Nanoscale Surface Topography Modulates hIAPP Aggregation Pathways at Solid–Liquid Interfaces
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Flat and Nanopatterned Surfaces
2.2. hIAPP Aggregation in Bulk Solution
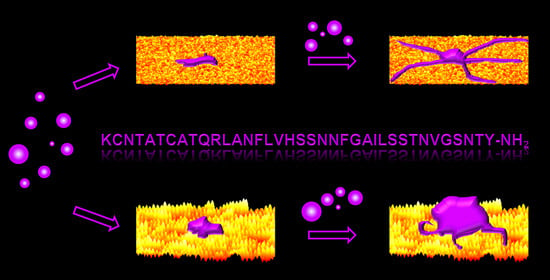
2.3. hIAPP Aggregation in Contact with Flat and Nanopatterned Surfaces
3. Discussion
4. Materials and Methods
4.1. Substrate Preparation
4.2. Peptide Preparation
4.3. hIAPP Aggregation in Bulk Solution
4.4. hIAPP Aggregation in Contact with Flat and Nanopatterned Surfaces
4.5. AFM Imaging and Image Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prigent-Combaret, C.; Prensier, G.; Le Thi, T.T.; Vidal, O.; Lejeune, P.; Dorel, C. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: Role of flagella, curli and colanic acid. Environ. Microbiol. 2000, 2, 450–464. [Google Scholar] [CrossRef]
- Balcells, M.; Edelman, E.R. Effect of pre-adsorbed proteins on attachment, proliferation, and function of endothelial cells. J. Cell. Physiol. 2002, 191, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, M.S.; Friedman, A.E.; Finkelstein, J.N.; Oberdörster, G.; McGrath, J.L. The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomaterials 2009, 30, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Vaisocherová, H.; Zhang, Z.; Yang, W.; Cao, Z.; Cheng, G.; Taylor, A.D.; Piliarik, M.; Homola, J.; Jiang, S. Functionalizable surface platform with reduced nonspecific protein adsorption from full blood plasma--material selection and protein immobilization optimization. Biosens. Bioelectron. 2009, 24, 1924–1930. [Google Scholar] [CrossRef]
- Pezzella, A.; Barra, M.; Musto, A.; Navarra, A.; Alfè, M.; Manini, P.; Parisi, S.; Cassinese, A.; Criscuolo, V.; d’Ischia, M. Stem cell-compatible eumelanin biointerface fabricated by chemically controlled solid state polymerization. Mater. Horiz. 2015, 2, 212–220. [Google Scholar] [CrossRef]
- Shen, Z.; Stryker, G.A.; Mernaugh, R.L.; Yu, L.; Yan, H.; Zeng, X. Single-chain fragment variable antibody piezoimmunosensors. Anal. Chem. 2005, 77, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Dee, K.C.; Puleo, D.A.; Bizios, R. Protein-Surface Interactions. In An Introduction to Tissue-Biomaterial Interactions; Dee, K.C., Puleo, D.A., Bizios, R., Eds.; John Wiley & Sons, Inc: New York, NY, USA, 2002; pp. 37–52. ISBN 9780471270591. [Google Scholar]
- Rechendorff, K.; Hovgaard, M.B.; Foss, M.; Zhdanov, V.P.; Besenbacher, F. Enhancement of Protein Adsorption Induced by Surface Roughness. Langmuir 2006, 22, 10885–10888. [Google Scholar] [CrossRef]
- Cai, K.; Bossert, J.; Jandt, K.D. Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surf. B Biointerfaces 2006, 49, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Dolatshahi-Pirouz, A.; Jensen, T.; Kraft, D.C.; Foss, M.; Kingshott, P.; Hansen, J.L.; Larsen, A.N.; Chevallier, J.; Besenbacher, F. Fibronectin adsorption, cell adhesion, and proliferation on nanostructured tantalum surfaces. ACS Nano 2010, 4, 2874–2882. [Google Scholar] [CrossRef]
- Rockwell, G.P.; Lohstreter, L.B.; Dahn, J.R. Fibrinogen and albumin adsorption on titanium nanoroughness gradients. Colloids Surf. B Biointerfaces 2012, 91, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Hovgaard, M.B.; Rechendorff, K.; Chevallier, J.; Foss, M.; Besenbacher, F. Fibronectin adsorption on tantalum: The influence of nanoroughness. J. Phys. Chem. B 2008, 112, 8241–8249. [Google Scholar] [CrossRef] [PubMed]
- Richert, L.; Variola, F.; Rosei, F.; Wuest, J.D.; Nanci, A. Adsorption of proteins on nanoporous Ti surfaces. Surf. Sci. 2010, 604, 1445–1451. [Google Scholar] [CrossRef]
- Sommerfeld, J.; Richter, J.; Niepelt, R.; Kosan, S.; Keller, T.F.; Jandt, K.D.; Ronning, C. Protein adsorption on nano-scaled, rippled TiO2 and Si surfaces. Biointerphases 2012, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Yu, M.; Böke, F.; Qin, Q.; Hübner, R.; Knust, S.; Schwiderek, S.; Grundmeier, G.; Fischer, H.; Keller, A. Effect of nanoscale surface topography on the adsorption of globular proteins. Appl. Surf. Sci. 2021, 535, 147671. [Google Scholar] [CrossRef]
- Yang, Y.; Knust, S.; Schwiderek, S.; Qin, Q.; Yun, Q.; Grundmeier, G.; Keller, A. Protein Adsorption at Nanorough Titanium Oxide Surfaces: The Importance of Surface Statistical Parameters beyond Surface Roughness. Nanomaterials 2021, 11, 357. [Google Scholar] [CrossRef]
- González-García, C.; Sousa, S.R.; Moratal, D.; Rico, P.; Salmerón-Sánchez, M. Effect of nanoscale topography on fibronectin adsorption, focal adhesion size and matrix organisation. Colloids Surf. B Biointerfaces 2010, 77, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kam, K.R.; Walsh, L.A.; Bock, S.M.; Ollerenshaw, J.D.; Ross, R.F.; Desai, T.A. The effect of nanotopography on modulating protein adsorption and the fibrotic response. Tissue Eng. Part A 2014, 20, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Lord, M.S.; Whitelock, J.M.; Simmons, A.; Williams, R.L.; Milthorpe, B.K. Fibrinogen adsorption and platelet adhesion to silica surfaces with stochastic nanotopography. Biointerphases 2014, 9, 41002. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.K.; Pham, V.T.H.; Al Kobaisi, M.; Bhadra, C.; Orlowska, A.; Ghanaati, S.; Manzi, B.M.; Baulin, V.A.; Joudkazis, S.; Kingshott, P.; et al. Adsorption of Human Plasma Albumin and Fibronectin onto Nanostructured Black Silicon Surfaces. Langmuir 2016, 32, 10744–10751. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, P.E.; Borgonovo, A.; Indrieri, M.; Giorgetti, L.; Bongiorno, G.; Carbone, R.; Podestà, A.; Milani, P. The effect of surface nanometre-scale morphology on protein adsorption. PLoS ONE 2010, 5, e11862. [Google Scholar] [CrossRef] [Green Version]
- Manzi, B.M.; Werner, M.; Ivanova, E.P.; Crawford, R.J.; Baulin, V.A. Simulations of Protein Adsorption on Nanostructured Surfaces. Sci. Rep. 2019, 9, 4694. [Google Scholar] [CrossRef] [PubMed]
- Shezad, K.; Zhang, K.; Hussain, M.; Dong, H.; He, C.; Gong, X.; Xie, X.; Zhu, J.; Shen, L. Surface Roughness Modulates Diffusion and Fibrillation of Amyloid-β Peptide. Langmuir 2016, 32, 8238–8244. [Google Scholar] [CrossRef]
- Co, N.T.; Li, M.S. Effect of Surface Roughness on Aggregation of Polypeptide Chains: A Monte Carlo Study. Biomolecules 2021, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Huber, F.; Schnauß, J.; Rönicke, S.; Rauch, P.; Müller, K.; Fütterer, C.; Käs, J. Emergent complexity of the cytoskeleton: From single filaments to tissue. Adv. Phys. 2013, 62, 1–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Biophys. Acta 2013, 1832, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Erskine, E.; MacPhee, C.E.; Stanley-Wall, N.R. Functional Amyloid and Other Protein Fibers in the Biofilm Matrix. J. Mol. Biol. 2018, 430, 3642–3656. [Google Scholar] [CrossRef]
- Ke, P.C.; Sani, M.-A.; Ding, F.; Kakinen, A.; Javed, I.; Separovic, F.; Davis, T.P.; Mezzenga, R. Implications of peptide assemblies in amyloid diseases. Chem. Soc. Rev. 2017, 46, 6492–6531. [Google Scholar] [CrossRef]
- Sciacca, M.F.M.; Tempra, C.; Scollo, F.; Milardi, D.; La Rosa, C. Amyloid growth and membrane damage: Current themes and emerging perspectives from theory and experiments on Aβ and hIAPP. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1625–1638. [Google Scholar] [CrossRef]
- Eichner, T.; Radford, S.E. A diversity of assembly mechanisms of a generic amyloid fold. Mol. Cell 2011, 43, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Sluzky, V.; Tamada, J.A.; Klibanov, A.M.; Langer, R. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc. Natl. Acad. Sci. USA 1991, 88, 9377–9381. [Google Scholar] [CrossRef] [Green Version]
- Stefani, M. Protein folding and misfolding on surfaces. Int. J. Mol. Sci. 2008, 9, 2515–2542. [Google Scholar] [CrossRef]
- Keller, A.; Grundmeier, G. Amyloid aggregation at solid-liquid interfaces: Perspectives of studies using model surfaces. Appl. Surf. Sci. 2020, 506, 144991. [Google Scholar] [CrossRef]
- Grigolato, F.; Arosio, P. The role of surfaces on amyloid formation. Biophys. Chem. 2021, 270, 106533. [Google Scholar] [CrossRef]
- Cabaleiro-Lago, C.; Quinlan-Pluck, F.; Lynch, I.; Lindman, S.; Minogue, A.M.; Thulin, E.; Walsh, D.M.; Dawson, K.A.; Linse, S. Inhibition of amyloid beta protein fibrillation by polymeric nanoparticles. J. Am. Chem. Soc. 2008, 130, 15437–15443. [Google Scholar] [CrossRef]
- Cabaleiro-Lago, C.; Lynch, I.; Dawson, K.A.; Linse, S. Inhibition of IAPP and IAPP(20–29) fibrillation by polymeric nanoparticles. Langmuir 2010, 26, 3453–3461. [Google Scholar] [CrossRef]
- Wang, M.; Kakinen, A.; Pilkington, E.H.; Davis, T.P.; Ke, P.C. Differential effects of silver and iron oxide nanoparticles on IAPP amyloid aggregation. Biomater. Sci. 2017, 5, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.M.; Cardoso, I.; Saraiva, M.J.; Tauer, K.; Pereira, M.C.; Coelho, M.A.N.; Möhwald, H.; Brezesinski, G. Randomization of amyloid-β-peptide(1–42) conformation by sulfonated and sulfated nanoparticles reduces aggregation and cytotoxicity. Macromol. Biosci. 2010, 10, 1152–1163. [Google Scholar] [CrossRef]
- Hanke, M.; Gonzalez Orive, A.; Grundmeier, G.; Keller, A. Effect of DNA Origami Nanostructures on hIAPP Aggregation. Nanomaterials 2020, 10, 2200. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Kalhor, H.R.; Laurent, S.; Lynch, I. Protein fibrillation and nanoparticle interactions: Opportunities and challenges. Nanoscale 2013, 5, 2570–2588. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Chang, Y.-J.; Yoshiike, Y.; Chang, Y.-C.; Chen, Y.-R. Negatively charged gold nanoparticles inhibit Alzheimer’s amyloid-β fibrillization, induce fibril dissociation, and mitigate neurotoxicity. Small 2012, 8, 3631–3639. [Google Scholar] [CrossRef]
- Ke, P.C.; Pilkington, E.H.; Sun, Y.; Javed, I.; Kakinen, A.; Peng, G.; Ding, F.; Davis, T.P. Mitigation of Amyloidosis with Nanomaterials. Adv. Mater. 2019, 1901690. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.-H.; Lee, H.; Nam, J.-M. How Do the Size, Charge and Shape of Nanoparticles Affect Amyloid β Aggregation on Brain Lipid Bilayer? Sci. Rep. 2016, 6, 19548. [Google Scholar] [CrossRef]
- Li, C.; Born, A.-K.; Schweizer, T.; Zenobi-Wong, M.; Cerruti, M.; Mezzenga, R. Amyloid-hydroxyapatite bone biomimetic composites. Adv. Mater. 2014, 26, 3207–3212. [Google Scholar] [CrossRef]
- Shen, Y.; Nyström, G.; Mezzenga, R. Amyloid Fibrils form Hybrid Colloidal Gels and Aerogels with Dispersed CaCO3 Nanoparticles. Adv. Funct. Mater. 2017, 27, 1700897. [Google Scholar] [CrossRef]
- Li, C.; Qin, R.; Liu, R.; Miao, S.; Yang, P. Functional amyloid materials at surfaces/interfaces. Biomater. Sci. 2018, 6, 462–472. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Wang, X.; An, B.; Cui, M.; Pu, J.; Wei, S.; Xue, S.; Ye, H.; Zhao, Y.; et al. Patterned Amyloid Materials Integrating Robustness and Genetically Programmable Functionality. Nano Lett. 2019, 19, 8399–8408. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Mezzenga, R. Amyloid Fibrils as Building Blocks for Natural and Artificial Functional Materials. Adv. Mater. 2016, 28, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Hajiraissi, R.; Hanke, M.; Yang, Y.; Duderija, B.; Gonzalez Orive, A.; Grundmeier, G.; Keller, A. Adsorption and Fibrillization of Islet Amyloid Polypeptide at Self-Assembled Monolayers Studied by QCM-D, AFM, and PM-IRRAS. Langmuir 2018, 34, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Hajiraissi, R.; Giner, I.; Grundmeier, G.; Keller, A. Self-Assembly, Dynamics, and Polymorphism of hIAPP(20-29) Aggregates at Solid-Liquid Interfaces. Langmuir 2017, 33, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Shalabaeva, V.; Di Cola, E.; Burghammer, M.; Krahne, R.; Riekel, C.; Dante, S. Superhydrophobic Surfaces Boost Fibril Self-Assembly of Amyloid β Peptides. ACS Appl. Mater. Interfaces 2015, 7, 20875–20884. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, T.; Holtzman, D.M. In situ atomic force microscopy study of Alzheimer’s-amyloid peptide on different substrates: New insights into mechanism of -sheet formation. Proc. Natl. Acad. Sci. USA 1999, 96, 3688–3693. [Google Scholar] [CrossRef]
- Moores, B.; Drolle, E.; Attwood, S.J.; Simons, J.; Leonenko, Z. Effect of surfaces on amyloid fibril formation. PLoS ONE 2011, 6, e25954. [Google Scholar] [CrossRef]
- Wang, Q.; Shah, N.; Zhao, J.; Wang, C.; Zhao, C.; Liu, L.; Li, L.; Zhou, F.; Zheng, J. Structural, morphological, and kinetic studies of β-amyloid peptide aggregation on self-assembled monolayers. Phys. Chem. Chem. Phys. 2011, 13, 15200–15210. [Google Scholar] [CrossRef]
- Keller, A.; Fritzsche, M.; Yu, Y.-P.; Liu, Q.; Li, Y.-M.; Dong, M.; Besenbacher, F. Influence of hydrophobicity on the surface-catalyzed assembly of the islet amyloid polypeptide. ACS Nano 2011, 5, 2770–2778. [Google Scholar] [CrossRef]
- Giacomelli, C.E.; Norde, W. Conformational changes of the amyloid beta-peptide (1–40) adsorbed on solid surfaces. Macromol. Biosci. 2005, 5, 401–407. [Google Scholar] [CrossRef]
- Nayak, A.; Dutta, A.K.; Belfort, G. Surface-enhanced nucleation of insulin amyloid fibrillation. Biochem. Biophys. Res. Commun. 2008, 369, 303–307. [Google Scholar] [CrossRef]
- Rabe, M.; Soragni, A.; Reynolds, N.P.; Verdes, D.; Liverani, E.; Riek, R.; Seeger, S. On-surface aggregation of α-synuclein at nanomolar concentrations results in two distinct growth mechanisms. ACS Chem. Neurosci. 2013, 4, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Souillac, P.O.; Ionescu-Zanetti, C.; Carter, S.A.; Fink, A.L. Surface-catalyzed amyloid fibril formation. J. Biol. Chem. 2002, 277, 50914–50922. [Google Scholar] [CrossRef] [Green Version]
- Hajiraissi, R.; Hanke, M.; Gonzalez Orive, A.; Duderija, B.; Hofmann, U.; Zhang, Y.; Grundmeier, G.; Keller, A. Effect of Terminal Modifications on the Adsorption and Assembly of hIAPP(20–29). ACS Omega 2019, 4, 2649–2660. [Google Scholar] [CrossRef]
- Cheung, D.L. Effect of surface chemistry on islet amyloid polypeptide conformation. Biointerphases 2020, 15, 51001. [Google Scholar] [CrossRef]
- Pandey, L.M.; Le Denmat, S.; Delabouglise, D.; Bruckert, F.; Pattanayek, S.K.; Weidenhaupt, M. Surface chemistry at the nanometer scale influences insulin aggregation. Colloids Surf. B Biointerfaces 2012, 100, 69–76. [Google Scholar] [CrossRef]
- Dove, P.M.; Craven, C.M. Surface charge density on silica in alkali and alkaline earth chloride electrolyte solutions. Geochim. Cosmochim. Acta 2005, 69, 4963–4970. [Google Scholar] [CrossRef]
- Nishino, M.; Matsuzaki, I.; Musangile, F.Y.; Takahashi, Y.; Iwahashi, Y.; Warigaya, K.; Kinoshita, Y.; Kojima, F.; Murata, S.-I. Measurement and visualization of cell membrane surface charge in fixed cultured cells related with cell morphology. PLoS ONE 2020, 15, e0236373. [Google Scholar] [CrossRef]
- Srinivasan, S.; Sawyer, P. Role of surface charge of the blood vessel wall, blood cells, and prosthetic materials in intravascular thrombosis. J. Colloid Interface Sci. 1970, 32, 456–463. [Google Scholar] [CrossRef]
- Höppener, J.W.; Ahrén, B.; Lips, C.J. Islet amyloid and type 2 diabetes mellitus. N. Engl. J. Med. 2000, 343, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Brender, J.R.; Krishnamoorthy, J.; Sciacca, M.F.M.; Vivekanandan, S.; D’Urso, L.; Chen, J.; La Rosa, C.; Ramamoorthy, A. Probing the sources of the apparent irreproducibility of amyloid formation: Drastic changes in kinetics and a switch in mechanism due to micellelike oligomer formation at critical concentrations of IAPP. J. Phys. Chem. B 2015, 119, 2886–2896. [Google Scholar] [CrossRef]
- Goldsbury, C.S.; Cooper, G.J.; Goldie, K.N.; Müller, S.A.; Saafi, E.L.; Gruijters, W.T.; Misur, M.P.; Engel, A.; Aebi, U.; Kistler, J. Polymorphic fibrillar assembly of human amylin. J. Struct. Biol. 1997, 119, 17–27. [Google Scholar] [CrossRef]
- Goldsbury, C.; Goldie, K.; Pellaud, J.; Seelig, J.; Frey, P.; Müller, S.A.; Kistler, J.; Cooper, G.J.; Aebi, U. Amyloid fibril formation from full-length and fragments of amylin. J. Struct. Biol. 2000, 130, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Green, J.D.; Goldsbury, C.; Kistler, J.; Cooper, G.J.S.; Aebi, U. Human amylin oligomer growth and fibril elongation define two distinct phases in amyloid formation. J. Biol. Chem. 2004, 279, 12206–12212. [Google Scholar] [CrossRef] [Green Version]
- Kayed, R.; Bernhagen, J.; Greenfield, N.; Sweimeh, K.; Brunner, H.; Voelter, W.; Kapurniotu, A. Conformational transitions of islet amyloid polypeptide (IAPP) in amyloid formation in vitro. J. Mol. Biol. 1999, 287, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Marek, P.; Abedini, A.; Song, B.; Kanungo, M.; Johnson, M.E.; Gupta, R.; Zaman, W.; Wong, S.S.; Raleigh, D.P. Aromatic interactions are not required for amyloid fibril formation by islet amyloid polypeptide but do influence the rate of fibril formation and fibril morphology. Biochemistry 2007, 46, 3255–3261. [Google Scholar] [CrossRef]
- Pilkington, E.H.; Xing, Y.; Wang, B.; Kakinen, A.; Wang, M.; Davis, T.P.; Ding, F.; Ke, P.C. Effects of Protein Corona on IAPP Amyloid Aggregation, Fibril Remodelling, and Cytotoxicity. Sci. Rep. 2017, 7, 2455. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez Camargo, D.C.; Garg, D.; Buday, K.; Franko, A.; Rodriguez Camargo, A.; Schmidt, F.; Cox, S.J.; Suladze, S.; Haslbeck, M.; Mideksa, Y.G.; et al. hIAPP forms toxic oligomers in plasma. Chem. Commun. 2018, 54, 5426–5429. [Google Scholar] [CrossRef] [PubMed]
- Sivanesam, K.; Andersen, N.H. Inhibition of Human Amylin Amyloidogenesis by Human Amylin-Fragment Peptides: Exploring the Effects of Serine Residues and Oligomerization upon Inhibitory Potency. Biochemistry 2017, 56, 5373–5379. [Google Scholar] [CrossRef]
- Tu, L.-H.; Serrano, A.L.; Zanni, M.T.; Raleigh, D.P. Mutational analysis of preamyloid intermediates: The role of his-tyr interactions in islet amyloid formation. Biophys. J. 2014, 106, 1520–1527. [Google Scholar] [CrossRef] [Green Version]
- Goldsbury, C.; Kistler, J.; Aebi, U.; Arvinte, T.; Cooper, G.J. Watching amyloid fibrils grow by time-lapse atomic force microscopy. J. Mol. Biol. 1999, 285, 33–39. [Google Scholar] [CrossRef]
- Yu, Y.-P.; Zhang, S.; Liu, Q.; Li, Y.-M.; Wang, C.; Besenbacher, F.; Dong, M. 2D amyloid aggregation of human islet amyloid polypeptide at the solid–liquid interface. Soft Matter 2012, 8, 1616–1622. [Google Scholar] [CrossRef]
- Jeworrek, C.; Hollmann, O.; Steitz, R.; Winter, R.; Czeslik, C. Interaction of IAPP and insulin with model interfaces studied using neutron reflectometry. Biophys. J. 2009, 96, 1115–1123. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.; Facsko, S. Ion-Induced Nanoscale Ripple Patterns on Si Surfaces: Theory and Experiment. Materials 2010, 3, 4811–4841. [Google Scholar] [CrossRef] [Green Version]
- Wittenbrink, I.; Hausmann, A.; Schickle, K.; Lauria, I.; Davtalab, R.; Foss, M.; Keller, A.; Fischer, H. Low-aspect ratio nanopatterns on bioinert alumina influence the response and morphology of osteoblast-like cells. Biomaterials 2015, 62, 58–65. [Google Scholar] [CrossRef]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef] [Green Version]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, Y.; Lin, Y.; Yagi, H.; Lee, Y.-H.; Kitayama, H.; Sakurai, K.; So, M.; Ogi, H.; Naiki, H.; Goto, Y. Distinguishing crystal-like amyloid fibrils and glass-like amorphous aggregates from their kinetics of formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14446–14451. [Google Scholar] [CrossRef] [Green Version]
- Kielar, C.; Ramakrishnan, S.; Fricke, S.; Grundmeier, G.; Keller, A. Dynamics of DNA Origami Lattice Formation at Solid-Liquid Interfaces. ACS Appl. Mater. Interfaces 2018, 10, 44844–44853. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Banerjee, S.; Zagorski, K.; Shlyakhtenko, L.S.; Kolomeisky, A.B.; Lyubchenko, Y.L. Molecular Model for the Surface-Catalyzed Protein Self-Assembly. J. Phys. Chem. B 2020, 124, 366–372. [Google Scholar] [CrossRef]
- Jayasinghe, S.A.; Langen, R. Lipid membranes modulate the structure of islet amyloid polypeptide. Biochemistry 2005, 44, 12113–12119. [Google Scholar] [CrossRef]
- Knight, J.D.; Miranker, A.D. Phospholipid catalysis of diabetic amyloid assembly. J. Mol. Biol. 2004, 341, 1175–1187. [Google Scholar] [CrossRef]
- Caillon, L.; Lequin, O.; Khemtémourian, L. Evaluation of membrane models and their composition for islet amyloid polypeptide-membrane aggregation. Biochim. Biophys. Acta 2013, 1828, 2091–2098. [Google Scholar] [CrossRef]
- Cuddy, M.F.; Poda, A.R.; Brantley, L.N. Determination of isoelectric points and the role of pH for common quartz crystal microbalance sensors. ACS Appl. Mater. Interfaces 2013, 5, 3514–3518. [Google Scholar] [CrossRef]
- Hirota, N.; Edskes, H.; Hall, D. Unified theoretical description of the kinetics of protein aggregation. Biophys. Rev. 2019, 11, 191–208. [Google Scholar] [CrossRef]
- Keller, A.; Cuerno, R.; Facsko, S.; Möller, W. Anisotropic scaling of ripple morphologies on high-fluence sputtered silicon. Phys. Rev. B 2009, 79, 115437. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.; Roßbach, S.; Facsko, S.; Möller, W. Simultaneous formation of two ripple modes on ion sputtered silicon. Nanotechnology 2008, 19, 135303. [Google Scholar] [CrossRef]
- Keller, A.; Fritzsche, M.; Ogaki, R.; Bald, I.; Facsko, S.; Dong, M.; Kingshott, P.; Besenbacher, F. Tuning the hydrophobicity of mica surfaces by hyperthermal Ar ion irradiation. J. Chem. Phys. 2011, 134, 104705. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanke, M.; Yang, Y.; Ji, Y.; Grundmeier, G.; Keller, A. Nanoscale Surface Topography Modulates hIAPP Aggregation Pathways at Solid–Liquid Interfaces. Int. J. Mol. Sci. 2021, 22, 5142. https://doi.org/10.3390/ijms22105142
Hanke M, Yang Y, Ji Y, Grundmeier G, Keller A. Nanoscale Surface Topography Modulates hIAPP Aggregation Pathways at Solid–Liquid Interfaces. International Journal of Molecular Sciences. 2021; 22(10):5142. https://doi.org/10.3390/ijms22105142
Chicago/Turabian StyleHanke, Marcel, Yu Yang, Yuxin Ji, Guido Grundmeier, and Adrian Keller. 2021. "Nanoscale Surface Topography Modulates hIAPP Aggregation Pathways at Solid–Liquid Interfaces" International Journal of Molecular Sciences 22, no. 10: 5142. https://doi.org/10.3390/ijms22105142
APA StyleHanke, M., Yang, Y., Ji, Y., Grundmeier, G., & Keller, A. (2021). Nanoscale Surface Topography Modulates hIAPP Aggregation Pathways at Solid–Liquid Interfaces. International Journal of Molecular Sciences, 22(10), 5142. https://doi.org/10.3390/ijms22105142