Deregulation of Secreted Frizzled-Related Protein 5 in Nonalcoholic Fatty Liver Disease Associated with Obesity
Abstract
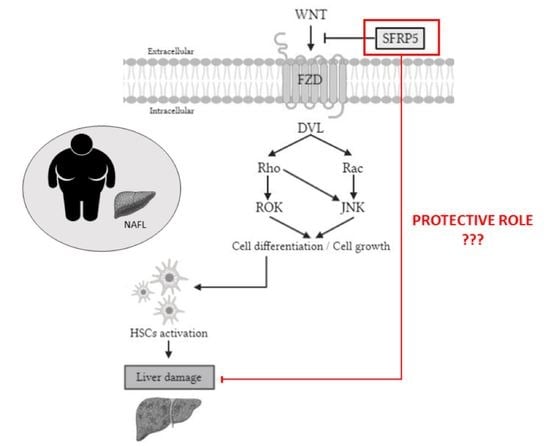
:1. Introduction
2. Results
2.1. Baseline Characteristics of Subjects
2.2. Evaluation of Serum SFRP5 Levels According to BMI and Hepatic Histology
2.3. Evaluation of Relative mRNA Abundance of SFRP5 in Liver According to Hepatic Histology
2.4. Evaluation of the Relative mRNA Abundance of SFRP5 in Liver According to Liver Inflammation-Related Parameters
2.5. Hepatic Expression of Validated Proinflammatory Molecules in SS and NASH Groups
2.6. Correlations of Relative Hepatic mRNA Abundance of SFRP5 with Clinical and Biochemical Parameters and with the Studied Adipocytokines
2.7. Evaluation of Relative mRNA Abundance of WNT5A and JNK in Liver According to Hepatic Histology
2.8. Correlations of Relative Hepatic mRNA Abundance of WNT5A and JNK with Clinical, Biochemical, and Other Parameters
2.9. Evaluation of Relative mRNA Abundance of SFRP5, WNT5A and JNK in Liver According to Hepatic Histology
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Sample Size
4.3. Liver Pathology
4.4. Biochemical Analyses
4.5. Gene Expression in the Liver
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| DBP | Diastolic blood pressure |
| GGT | Gamma-glutamyltransferase |
| HbA1c | Glycosylated hemoglobin |
| HCC | Hepatocellular carcinoma |
| HDL-C | High-density lipoprotein cholesterol |
| HOMA2-IR | Homeostatic model assessment method-insulin resistance |
| IL | Interleukin |
| JNK | Jun N-terminal kinase |
| LDL-C | Low-density lipoprotein cholesterol |
| MetS | Metabolic syndrome |
| MO | Morbid obesity |
| NAFLD | Nonalcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| NL | Normal liver |
| NW | Normal weigh |
| SBP | Systolic blood pressure |
| SFRP5 | Secreted frizzled-related protein 5 |
| SS | Simple steatosis |
| T2DM | Type 2 diabetes mellitus |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor α |
| WNT | Wingless-MMTV integration site |
| WNT5A | WNT family member 5a |
References
- Araújo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global Epidemiology of Non-Alcoholic Fatty Liver Disease/Non-Alcoholic Steatohepatitis: What We Need in the Future. Liver Int. 2018, 38, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and Natural History of Non-Alcoholic Fatty Liver Disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuman, M.G.; Cohen, L.B.; Nanau, R.M. Biomarkers in Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2014, 28, 607–618. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, X.; Liang, G.; Sun, J.; Lin, Z.; Hu, R.; Chen, P.; Zhang, Z.; Zhou, L.; Li, Y. Recombinant SFRP5 Protein Significantly Alleviated Intrahepatic Inflammation of Nonalcoholic Steatohepatitis. Nutr. Metab. 2017, 14, 56. [Google Scholar] [CrossRef] [Green Version]
- Zhao, A.; Jiang, F.; Yang, G.; Liu, H.; Li, L. Sfrp5 Interacts with Slurp1 to Regulate the Accumulation of Triglycerides in Hepatocyte Steatosis Model. Biochem. Biophys. Res. Commun. 2019, 512, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-B.; Chen, X.-D.; Zhou, X.-Y.; Zhu, Q. The Wnt Antagonist and Secreted Frizzled-Related Protein 5: Implications on Lipid Metabolism, Inflammation, and Type 2 Diabetes Mellitus. Biosci. Rep. 2018, 38, BSR20180011. [Google Scholar] [CrossRef]
- Mori, H.; Prestwich, T.C.; Reid, M.A.; Longo, K.A.; Gerin, I.; Cawthorn, W.P.; Susulic, V.S.; Krishnan, V.; Greenfield, A.; MacDougald, O.A. Secreted Frizzled-Related Protein 5 Suppresses Adipocyte Mitochondrial Metabolism through WNT Inhibition. J. Clin. Investig. 2012, 122, 2405–2416. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.; Ji, Q.; Du, Y.; Zhu, X.; Zhu, C.; Zhou, Y. Sfrp5/Wnt Pathway: A Protective Regulatory System in Atherosclerotic Cardiovascular Disease. J. Interferon Cytokine Res. 2019, 39, 472–482. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, L.; Li, L.; Yan, Q.; Li, J.; Xu, T. Emerging Role and Therapeutic Implication of Wnt Signaling Pathways in Liver Fibrosis. Gene 2018, 674, 57–69. [Google Scholar] [CrossRef]
- Delogu, W.; Caligiuri, A.; Provenzano, A.; Rosso, C.; Bugianesi, E.; Coratti, A.; Macias-Barragan, J.; Galastri, S.; Di Maira, G.; Marra, F. Myostatin Regulates the Fibrogenic Phenotype of Hepatic Stellate Cells via C-Jun N-Terminal Kinase Activation. Dig. Liver Dis. 2019, 51, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, L.; Yang, M.; Luo, X.; Ran, W.; Liu, D.; Xiong, Z.; Liu, H.; Yang, G. Circulating Sfrp5 Is a Signature of Obesity-Related Metabolic Disorders and Is Regulated by Glucose and Liraglutide in Humans. J. Clin. Endocrinol. Metab. 2013, 98, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Higuchi, A.; Ohashi, K.; Oshima, Y.; Gokce, N.; Shibata, R.; Akasaki, Y.; Shimono, A.; Walsh, K. Sfrp5 Is an Anti-Inflammatory Adipokine That Modulates Metabolic Dysfunction in Obesity. Science 2010, 329, 454–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, A.; Mandrup, S. Lighting the Fat Furnace without SFRP5. J. Clin. Investig. 2012, 122, 2349–2352. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tian, M.; Yang, M.; Yang, G.; Chen, J.; Wang, H.; Liu, D.; Wang, H.; Deng, W.; Zhu, Z.; et al. Central Sfrp5 Regulates Hepatic Glucose Flux and VLDL-Triglyceride Secretion. Metabolism 2020, 103, 154029. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Wang, X.; Chu, H.; Liu, H.; Yi, X.; Xiao, Y. SFRP5 Correlates with Obesity and Metabolic Syndrome and Increases after Weight Loss in Children. Clin. Endocrinol. 2014, 81, 363–369. [Google Scholar] [CrossRef]
- Schulte, D.M.; Müller, N.; Neumann, K.; Oberhäuser, F.; Faust, M.; Güdelhöfer, H.; Brandt, B.; Krone, W.; Laudes, M. Pro-Inflammatory Wnt5a and Anti-Inflammatory SFRP5 Are Differentially Regulated by Nutritional Factors in Obese Human Subjects. PLoS ONE 2012, 7, e32437. [Google Scholar] [CrossRef]
- Gutiérrez-Vidal, R.; Vega-Badillo, J.; Reyes-Fermín, L.M.; Hernández-Pérez, H.A.; Sánchez-Muñoz, F.; López-Álvarez, G.S.; Larrieta-Carrasco, E.; Fernández-Silva, I.; Méndez-Sánchez, N.; Tovar, A.R.; et al. SFRP5 Hepatic Expression Is Associated with Non-Alcoholic Liver Disease in Morbidly Obese Women. Ann. Hepatol. 2015, 14, 666–674. [Google Scholar] [CrossRef]
- Farrell, G.C.; van Rooyen, D.; Gan, L.; Chitturi, S. NASH Is an Infl Ammatory Disorder: Pathogenic, Prognostic and Therapeutic Implications. Gut Liver 2012, 6, 149–171. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Song, K.; Srivastava, R.; Dong, C.; Go, G.-W.; Li, N.; Iwakiri, Y.; Mani, A. Nonalcoholic Fatty Liver Disease Induced by Noncanonical Wnt and Its Rescue by Wnt3a. FASEB J. 2015, 29, 3436–3445. [Google Scholar] [CrossRef] [Green Version]
- Kodama, Y.; Kisseleva, T.; Iwaisako, K.; Miura, K.; Taura, K.; De Minicis, S.; Österreicher, C.H.; Schnabl, B.; Seki, E.; Brenner, D.A. C-Jun N-Terminal Kinase-1 From Hematopoietic Cells Mediates Progression From Hepatic Steatosis to Steatohepatitis and Fibrosis in Mice. Gastroenterology 2009, 137, 1467–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A Central Role for JNK in Obesity and Insulin Resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Coccia, F.; Testa, M.; Guarisco, G.; Bonci, E.; Di Cristofano, C.; Silecchia, G.; Leonetti, F.; Gastaldelli, A.; Capoccia, D. Noninvasive Assessment of Hepatic Steatosis and Fibrosis in Patients with Severe Obesity. Endocrine 2020, 67, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Neuschwander-Tetri, B.A. Nonalcoholic Steatohepatitis: A Proposal for Grading and Staging the Histological Lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]






| MO (n = 69) | ||||
|---|---|---|---|---|
| NW | NL | SS | NASH | |
| Variables | (n = 20) | (n = 28) | (n = 24) | (n = 17) |
| Weight (kg) | 54.20(51.00–64.25) | 116.50(107.25–130.50) * | 113.20(108.33–128.00) & | 112.00(104.65–125.00) ^ |
| BMI (kg/m2) | 21.97(20.47–23.90) | 43.30(40.94–46.47) * | 44.35(40.82–46.83) & | 44.46(40.76–46.03) ^ |
| Glucose (mg/dL) | 90.00(84.00–97.00) | 85.50(76.25–93.00) | 93.50(85.75–107.00) $ | 91.00(82.50–101.20) |
| Insulin (mUI/L) | 7.80(4.80–9.90) | 9.43(5.59–16.21) | 11.27(7.81–14.51) & | 6.57(5.09–23.04) |
| HOMA 2-IR | 1.05(0.60–1.30) | 1.23(0.75–2.05) | 1.49(0.95–2.18) | 0.86(0.61–3.00) |
| HbA1c (%) | 4.75(4.50–5.03) | 5.40(5.30–5.70) * | 5.60(5.25–6.03) & | 5.50(5.20–6.10) ^ |
| Cholesterol (mg/dL) | 193.98 ± 30.66 | 171.88 ± 36.20 | 174.42 ± 35.41 & | 185.28 ± 43.39 |
| HDL-C (mg/dL) | 63.75 ± 16.03 | 40.84 ± 9.89 * | 42.56 ± 12.38 & | 38.89 ± 8.47 ^ |
| LDL-C (mg/dL) | 109.99 ± 30.67 | 108.16±27.94 | 104.39 ± 31.21 | 104.62 ± 31.58 |
| TG (mg/dL) | 79.50(49.50–149.25) | 106.00(89.00–136.00) * | 129.50(85.75––175.50) & | 140.00(106.00–247.00) ^ |
| AST (UI/L) | 19.50(15.75–23.00) | 20.50(15.75–36.25) | 23.00(17.00–35.00) | 24.00(17.00–43.00) ^ |
| ALT (UI/L) | 15.00(12.00–21.00) | 22.00(16.00–27.00) * | 31.00(23.00–35.75) $,& | 30.00(15.50–40.00) ^ |
| GGT (UI/L) | 12.00(9.00–20.00) | 18.00(16.00–27.00) | 21.00(16.25–30.50) & | 25.00(15.00–27.00) ^ |
| ALP (Ul/L) | 54.44 ± 14.10 | 60.42 ± 13.09 | 75.80 ± 11.66 $,& | 62.77 ± 11.16 ” |
| SBP (mmHg) | 118.56 ± 10.92 | 119.00 ± 18.26 | 120.09 ± 13.41 | 113.44 ± 13.96 |
| DBP (mmHg) | 72.00(68.50–75.00) | 63.00(57.75–75.75) | 62.00(59.00–72.50) | 65.50(56.75–70.75) |
| Variables | WNT5A mRNA Hepatic R.E. | |
|---|---|---|
| rho | p-Value | |
| GGT (Ul/L) | 0.318 | 0.033 |
| SFRP5 hepatic R.E. | 0.535 | <0.001 |
| JNK hepatic R.E. | 0.846 | <0.001 |
| Variables | JNK mRNA Hepatic R.E. | |
|---|---|---|
| rho | p-Value | |
| SFRP5 hepatic R.E. | 0.513 | 0.001 |
| WNT5A hepatic R.E. | 0.846 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertran, L.; Portillo-Carrasquer, M.; Aguilar, C.; Porras, J.A.; Riesco, D.; Martínez, S.; Vives, M.; Sabench, F.; Gonzalez, E.; Del Castillo, D.; et al. Deregulation of Secreted Frizzled-Related Protein 5 in Nonalcoholic Fatty Liver Disease Associated with Obesity. Int. J. Mol. Sci. 2021, 22, 6895. https://doi.org/10.3390/ijms22136895
Bertran L, Portillo-Carrasquer M, Aguilar C, Porras JA, Riesco D, Martínez S, Vives M, Sabench F, Gonzalez E, Del Castillo D, et al. Deregulation of Secreted Frizzled-Related Protein 5 in Nonalcoholic Fatty Liver Disease Associated with Obesity. International Journal of Molecular Sciences. 2021; 22(13):6895. https://doi.org/10.3390/ijms22136895
Chicago/Turabian StyleBertran, Laia, Marta Portillo-Carrasquer, Carmen Aguilar, José Antonio Porras, David Riesco, Salomé Martínez, Margarita Vives, Fàtima Sabench, Eva Gonzalez, Daniel Del Castillo, and et al. 2021. "Deregulation of Secreted Frizzled-Related Protein 5 in Nonalcoholic Fatty Liver Disease Associated with Obesity" International Journal of Molecular Sciences 22, no. 13: 6895. https://doi.org/10.3390/ijms22136895
APA StyleBertran, L., Portillo-Carrasquer, M., Aguilar, C., Porras, J. A., Riesco, D., Martínez, S., Vives, M., Sabench, F., Gonzalez, E., Del Castillo, D., Richart, C., & Auguet, T. (2021). Deregulation of Secreted Frizzled-Related Protein 5 in Nonalcoholic Fatty Liver Disease Associated with Obesity. International Journal of Molecular Sciences, 22(13), 6895. https://doi.org/10.3390/ijms22136895