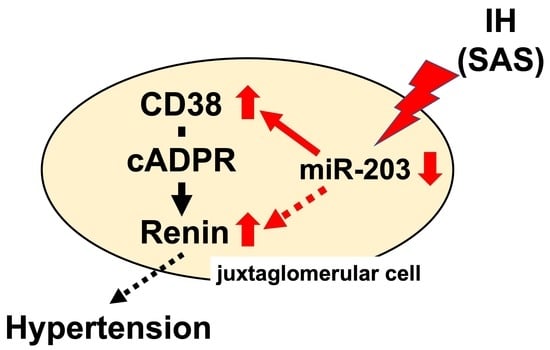
Intermittent Hypoxia Upregulates the Renin and Cd38 mRNAs in Renin-Producing Cells via the Downregulation of miR-203
Abstract
:1. Introduction
2. Results
2.1. Gene Expression Levels of Ren and Cd38 in Human and Mouse Renin-Producing Cells Were Increased by IH
2.2. Downregulation of Cd38 Attenuated the Ren Increase in As4.1 Cells Treated with Small Interfering RNA (siRNA) for Cd38
2.3. 8-Bromo-cADPR (8-Br-cADPR), a Cell-Permeable Antagonist of cADPR, Suppressed the Increases in the Ren and Cd38 Levels Induced by IH
2.4. Gene Expression Levels of the Ryanodine Receptor (RyR)s Were Not Changed by IH
2.5. Promoter Activities of Ren and Cd38 Were Not Increased by IH
2.6. The miR-203 Level Was Significantly Decreased by IH
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. RT-PCR
4.3. Measurement of Ren in the Culture Medium by ELISA
4.4. Immunoblot Analysis
4.5. RNA Interference
4.6. Addition of 8-Br-cADPR
4.7. Construction of Reporter Plasmids and Luciferase Assay
4.8. MiR-203 Mimic Transfection
4.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGT | Angiotensinogen |
| AGTR1 | Angiotensin II receptor type 1 |
| AGTR2 | Angiotensin II receptor type 2 |
| cADPR | Cyclic ADP-ribose |
| DICER | Endoribonuclease Dicer |
| DROSHA | Ribonuclease type III |
| ELISA | Enzyme-linked immunosorbent assay |
| FCS | Fetal calf serum |
| FGF2 | Fibroblast growth factor 2 |
| IH | Intermittent hypoxia |
| JG | Juxtaglomerular |
| miRNA | MicroRNA |
| RAS | Renin-angiotensin system |
| REN | Renin |
| Rig | Rat insulinoma gene |
| RpS15 | Ribosomal protein S15 |
| RyR | Ryanodine receptor |
| SAS | Sleep apnea syndrome |
| siRNA | Small interfering RNA |
References
- Ahmad, M.; Makati, D.; Akbar, S. Review of and updates on hypertension in obstructive sleep apnea. Int. J. Hypertens. 2017, 2017, 1848375. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.-N.; Wei, Y.-X. Meta-analysis of effects of obstructive sleep apnea on the renin-angiotensin-aldosterone system. J. Geriatr. Cardiol. 2016, 13, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.C.; Bao, G.; Li, R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 1999, 34, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iturriaga, R.; Castillo-Galán, S. Potential contribution of carotid body-induced sympathetic and renin-angiotensin system overflow to pulmonary hypertension in intermittent hypoxia. Curr. Hypertens. Rep. 2019, 21, 89. [Google Scholar] [CrossRef]
- Xiong, J.; Xia, M.; Yi, F.; Abais, J.M.; Li, N.; Boini, K.M.; Li, P.-L. Regulation of renin release via cyclic ADP-ribose-mediated signaling: Evidence from mice lacking CD38 gene. Cell. Physiol. Biochem. 2013, 31, 44–55. [Google Scholar] [CrossRef]
- Galione, A.; Lee, H.C.; Busa, W.B. Ca2+-induced Ca2+ release in sea urchin egg homogenates: Modulation by cyclic ADP-ribose. Science 1991, 253, 1143–1146. [Google Scholar] [CrossRef]
- Takasawa, S.; Nata, K.; Yonekura, H.; Okamoto, H. Cyclic ADP-ribose in insulin secretion from pancreatic β cells. Science 1993, 259, 370–373. [Google Scholar] [CrossRef]
- Takasawa, S.; Akiyama, T.; Nata, K.; Kuroki, M.; Tohgo, A.; Noguchi, N.; Kobayashi, S.; Kato, I.; Katada, T.; Okamoto, H. Cyclic ADP-ribose and inositol 1,4,5-trisphosphate as alternate second messengers for intracellular Ca2+ mobilization in normal and diabetic β-cells. J. Biol. Chem. 1998, 273, 2497–2500. [Google Scholar] [CrossRef] [Green Version]
- Takasawa, S.; Kuroki, M.; Nata, K.; Noguchi, N.; Ikeda, T.; Yamauchi, A.; Ota, H.; Itaya-Hironaka, A.; Sakuramoto-Tsuchida, S.; Takahashi, I.; et al. A novel ryanodine receptor expressed in pancreatic islets by alternative splicing from type 2 ryanodine receptor gene. Biochem. Biophys. Res. Commun. 2010, 397, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, S.; Tohgo, A.; Noguchi, N.; Koguma, T.; Nata, K.; Sugimoto, T.; Yonekura, H.; Okamoto, H. Synthesis and hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and inhibition of the hydrolysis by ATP. J. Biol. Chem. 1993, 268, 26052–26054. [Google Scholar] [CrossRef]
- Takasawa, S.; Okamoto, H. Pancreatic β-cell death, regeneration and insulin secretion: Roles of poly(ADP-ribose) polymerase and cyclic ADP-ribose. Int. J. Exp. Diabetes Res. 2002, 3, 79–96. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, H.; Takasawa, S. Okamoto model for necrosis and its expansions, CD38-cyclic ADP-ribose signal system for intracellular Ca2+ mobilization and Reg ([Regenerating gene] protein)-Reg receptor system for cell regeneration. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2021, 97. in press. [Google Scholar]
- Okamoto, H.; Takasawa, S. Recent advances in the Okamoto model: The CD38-cyclic ADP-ribose signal system and the regenerating gene protein (Reg)-Reg receptor system in β-cells. Diabetes 2002, 51, S462–S473. [Google Scholar] [CrossRef] [Green Version]
- Yi, F.; Zhang, A.Y.; Li, N.; Zhang, F.; Xia, M.; Li, P.-L. Role of cyclic ADP-ribose-Ca2+ signaling in mediating renin production and release in As4.1 cells. Cell. Physiol. Biochem. 2007, 19, 293–302. [Google Scholar] [CrossRef]
- Sparks, M.A.; Crowley, S.D.; Gurley, S.B.; Mirotsou, M.; Coffman, T.M. Classical renin-angiotensin system in kidney physiology. Compr. Physiol. 2014, 4, 1201–1228. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.-J.; Sun, H.-Y.; Ting, K.Y.; Zhang, L.-H.; Lee, H.-C.; Li, G.-R.; Yue, J. Inhibition of cardiomyocytes differentiation of mouse embryonic stem cells by CD38/cADPR/Ca2+ signaling pathway. J. Biol. Chem. 2012, 287, 35599–35611. [Google Scholar] [CrossRef] [Green Version]
- Francia, S.; Michelini, F.; Saxena, A.; Tang, D.; de Hoon, M.; Anelli, V.; Mione, M.; Carninci, P.; d’Adda di Fagagna, F. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature 2012, 488, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.-H.; Wang, C.-X.; Liu, B.; Fan, X.-S.; Wen, J.-J.; Shi, Q.-L.; Zhou, X.-J. Dysregulation of microRNA biosynthesis enzyme Dicer plays an important role in gastric cancer progression. Int. J. Clin. Exp. Pathol. 2014, 7, 1702–1707. [Google Scholar]
- Pamidi, S.; Pinto, L.M.; Marc, I.; Benedetti, A.; Schwartzman, K.; Kimoff, R.J. Maternal sleep-disordered breathing and adverse pregnancy outcomes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2014, 210, 52.e1–52.e14. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Shinohara, H.; Kodama, H. Nocturnal oxygen desaturation in the late third trimester of uncomplicated pregnancy for prediction of late-onset gestational hypertension. J. Obstet. Gynaecol. Res. 2020, 46, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Feng, Y.; Peng, H.; Guo, D.; Li, T. Obstructive sleep apnea and the risk of perinatal outcomes: A meta-analysis of cohort studies. Sci. Rep. 2014, 4, 6982. [Google Scholar] [CrossRef] [Green Version]
- Facco, F.L.; Parker, C.B.; Reddy, U.M.; Silver, R.M.; Koch, M.A.; Louis, J.M.; Basner, R.C.; Chung, J.H.; Nhan-Chang, C.-L.; Pien, G.W.; et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet. Gynecol. 2017, 129, 31–41. [Google Scholar] [CrossRef]
- Jaimchariyatam, N.; Na-rungsri, K.; Tungsanga, S.; Lertmaharit, S.; Lohsoonthorn, V.; Totienchai, S. Obstructive sleep apnea as a risk factor for preeclamsia-eclampsia. Sleep Breath. 2019, 23, 687–693. [Google Scholar] [CrossRef]
- Foster, G.E.; Hanly, P.J.; Ahmed, S.B.; Beaudin, A.E.; Pialoux, V.; Poulin, M.J. Intermittent hypoxia increases arterial blood pressure in humans through a renin-angiotensin system-dependent mechanism. Hypertension 2010, 56, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ritthaler, T.; Schricker, K.; Kees, F.; Krämer, B.; Kurtz, A. Acute hypoxia stimulates renin secretion and renin gene expression in vivo but not in vitro. Am. J. Physiol. 1997, 272, R1105–R1111. [Google Scholar] [CrossRef]
- Krämer, B.K.; Ritthaler, T.; Schweda, F.; Kees, F.; Schricker, K.; Holmer, S.R.; Kurtz, A. Effects of hypoxia on renin secretion and renal renin gene expression. Kidney Int. 1998, 54, S155–S158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweda, F.; Blumberg, F.C.; Schweda, A.; Kammerl, M.; Holmer, S.R.; Riegger, G.A.J.; Pfeifer, M.; Krämer, B.K. Effects of chronic hypoxia on renal renin gene expression in rats. Nephrol. Dial. Transplant. 2000, 15, 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muxfeldt, E.S.; Margallo, V.S.; Guimarães, G.M.; Salles, G.F. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am. J. Hypertens. 2014, 27, 1069–1078. [Google Scholar] [CrossRef]
- Svatikova, A.; Olson, L.J.; Wolk, R.; Phillips, B.G.; Adachi, T.; Schwartz, G.L.; Somers, V.K. Obstructive sleep apnea and aldosterone. Sleep 2009, 32, 1589–1592. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.H.; Hong, X.; Zhao, N.; Liu, X.H.; Xiao, Y.F.; Chen, T.T.; Deng, L.B.; Wang, X.L.; Wang, J.B.; Ji, G.J.; et al. CD38 promotes angiotensin II-induced cardiac hypertrophy. J. Cell. Mol. Med. 2017, 21, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, B.H.; Kim, U.H. CD38-mediated Ca2+ signaling contributes to angiotensin II-induced activation of hepatic stellate cells: Attenuation of hepatic fibrosis by CD38 ablation. J. Biol. Chem. 2010, 285, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Patra, B.C.; Bhattacharya, M.; Sharma, G.; Lee, S.-S. MicroRNAs mediated regulation of MAPK signaling pathways in chronic myeloid leukemia. Oncotarget 2016, 7, 42683–42697. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Bhattacharya, S.; Steele, R.; Ray, R.B. Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOCS3. Oncotarget 2016, 7, 58595–58605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanzadeh, M.; Movahedi, M.; Rejali, M.; Maleki, F.; Moetamani-Ahmadi, M.; Seifi, S.; Hosseini, Z.; Khazaei, M.; Amerizadeh, F.; Ferns, G.A.; et al. The potential prognostic and therapeutic application of tissue and circulating microRNAs in cervical cancer. J. Cell. Physiol. 2019, 234, 1289–1294. [Google Scholar] [CrossRef]
- Braga, E.A.; Fridman, M.V.; Loginov, V.I.; Dmitriev, A.A.; Morozov, S.G. Molecular mechanisms in clear cell renal cell carcinoma: Role of miRNAs and hypermethylated miRNA genes in crucial oncogenic pathways and processes. Front. Genet. 2019, 10, 320. [Google Scholar] [CrossRef]
- Uchiyama, T.; Ota, H.; Itaya-Hironaka, A.; Shobatake, R.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Makino, M.; Kimura, H.; Takeda, M.; Ohbayashi, C.; et al. Up-regulation of selenoprotein P and HIP/PAP mRNAs in hepatocytes by intermittent hypoxia via down-regulation of miR-203. Biochem. Biophys. Rep. 2017, 11, 130–137. [Google Scholar] [CrossRef]
- Sánchez-de-la-Torre, M.; Khalyfa, A.; Sánchez-de-la-Torre, A.; Martinez-Alonso, M.; Martinez-García, M.Á.; Barceló, A.; Lloberes, P.; Campos-Rodriguez, F.; Capote, F.; Diaz-de-Atauri, M.J.; et al. Precision medicine in patients with resistant hypertension and obstructive sleep apnea: Blood pressure response to continuous positive airway pressure treatment. J. Am. Coll. Cardiol. 2015, 66, 1023–1032. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-N.; Yu, W.-C.; Du, C.-H.; Tong, L.; Cheng, Z.-Z. Hypoxia is involved in hypoxic pulmonary hypertension through inhibiting the activation of FGF2 by miR-203. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8866–8876. [Google Scholar] [CrossRef]
- Murakami-Kawaguchi, S.; Takasawa, S.; Onogawa, T.; Nata, K.; Itaya-Hironaka, A.; Sakuramoto-Tsuchida, S.; Yamauchi, A.; Ota, H.; Takeda, M.; Kato, M.; et al. Expression of Ins1 and Ins2 genes in mouse fetal liver. Cell Tissue Res. 2014, 355, 303–314. [Google Scholar] [CrossRef]
- Nakazawa, T.; Takasawa, S.; Noguchi, N.; Nata, K.; Tohgo, A.; Mori, M.; Nakagawara, K.; Akiyama, T.; Ikeda, T.; Yamauchi, A.; et al. Genomic organization, chromosomal localization, and promoter of human gene for FK506-binding protein 12.6. Gene 2005, 360, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Takasawa, S.; Yamauchi, M.; Yoshikawa, M.; Tomoda, K.; Kimura, H. Intermittent hypoxia in pancreatic beta cells. Pancreat. Disord. Ther. 2015, 5, S5-004. [Google Scholar] [CrossRef] [Green Version]
- Shobatake, R.; Takasawa, K.; Ota, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Uchiyama, T.; Makino, M.; Sugie, K.; Takasawa, S.; et al. Up-regulation of POMC and CART mRNAs by intermittent hypoxia via GATA transcription factors in human neuronal cells. Int. J. Biochem. Cell Biol. 2018, 95, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Tamaki, S.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Morioka, T.; Takasawa, S.; Kimura, H. Attenuation of glucose-induced insulin secretion by intermittent hypoxia via down-regulation of CD38. Life Sci. 2012, 90, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Fujita, Y.; Yamauchi, M.; Muro, S.; Kimura, H.; Takasawa, S. Relationship between intermittent hypoxia and Type 2 diabetes in sleep apnea syndrome. Int. J. Mol. Sci. 2019, 20, 4756. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Ota, H.; Kimura, Y.; Takasawa, S. Effects of intermittent hypoxia on pulmonary vascular and systemic diseases. Int. J. Environ. Res. Public Health 2019, 16, 3101. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.M.; Thomas, A.; Crinion, S.J.; Kent, B.D.; Tambuwala, M.M.; Fabre, A.; Pepin, J.-L.; Roche, H.M.; Arnaud, C.; Ryan, S. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur. Respir. J. 2017, 49, 1601731. [Google Scholar] [CrossRef] [Green Version]
- Ota, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Miyaoka, T.; Fujimura, T.; Tsujinaka, H.; Yoshimoto, K.; Nakagawara, K.; Tamaki, S.; et al. Pancreatic β cell proliferation by intermittent hypoxia via up-regulation of Reg family genes and HGF gene. Life Sci. 2013, 93, 664–672. [Google Scholar] [CrossRef]
- Kyotani, Y.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Makino, M.; Takasawa, S.; Yoshizumi, M. Intermittent hypoxia-induced epiregulin expression by IL-6 production in human coronary artery smooth muscle cells. FEBS Open Bio 2018, 8, 868–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shobatake, R.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Uchiyama, T.; Ota, H.; Takahashi, N.; Ueno, S.; Sugie, K.; et al. Intermittent hypoxia up-regulates gene expressions of peptide YY (PYY), glucagon-like peptide-1 (GLP-1), and neurotensin (NTS) in enteroendocrine cells. Int. J. Mol. Sci. 2019, 20, 1849. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, T.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Shobatake, R.; Ota, H.; Takeda, M.; Ohbayashi, C.; Takasawa, S. Intermittent hypoxia up-regulates CCL2, RETN, and TNFα mRNAs in adipocytes via down-regulation of miR-452. Int. J. Mol. Sci. 2019, 20, 1960. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, K.; Fujimoto, T.; Itaya-Hironaka, A.; Miyaoka, T.; Sakuramoto-Tsuchida, S.; Yamauchi, A.; Takeda, M.; Kasai, T.; Nakagawara, K.; Nonomura, A.; et al. Involvement of autoimmunity to REG, a regeneration factor, in patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2013, 174, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, A.; Itaya-Hironaka, A.; Sakuramoto-Tsuchida, S.; Takeda, M.; Yoshimoto, K.; Miyaoka, T.; Fujimura, T.; Tsujinaka, H.; Tsuchida, C.; Ota, H.; et al. Synergistic activations of REG Iα and REG Iβ promoters by IL-6 and glucocorticoids through JAK/STAT pathway in human pancreatic β cells. J. Diabetes Res. 2015, 2015, 173058. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, T.; Fujimoto, T.; Itaya-Hironaka, A.; Miyaoka, T.; Yoshimoto, K.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Kondo, S.; Takeda, M.; Tsujinaka, H.; et al. Interleukin-6/STAT pathway is responsible for the induction of gene expression of REG Iα, a new auto-antigen in Sjögren׳s syndrome patients, in salivary duct epithelial cells. Biochem. Biophys. Rep. 2015, 2, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Tsujinaka, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Ota, H.; Takeda, M.; Fujimura, T.; Takasawa, S.; Ogata, N. Human retinal pigment epithelial cell proliferation by the combined stimulation of hydroquinone and advanced glycation end-products via up-regulation of VEGF gene. Biochem. Biophys. Rep. 2015, 2, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, C.; Sakuramoto-Tsuchida, S.; Takeda, M.; Itaya-Hironaka, A.; Yamauchi, A.; Misu, M.; Shobatake, R.; Uchiyama, T.; Makino, M.; Pujol-Autonell, I.; et al. Expression of REG family genes in human inflammatory bowel diseases and its regulation. Biochem. Biophys. Rep. 2017, 12, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Tohma, Y.; Dohi, Y.; Shobatake, R.; Uchiyama, T.; Takeda, M.; Takasawa, S.; Tanaka, Y.; Ohgushi, H. Reg gene expression in periosteum after fracture and its in vitro induction triggered by IL-6. Int. J. Mol. Sci. 2017, 18, 2257. [Google Scholar] [CrossRef] [Green Version]
- Takasawa, S.; Tsuchida, C.; Sakuramoto-Tsuchida, S.; Takeda, M.; Itaya-Hironaka, A.; Yamauchi, A.; Misu, M.; Shobatake, R.; Uchiyama, T.; Makino, M.; et al. Expression of human REG family genes in inflammatory bowel disease and their molecular mechanism. Immunol. Res. 2018, 66, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, J.; Naruse, K.; Sado, T.; Uchiyama, T.; Makino, M.; Yamauchi, A.; Ota, H.; Sakuramoto-Tsuchida, S.; Itaya-Hironaka, A.; Takasawa, S.; et al. Involvement of receptor for advanced glycation endproducts in hypertensive disorders of pregnancy. Int. J. Mol. Sci. 2019, 20, 5462. [Google Scholar] [CrossRef] [Green Version]
- Kato, I.; Yamamoto, Y.; Fujimura, M.; Noguchi, N.; Takasawa, S.; Okamoto, H. CD38 disruption impairs glucose-induced increases in cyclic ADP-ribose, [Ca2+]i, and insulin secretion. J. Biol. Chem. 1999, 274, 1869–1872. [Google Scholar] [CrossRef] [Green Version]
- Harada, N.; Santos-Argumedo, L.; Chang, R.; Grimaldi, J.C.; Lund, F.E.; Brannan, C.I.; Copeland, N.G.; Jenkins, N.A.; Heath, A.W.; Parkhouse, R.M.; et al. Expression cloning of a cDNA encoding a novel murine B cell activation marker. Homology to human CD38. J. Immunol. 1993, 151, 3111–3118. [Google Scholar] [PubMed]
- Ikehata, F.; Satoh, J.; Nata, K.; Tohgo, A.; Nakazawa, T.; Kato, I.; Kobayashi, S.; Akiyama, T.; Takasawa, S.; Toyota, T.; et al. Autoantibodies against CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) that impair glucose-induced insulin secretion in noninsulin-dependent diabetes patients. J. Clin. Investig. 1998, 102, 395–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shervani, N.J.; Takasawa, S.; Uchigata, Y.; Akiyama, T.; Nakagawa, K.; Noguchi, N.; Takada, H.; Takahashi, I.; Yamauchi, A.; Ikeda, T.; et al. Autoantibodies to REG, a beta-cell regeneration factor, in diabetic patients. Eur. J. Clin. Investig. 2004, 34, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Takasawa, S.; Nata, K.; Yamauchi, A.; Itaya-Hironaka, A.; Ota, H.; Yoshimoto, K.; Sakuramoto-Tsuchida, S.; Miyaoka, T.; Takeda, M.; et al. Prevention of Reg I-induced β-cell apoptosis by IL-6/dexamethasone through activation of HGF gene regulation. Biochim. Biophys. Acta 2013, 1833, 2988–2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Target mRNA | Primer Sequence |
|---|---|
| REN (NM_000537) | 5′-AAATGAAGGGGGTGTCTGTGG-3′ |
| 5′-AAGCCAATGCGGTTGTTACGC-3′ | |
| CD38 (NM_001775) | 5′-ACAAACCCTGCTGCCGGCTCTC-3′ |
| 5′-GCATCGCGCCAGGACGGTCT-3′ | |
| AGT (NM_001382817) | 5′-AACTGGTGCTGCAAGGATCT-3′ |
| 5′-TCTCTCTCATCCGCTTCAAG-3′ | |
| AGTR1 (NM_000685) | 5′-ATCCACCAAGAAGCCTGCAC-3′ |
| 5′-TGAAGTGCTGCAGAGGAATG-3′ | |
| AGTR2 (NM_000686) | 5′-CCTCGCTGTGGCTGATTTACTCCTT-3′ |
| 5′-TTGCACATCACAGGTCCAA-3′ | |
| β-actin (NM_001101) | 5′-GCGAGAAGATGACCCAGA-3′ |
| 5′-CAGAGGCGTACAGGGATA-3′ |
| Target mRNA/miR | Primer Sequence |
|---|---|
| Ren (NM_031192) | 5′-CCTCTACCTTGCTTGTGGGATT-3′ |
| 5′-CTGGCTGAGGAAACCTTTGACT-3′ | |
| Cd38 (NM_007646) | 5′-ACAGACCTGGCTGCCGCCTCTCTAG-3′ |
| 5′-GGGGCGTAGTCTTCTCTTGTGATGT-3′ | |
| Agt (NM_007428) | 5′-CACCCCTGCTACAGTCCATT-3′ |
| 5′-GTCTGTACTGACCCCCTCCA-3′ | |
| Agtr1 (NM_177322) | 5′-GGCTGGCATTTTGTCTGGATA-3′ |
| 5′-CTTTTCTGGGTTGAGTTGGTCT-3′ | |
| Agtr2 (NM_007429) | 5′-AGCTTACTTCAGCCTGCATT-3′ |
| 5′-CAGCAACTCCAAATTCTTACACC-3′ | |
| Ryr1 (NM_009109) | 5′-AAGTCCCACAACTTTAAGCG-3′ |
| 5′-TCTTCTTGGTGCGTTCCTG-3′ | |
| Islet-type Ryr2 (NM_023868) | 5′-GACAGTCGAGCGTGTCCTGGGTATA-3′ |
| 5′-TGCTTAGAGAGTAGTTTGTGCCACA-3′ | |
| Cardiac-type Ryr2 (NM_023868) | 5′-GACAGTCGAGCGTGTCCTGGGTATA-3′ |
| 5′-TGCTTAGAGAGTAGTTTGTGCCACA-3′ | |
| Ryr3 (NM_001319156) | 5′-AGAAGAGGCCAAAGCAGAGG-3′ |
| 5′-GGAGGCCAACGGTCAGA-3′ | |
| Rig/RpS15 (NM_009091) | 5′-ACGGCAAGACCTTCAACCAG-3′ |
| 5′-ATGGAGAACTCGCCCAGGTAG-3′ | |
| Dicer (NM_148948) | 5′-ATGCAAAAAGGACCGTGTTC-3′ |
| 5′-CAAGGCGACATAGCAAGTCA-3′ | |
| Drosha (NM_001130149) | 5′-CTCTTTCCCACCCAGTGCTA-3′ |
| 5′-TGGTCGTCGTAGTGCTTGAG-3′ | |
| miR-203 (NR_029590) | 5′-TCCAGTGGTTCTTGACAGTTCA-3′ |
| 5′-GGTCTAGTGGTCCTAAACATTTC-3′ | |
| U6 (XR_003953458) | 5´-CGCTTCGGCAGCACATATAC-3′ |
| 5′-AAATATGGAACGCTTCACGA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, Y.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Ota, H.; Kawaguchi, R.; Takasawa, S. Intermittent Hypoxia Upregulates the Renin and Cd38 mRNAs in Renin-Producing Cells via the Downregulation of miR-203. Int. J. Mol. Sci. 2021, 22, 10127. https://doi.org/10.3390/ijms221810127
Takeda Y, Itaya-Hironaka A, Yamauchi A, Makino M, Sakuramoto-Tsuchida S, Ota H, Kawaguchi R, Takasawa S. Intermittent Hypoxia Upregulates the Renin and Cd38 mRNAs in Renin-Producing Cells via the Downregulation of miR-203. International Journal of Molecular Sciences. 2021; 22(18):10127. https://doi.org/10.3390/ijms221810127
Chicago/Turabian StyleTakeda, Yoshinori, Asako Itaya-Hironaka, Akiyo Yamauchi, Mai Makino, Sumiyo Sakuramoto-Tsuchida, Hiroyo Ota, Ryuji Kawaguchi, and Shin Takasawa. 2021. "Intermittent Hypoxia Upregulates the Renin and Cd38 mRNAs in Renin-Producing Cells via the Downregulation of miR-203" International Journal of Molecular Sciences 22, no. 18: 10127. https://doi.org/10.3390/ijms221810127
APA StyleTakeda, Y., Itaya-Hironaka, A., Yamauchi, A., Makino, M., Sakuramoto-Tsuchida, S., Ota, H., Kawaguchi, R., & Takasawa, S. (2021). Intermittent Hypoxia Upregulates the Renin and Cd38 mRNAs in Renin-Producing Cells via the Downregulation of miR-203. International Journal of Molecular Sciences, 22(18), 10127. https://doi.org/10.3390/ijms221810127