In Vivo Study of the Efficacy and Safety of 5-Aminolevulinic Radiodynamic Therapy for Glioblastoma Fractionated Radiotherapy
Abstract
:1. Introduction
2. Results
2.1. 5-ALA Enhanced Cellular Response to Single-Dose Ionizing Radiation
2.2. 5-ALA Enhanced the Response to Fractionated Irradiation in Terms of Tumor Growth
2.3. Morphological Observation of Tumor Tissues
2.4. Effect of 5-ALA on Fractionated Irradiation in Normal Tissues
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. X-ray Irradiation Conditions
4.3. Clonogenic Assay
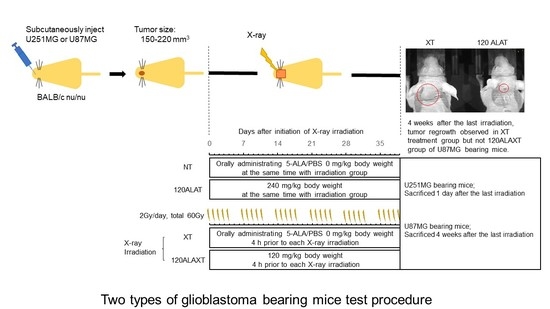
4.4. RDT Treatment for Two Types of Glioblastoma-Bearing Mice
4.4.1. Grouping and RDT Fractionated Irradiation
4.4.2. Evaluation during the Irradiation Period
4.5. Determination of PpIX Concentration in Cells and Tissue
4.6. Immunocytochemistry or Morphological Observation of Tumor Tissues
4.7. Safety Test of 5-ALA on Fractionated Irradiation
4.7.1. Mice and Breeding Condition
4.7.2. Grouping and RDT Fractionated Irradiation
4.7.3. Evaluation after RDT Fractionated Irradiation
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-ALA | 5-aminolevulinic acid |
| APTIF1 | ATPase inhibitory factor 1 |
| PDD | Photodynamic diagnosis |
| PDT | Photodynamic therapy |
| PpIX | Protoporphyrin IX |
| RDT | Radiodynamic therapy |
| ROS | Reactive oxygen spices |
| RT | Radiation therapy, radiotherapy |
| TMZ | Temozolomide |
References
- Ogura, K.; Mizowaki, T.; Arakawa, Y.; Ogura, M.; Sakanaka, K.; Miyamoto, S.; Hiraoka, M. Initial and cumulative recurrence patterns of glioblastoma after temozolomide-based chemoradiotherapy and salvage treatment: A retrospective cohort study in a single institution. Radiat. Oncol. 2013, 8, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Lawrence, Y.R.; Li, X.A.; el Naqa, I.; Hahn, C.A.; Marks, L.B.; Merchant, T.E.; Dicker, A.P. Radiation dose-volume effects in the brain. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S20–S27. [Google Scholar] [CrossRef] [Green Version]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Kaneko, S.; Kaneko, S. Fluorescence-Guided Resection of Malignant Glioma with 5-ALA. Int. J. Biomed. Imaging 2016, 2016, 6135293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, J.C.; Pottier, R.H.; Pross, D.C. Photodynamic therapy with endogenous protoporphyrin IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Morgan, J.; Oseroff, A.R. Mitochondria-based photodynamic anti-cancer therapy. Adv. Drug Deliv. Rev. 2001, 49, 71–86. [Google Scholar] [CrossRef]
- Stummer, W.; Beck, T.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Etminan, N.; Stepp, H.; Tonn, J.C.; Baumgartner, R.; Herms, J.; et al. Long-sustaining response in a patient with non-resectable, distant recurrence of glioblastoma multiforme treated by interstitial photodynamic therapy using 5-ALA: Case report. J. Neurooncol. 2008, 87, 103–109. [Google Scholar] [CrossRef]
- Johansson, A.; Faber, F.; Kniebühler, G.; Stepp, H.; Sroka, R.; Egensperger, R.; Beyer, W.; Kreth, F.W. Protoporphyrin IX fluorescence and photobleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Lasers Surg. Med. 2013, 45, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Vermandel, M.; Leroy, H.A.; Quidet, M.; Lecomte, F.; Delhem, N.; Mordon, S.; Reyns, N. INtraoperative photoDYnamic Therapy for GliOblastomas (INDYGO): Study Protocol for a Phase I Clinical Trial. Neurosurgery 2019, 84, E414–E419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, A. Clinical uses of 5-aminolaevulinic acid in photodynamic treatment and photodetection of cancer: A review. Cancer Lett. 2020, 490, 165–173. [Google Scholar] [CrossRef]
- Lietke, S.; Schmutzer, M.; Schwartz, C.; Weller, J.; Siller, S.; Aumiller, M.; Heckl, C.; Forbrig, R.; Niyazi, M.; Egensperger, R.; et al. Interstitial Photodynamic Therapy Using 5-ALA for Malignant Glioma Recurrences. Cancers 2021, 13, 1767. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Berg, K.; Selbo, P.K.; Weyergang, A.; Dietze, A.; Prasmickaite, L.; Bonsted, A.; Engesaeter, B.Ø.; Angell-Petersen, E.; Warloe, T.; Frandsen, N.; et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J. Microsc. 2005, 218 Pt 2, 133–147. [Google Scholar] [CrossRef]
- Takahashi, J.; Misawa, M. Characterization of reactive oxygen species generated by protoporphyrin IX under X-ray irradiation. Radiat. Phys. Chem. 2009, 78, 889–898. [Google Scholar] [CrossRef]
- Takahashi, J.; Murakami, M.; Mori, T.; Iwahashi, H. Verification of radiodynamic therapy by medical linear accelerator using a mouse melanoma tumor model. Sci. Rep. 2018, 8, 2728. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Nagasawa, S.; Ikemoto, M.J.; Sato, C.; Sato, M.; Iwahashi, H. Verification of 5-Aminolevurinic Radiodynamic Therapy Using a Murine Melanoma Brain Metastasis Model. Int. J. Mol. Sci. 2019, 20, 5155. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef]
- Schröder, C.; Gramatzki, D.; Vu, E.; Guckenberger, M.; Andratschke, N.; Weller, M.; Hertler, C. Radiotherapy for glioblastoma patients with poor performance status. J. Cancer Res. Clin. Oncol. 2021, 1–10. [Google Scholar] [CrossRef]
- García, S.C.; Moretti, M.B.; Garay, M.V.; Batlle, A. Delta-aminolevulinic acid transport through blood-brain barrier. Gen. Pharmacol. 1998, 31, 579–582. [Google Scholar] [CrossRef]
- Terr, L.; Weiner, L.P. An autoradiographic study of delta-aminolevulinic acid uptake by mouse brain. Exp. Neurol. 1983, 79, 564–568. [Google Scholar] [CrossRef]
- Chan, J.L.; Lee, S.W.; Fraass, B.A.; Normolle, D.P.; Greenberg, H.S.; Junck, L.R.; Gebarski, S.S.; Sandler, H.M. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J. Clin. Oncol. 2002, 20, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ino, Y.; Nakagawa, K.; Tago, M.; Todo, T. High-dose conformal radiotherapy for supratentorial malignant glioma: A historical comparison. Lancet Oncol. 2005, 6, 953–960. [Google Scholar] [CrossRef]
- Navarria, P.; Pessina, F.; Tomatis, S.; Soffietti, R.; Grimaldi, M.; Lopci, E.; Chiti, A.; Leonetti, A.; Casarotti, A.; Rossi, M.; et al. Are three weeks hypofractionated radiation therapy (HFRT) comparable to six weeks for newly diagnosed glioblastoma patients? Results of a phase II study. Oncotarget 2017, 8, 67696–67708. [Google Scholar] [CrossRef]
- Wang, T.; Wu, C.C.; Jani, A.; Estrada, J.; Ung, T.; Chow, D.S.; Soun, J.E.; Saad, S.; Qureshi, Y.H.; Gartrell, R.; et al. Hypofractionated radiation therapy versus standard fractionated radiation therapy with concurrent temozolomide in elderly patients with newly diagnosed glioblastoma. Pract. Radiat. Oncol. 2016, 6, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kusuzaki, K.; Matsubara, T.; Matsumine, A.; Kumamoto, T.; Komada, Y.; Naka, N.; Uchida, A. Periosteal Ewing’s sarcoma treated by photodynamic therapy with acridine orange. Oncol. Rep. 2005, 13, 279–282. [Google Scholar]
- Kusuzaki, K.; Murata, H.; Matsubara, T.; Satonaka, H.; Wakabayashi, T.; Matsumine, A.; Uchida, A. Review. Acridine orange could be an innovative anticancer agent under photon energy. In Vivo 2007, 21, 205–214. [Google Scholar]
- Takahashi, J.; Misawa, M.; Murakami, M.; Mori, T.; Nomura, K.; Iwahashi, H. 5-Aminolevulinic acid enhances cancer radiotherapy in a mouse tumor model. SpringerPlus 2013, 2, 602. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.; Misawa, M.; Iwahashi, H. Combined treatment with X-ray irradiation and 5-aminolevulinic acid elicits better transcriptomic response of cell cycle-related factors than X-ray irradiation alone. Int. J. Radiat. Biol. 2016, 92, 774–789. [Google Scholar] [CrossRef]
- Yamamoto, J.; Ogura, S.; Tanaka, T.; Kitagawa, T.; Nakano, Y.; Saito, T.; Takahashi, M.; Akiba, D.; Nishizawa, S. Radiosensitizing effect of 5-aminolevulinic acid-induced protoporphyrin IX in glioma cells in vitro. Oncol. Rep. 2012, 27, 1748–1752. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, T.; Yamamoto, J.; Tanaka, T.; Nakano, Y.; Akiba, D.; Ueta, K.; Nishizawa, S. 5-Aminolevulinic acid strongly enhances delayed intracellular production of reactive oxygen species (ROS) generated by ionizing irradiation: Quantitative analyses and visualization of intracellular ROS production in glioma cells in vitro. Oncol. Rep. 2015, 33, 583–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, J.; Ogura, S.; Shimajiri, S.; Nakano, Y.; Akiba, D.; Kitagawa, T.; Ueta, K.; Tanaka, T.; Nishizawa, S. 5-aminolevulinic acid-induced protoporphyrin IX with multi-dose ionizing irradiation enhances host antitumor response and strongly inhibits tumor growth in experimental glioma in vivo. Mol. Med. Rep. 2015, 11, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Ueta, K.; Yamamoto, J.; Tanaka, T.; Nakano, Y.; Kitagawa, T.; Nishizawa, S. 5-Aminolevulinic acid enhances mitochondrial stress upon ionizing irradiation exposure and increases delayed production of reactive oxygen species and cell death in glioma cells. Int. J. Mol. Med. 2017, 39, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Nonoguchi, N.; Ikeda, N.; Yoshikawa, N.; Sato, T.; Mishima, Y.; Kajimoto, Y.; Narumi, Y.; Takahashi, J.; Kuroiwa, T. OS3.2 Delta-aminolevulinic acid can decrease the radioresistance of glioma stem cells with mesenchymal phenotypes in vitro and in vivo. Neuro Oncol. 2016, 18, iv7. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Murayama, Y.; Kamada, Y.; Arita, T.; Kosuga, T.; Konishi, H.; Morimura, R.; Shiozaki, A.; Kuriu, Y.; Ikoma, H.; et al. Radiosensitizing effect of 5-aminolevulinic acid in colorectal cancer in vitro and in vivo. Oncol Lett. 2019, 17, 5132–5138. [Google Scholar] [CrossRef] [Green Version]
- Miyake, M.; Tanaka, N.; Hori, S.; Ohnishi, S.; Takahashi, H.; Fujii, T.; Owari, T.; Ohnishi, K.; Iida, K.; Morizawa, Y.; et al. Dual benefit of supplementary oral 5-aminolevulinic acid to pelvic radiotherapy in a syngeneic prostate cancer model. Prostate 2019, 79, 340–351. [Google Scholar] [CrossRef]
- Panetta, J.V.; Cvetkovic, D.; Chen, X.; Chen, L.; Ma, C.C. Radiodynamic therapy using 15-MV radiation combined with 5-aminolevulinic acid and carbamide peroxide for prostate cancer in vivo. Phys. Med. Biol. 2020, 65, 165008. [Google Scholar] [CrossRef]
- Kaneko, T.; Tominaga, M.; Kouzaki, R.; Hanyu, A.; Ueshima, K.; Yamada, H.; Suga, M.; Yamashita, T.; Okimoto, T.; Uto, Y. Radiosensitizing effect of 5-aminolevulinic acid and protoporphyrin IX on carbon-ion beam irradiation. Anticancer Res. 2018, 38, 4313–4317. [Google Scholar] [CrossRef]
- Xia, W.; Zhu, J.; Tang, Y.; Wang, X.; Wei, X.; Zheng, X.; Hou, M.; Li, S. PD-L1 Inhibitor Regulates the miR-33a-5p/PTEN Signaling Pathway and Can Be Targeted to Sensitize Glioblastomas to Radiation. Front Oncol. 2020, 10, 821. [Google Scholar] [CrossRef]
- Albert, I.; Hefti, M.; Luginbuehl, V. Physiological oxygen concentration alters glioma cell malignancy and responsiveness to photodynamic therapy in vitro. Neurol Res. 2014, 36, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Miki, S.; Fujimoto, K.; Fukuoka, K.; Matsushita, Y.; Maida, Y.; Yasukawa, M.; Hayashi, M.; Shinkyo, R.; Kikuchi, K.; et al. Eribulin penetrates brain tumor tissue and prolongs survival of mice harboring intracerebral glioblastoma xenografts. Cancer Sci. 2019, 110, 2247–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Hong, S.H.; Kim, J.Y.; Kim, I.C.; Park, Y.W.; Lee, S.J.; Song, S.W.; Kim, J.J.; Park, G.; Kim, T.M.; et al. Preclinical development of a humanized neutralizing antibody targeting HGF. Exp. Mol. Med. 2017, 49, e309. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhang, L.; Hu, W.; Fan, M.; Jiang, N.; Duan, Y.; Jing, D.; Xiao, W.; Fragoso, R.C.; Lam, K.S.; et al. Dual blockage of STAT3 and ERK1/2 eliminates radioresistant GBM cells. Redox Biol. 2019, 24, 101189. [Google Scholar] [CrossRef]
- Singer, E.; Judkins, J.; Salomonis, N.; Matlaf, L.; Soteropoulos, P.; McAllister, S.; Soroceanu, L. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015, 6, e1601. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Cenizo, L.; Formentini, L.; Aldea, M.; Ortega, A.D.; García-Huerta, P.; Sánchez-Aragó, M.; Cuezva, J.M. Up-regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+-ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype. J. Biol. Chem. 2010, 285, 25308–25313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Shan, Q.; Li, P.; Wu, Y.; Xie, J.; Wang, X. ATPase inhibitory factor 1 is a potential prognostic marker for the migration and invasion of glioma. Oncol. Lett. 2015, 10, 2075–2080. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.E.; Hopper, C.; MacRobert, A.J.; Speight, P.M.; Bown, S.G. Photodynamic therapy of oral cancer: Photosensitisation with systemic aminolaevulinic acid. Lancet 1993, 342, 147–148. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; US Food and Drug Administration: Silver Spring, MD, USA, 2005; pp. 1–27. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-starting-dose-initial-clinical-trials-therapeutics-adult-healthy-volunteers (accessed on 1 July 2021).
- Offersen, C.M.; Skjoeth-Rasmussen, J. Evaluation of the risk of liver damage from the use of 5-aminolevulinic acid for intra-operative identification and resection in patients with malignant gliomas. Acta Neurochir. 2017, 159, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.H.; Jin, S.Z.; Liu, Y.; Xu, R.M.; Yang, J.Z.; Pan, X.N.; Liu, S.Z. Therapeutic effect of gene-therapy in combination with local X-irradiation in a mouse malignant melanoma model. Biochem. Biophys. Res. Commun. 2005, 330, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Doss, M.; Schmidt, A. Rapid determination of urinary total porphyrins by ion exchange chromatography. Z. Klin. Chem. Klin. Biochem. 1971, 9, 415–418. [Google Scholar] [CrossRef] [Green Version]






| 5-ALA (mg/mL) | U251MG (pM/mg Protein) | U87MG (pM/mg Protein) |
|---|---|---|
| 0 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| 30 | 7.1 ± 1.3 | 6.7 ± 1.2 |
| 100 | 23.6 ± 5.1 | 17.8 ± 1.7 |
| 5-ALA (mg/kg) | U251MG (pM/mg Protein) | U87MG (pM/mg Protein) |
|---|---|---|
| 0 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| 60 | 1.1 ± 0.7 | 1.6 ± 0.7 |
| 120 | 4.9 ± 0.6 | 3.5 ± 0.7 |
| Group | NT | 240ALAT | XT | 120ALA XT | 240ALA XT | |
|---|---|---|---|---|---|---|
| X-ray irradiation | 0 Gy | 0 Gy | 60 Gy | 60 Gy | 60 Gy | |
| 5-ALA (mg/kg/day) | 0 | 240 | 0 | 120 | 240 | |
| n | 4 | 4 | 5 | 5 | 5 | |
| Skin | Pathological findings | N | N | p | p | p |
| Acanthosis/fleshiness of epidermal cells | - | - | +++ | +++ | +++ | |
| Apoptosis of epidermal cells | - | - | + | + | + | |
| Atrophy or elimination of follicles of pile | - | - | ++ | ++ | ++ | |
| Atrophy or elimination of sebaceous gland | - | - | +++ | +++ | +++ | |
| Brain | Pathological findings | N | N | N | N | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, J.; Nagasawa, S.; Doi, M.; Takahashi, M.; Narita, Y.; Yamamoto, J.; Ikemoto, M.J.; Iwahashi, H. In Vivo Study of the Efficacy and Safety of 5-Aminolevulinic Radiodynamic Therapy for Glioblastoma Fractionated Radiotherapy. Int. J. Mol. Sci. 2021, 22, 9762. https://doi.org/10.3390/ijms22189762
Takahashi J, Nagasawa S, Doi M, Takahashi M, Narita Y, Yamamoto J, Ikemoto MJ, Iwahashi H. In Vivo Study of the Efficacy and Safety of 5-Aminolevulinic Radiodynamic Therapy for Glioblastoma Fractionated Radiotherapy. International Journal of Molecular Sciences. 2021; 22(18):9762. https://doi.org/10.3390/ijms22189762
Chicago/Turabian StyleTakahashi, Junko, Shinsuke Nagasawa, Motomichi Doi, Masamichi Takahashi, Yoshitaka Narita, Junkoh Yamamoto, Mitsushi J. Ikemoto, and Hitoshi Iwahashi. 2021. "In Vivo Study of the Efficacy and Safety of 5-Aminolevulinic Radiodynamic Therapy for Glioblastoma Fractionated Radiotherapy" International Journal of Molecular Sciences 22, no. 18: 9762. https://doi.org/10.3390/ijms22189762
APA StyleTakahashi, J., Nagasawa, S., Doi, M., Takahashi, M., Narita, Y., Yamamoto, J., Ikemoto, M. J., & Iwahashi, H. (2021). In Vivo Study of the Efficacy and Safety of 5-Aminolevulinic Radiodynamic Therapy for Glioblastoma Fractionated Radiotherapy. International Journal of Molecular Sciences, 22(18), 9762. https://doi.org/10.3390/ijms22189762