böhmer, A.; Großkopf, H.; Beckmann, B.; Dreißigacker, U.; Jordan, J.; Maassen, N. Clinical-chemistry laboratory relevant hemolysis is unlikely to compromise human plasma concentration of free asymmetric dimethylarginine (ADMA). Clin. Biochem. 2012, 45, 1536–1538. [Google Scholar] [CrossRef]
- Kang, E.S.; Cates, T.B.; Harper, D.N.; Chiang, T.M.; Myers, L.K.; Acchiardo, S.R.; Kimoto, M. An enzyme hydrolyzing methylated inhibitors of nitric oxide synthase is present in circulating human red blood cells. Free Radic. Res. 2001, 35, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Bollenbach, A.; Tsikas, D. Pharmacological activation of dimethylarginine dimethylaminohydrolase (DDAH) activity by inorganic nitrate and DDAH inhibition by NG-hydroxy-l-arginine, Nω,Nω-dimethyl-l-citrulline and Nω,Nω-dimethyl-Nδ-hydroxy-l-citrulline: Results and overview. Amino Acids 2019, 51, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Barbé, F.; Durán-Cantolla, J.; Sánchez-De-La-Torre, M.; Martínez-Alonso, M.; Carmona, C.; Barceló, A.; Chiner, E.; Masa, J.F.; Gonzalez, M.; Marín, J.M.; et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: A randomized controlled trial. JAMA J. Am. Med. Assoc. 2012, 307, 2161–2168. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.H.; Luo, Y.M.; McEvoy, R.D. The Sleep Apnea Cardiovascular Endpoints (SAVE) study: Implications for health services and sleep research in China and elsewhere. J. Thorac. Dis. 2017, 9, 2217–2220. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochol, J.; Gawrys, J.; Gajecki, D.; Szahidewicz-Krupska, E.; Martynowicz, H.; Doroszko, A. Cardiovascular Disorders Triggered by Obstructive Sleep Apnea—A Focus on Endothelium and Blood Components. Int. J. Mol. Sci. 2021, 22, 5139. https://doi.org/10.3390/ijms22105139
Mochol J, Gawrys J, Gajecki D, Szahidewicz-Krupska E, Martynowicz H, Doroszko A. Cardiovascular Disorders Triggered by Obstructive Sleep Apnea—A Focus on Endothelium and Blood Components. International Journal of Molecular Sciences. 2021; 22(10):5139. https://doi.org/10.3390/ijms22105139
Chicago/Turabian StyleMochol, Jakub, Jakub Gawrys, Damian Gajecki, Ewa Szahidewicz-Krupska, Helena Martynowicz, and Adrian Doroszko. 2021. "Cardiovascular Disorders Triggered by Obstructive Sleep Apnea—A Focus on Endothelium and Blood Components" International Journal of Molecular Sciences 22, no. 10: 5139. https://doi.org/10.3390/ijms22105139
APA StyleMochol, J., Gawrys, J., Gajecki, D., Szahidewicz-Krupska, E., Martynowicz, H., & Doroszko, A. (2021). Cardiovascular Disorders Triggered by Obstructive Sleep Apnea—A Focus on Endothelium and Blood Components. International Journal of Molecular Sciences, 22(10), 5139. https://doi.org/10.3390/ijms22105139