Plasma Lipid Profiling Contributes to Untangle the Complexity of Moyamoya Arteriopathy
Abstract
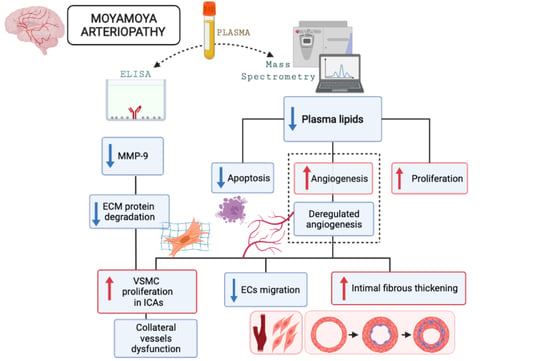
:1. Introduction
2. Results
2.1. MA Patients, Healthy Donors, and Unrelated Subjects Recruitment
2.2. Reduced MMP-9 Level in Plasma of MA Patients
2.3. Overall Lipid Content in Plasma of MA Patients: Untargeted Lipidomic Approach
2.4. Targeted Lipidomics Approach
3. Discussion
4. Materials and Methods
4.1. Moyamoya Patients and Healthy/ACVD Controls: Inclusion Criteria
4.2. Ethical Issues
4.3. Blood and Plasma Samples Collection
4.4. Clinical–Radiological Factors
4.5. ELISA
4.6. Chemicals and Reagents for Lipidomics
4.7. Untargeted Lipidomics
4.8. LC-HR-MS Data Processing
4.9. Sphingoid Long-Chain Bases Sphingoid Long-Chain Bases Determination
4.10. Statistics and Data Visualization
4.10.1. ELISA Statistical Analyses
4.10.2. Lipidomics Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACVD | Atherosclerotic cerebrovascular disease |
| ANG-2 | Angiopoietin-2 |
| CCL5 | Chemokine (C-C motif) ligand 5 |
| cEPCs | Circulating endothelial progenitor cells |
| EDTA | Ethylenediaminetetraacetic acid |
| HD | Healthy donors |
| ICAs | Internal carotid arteries |
| IL-6 | Interleukin 6 |
| IL-8 (CXCL8) | Interleukin 8 |
| MA | Moyamoya arteriopathy |
| MMP-9 | Matrix metalloproteinase 9 |
| MS | Mass Spectrometry |
| RNF213 | Ring Finger Protein 213 |
| TIA | Transient ischemic attack |
| VEGF-A | Vascular endothelial growth factor A |
| VIP | Variance importance in projection scores |
| VSMC | Vascular smooth muscle cell |
References
- Fukui, M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (’moyamoya’ disease). Research committee on spontaneous occlusion of the circle of willis (moyamoya disease) of the ministry of health and welfare, Japan. Clin. Neurol. Neurosurg. 1997, 99, S238–S240. [Google Scholar] [CrossRef]
- Bersano, A.; Guey, S.; Bedini, G.; Nava, S.; Hervé, D.; Vajkoczy, P.; Tatlisumak, T.; Sareela, M.; Van Der Zwan, A.; Klijn, C.J.; et al. Research progresses in understanding the pathophysiology of moyamoya disease. Cerebrovasc. Dis. 2016, 41, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Kossorotoff, M.; Tournier-Lasserve, E.; Herve, D.; Guey, S. Moyamoya disease and syndromes: From genetics to clinical management. Appl. Clin. Genet. 2015, 8, 49–68. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.; Saeki, N.; Oishi, H.; Hirai, S.; Yamaura, A. Long-term natural history of hemorrhagic type moyamoya disease in 42 patients. J. Neurosurg. 2000, 93, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.; Heienbrok, W.; Berlit, P. Moyamoya Disease in Europeans. Stroke 2008, 39, 3193–3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acker, G.; Goerdes, S.; Schneider, U.C.; Schmiedek, P.; Czabanka, M.; Vajkoczy, P. Distinct clinical and radiographic characteristics of moyamoya disease amongst European Caucasians. Eur. J. Neurol. 2015, 22, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Feghali, J.; Xu, R.; Yang, W.; Liew, J.; Tamargo, R.J.; Marsh, E.B.; Huang, J. Racial phenotypes in moyamoya disease: A comparative analysis of clinical presentation and natural history in a single multiethnic cohort of 250 hemispheres. J. Neurosurg. 2019, 133, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Houkin, K.; Ito, M.; Sugiyama, T.; Shichinohe, H.; Nakayama, N.; Kazumata, K.; Kuroda, S. Review of past research and current concepts on the etiology of moyamoya disease. Neurol. Med. Chir. 2012, 52, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedini, G.; Blecharz, K.; Nava, S.; Vajkoczy, P.; Alessandri, G.; Ranieri, M.; Acerbi, F.; Ferroli, P.; Riva, D.; Esposito, S.; et al. Vasculogenic and angiogenic pathways in moyamoya disease. Curr. Med. Chem. 2016, 23, 315–345. [Google Scholar] [CrossRef] [PubMed]
- Kamada, F.; Aoki, Y.; Narisawa, A.; Abe, Y.; Komatsuzaki, S.; Kikuchi, A.; Kanno, J.; Niihori, T.; Ono, M.; Ishii, N.; et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J. Hum. Genet. 2010, 56, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Guey, S.; Kraemer, M.; Herve, D.; Ludwig, T.E.; Kossorotoff, M.; Bergametti, F.; Schwitalla, J.C.; Choi, S.; Broseus, L.; Callebaut, I.; et al. Rare RNF213 variants in the C-terminal region encompassing the RING-finger domain are associated with moyamoya angiopathy in Caucasians. Eur. J. Hum. Genet. 2017, 25, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Raso, A.; Biassoni, R.; Mascelli, S.; Nozza, P.; Ugolotti, E.; Di Marco, E.; De Marco, P.; Merello, E.; Cama, A.; Pavanello, M.; et al. Moyamoya vasculopathy shows a genetic mutational gradient decreasing from East to West. J. Neurosurg. Sci. 2020, 64, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Fallen, S.; Zhou, Y.; Baxter, D.; Scherler, K.; Kuo, M.F.; Wang, K. The impact of moyamoya disease and RNF213 Mutations on the spectrum of plasma protein and microRNA. J. Clin. Med. 2019, 8, 1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, M.; Tezuka, T.; Kim, M.; Choi, J.; Oichi, Y.; Kobayashi, H.; Harada, K.H.; Mizushima, T.; Taketani, S.; Koizumi, A.; et al. Moyamoya disease patient mutations in the RING domain of RNF213 reduce its ubiquitin ligase activity and enhance NFκB activation and apoptosis in an AAA+ domain-dependent manner. Biochem. Biophys. Res. Commun. 2020, 525, 668–674. [Google Scholar] [CrossRef]
- Mertens, R.; Graupera, M.; Gerhardt, H.; Bersano, A.; Tournier-Lasserve, E.; Mensah, M.A.; Mundlos, S.; Vajkoczy, P. The Genetic Basis of Moyamoya Disease. Transl. Stroke Res. 2021, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Piccolis, M.; Bond, L.M.; Kampmann, M.; Pulimeno, P.; Chitraju, C.; Jayson, C.B.K.; Vaites, L.P.; Boland, S.; Lai, Z.W.; Gabriel, K.R.; et al. Probing the Global Cellular Responses to Lipotoxicity Caused by Saturated Fatty Acids. Mol. Cell 2019, 74, 32–44.e8. [Google Scholar] [CrossRef] [Green Version]
- Mineharu, Y.; Miyamoto, S. RNF213 and GUCY1A3 in Moyamoya Disease: Key Regulators of Metabolism, Inflammation, and Vascular Stability. Front. Neurol. 2021, 12, 687088. [Google Scholar] [CrossRef]
- Banh, R.S.; Iorio, C.; Marcotte, R.; Xu, Y.; Cojocari, D.; Rahman, A.A.; Pawling, J.; Zhang, W.; Sinha, A.; Rose, C.M.; et al. PTP1B controls non-mitochondrial oxygen consumption by regulating RNF213 to promote tumour survival during hypoxia. Nat. Cell Biol. 2016, 18, 803–813. [Google Scholar] [CrossRef]
- Tinelli, F.; Nava, S.; Arioli, F.; Bedini, G.; Scelzo, E.; Lisini, D.; Faragò, G.; Gioppo, A.; Ciceri, E.F.; Acerbi, F.; et al. Vascular Remodeling in Moyamoya Angiopathy: From Peripheral Blood Mononuclear Cells to Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 5763. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive lipids and chronic inflammation: Managing the fire within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Nishi, T.; Kobayashi, N.; Hisano, Y.; Kawahara, A.; Yamaguchi, A. Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim. Biophys. Acta. 2014, 1841, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Trostchansky, A.; Rubbo, H. Bioactive Lipids in Health and Disease, 1st ed.; Springer: Cham, Switzerland, 2019; Volume VIII, p. 198. [Google Scholar] [CrossRef]
- Fernandis, A.Z.; Wenk, M.R. Membrane lipids as signaling molecules. Curr. Opin. Lipidol. 2007, 18, 121–128. [Google Scholar] [CrossRef]
- Honn, K.V.; Zeldin, D.C. The Role of Bioactive Lipids in Cancer, Inflammation and Related Diseases. In Advances in Experimental Medicine and Biology, 1st ed.; Springer: Cham, Switzerland, 2019; Volume IX, p. 257. [Google Scholar] [CrossRef]
- Dei Cas, M.; Roda, G.; Li, F.; Secundo, F. Functional lipids in autoimmune inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 3074. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.W. Bishop-Bailey, Lipid mediators in immune regulation and resolution. Br. J. Pharmacol. 2019, 176, 1009–1023. [Google Scholar] [CrossRef] [Green Version]
- Tselepis, A.D.; Chapman, M.J. Inflammation, bioactive lipids and atherosclerosis: Potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase. Atheroscler. Suppl. 2002, 3, 57–68. [Google Scholar] [CrossRef]
- Leishman, E.; Kunkler, P.E.; Hurley, J.H.; Miller, S.; Bradshaw, H.B. Bioactive Lipids in Cancer, Inflammation and Related Diseases, Acute and Chronic Mild Traumatic Brain Injury Differentially Changes Levels of Bioactive Lipids in the CNS Associated with Headache. Adv. Exp. Med. Biol. 2019, 1161, 193–217. [Google Scholar] [CrossRef]
- Camerer, E.; Regard, J.B.; Cornelissen, I.; Srinivasan, Y.; Duong, D.N.; Palmer, D.; Pham, T.H.; Wong, J.S.; Pappu, R.; Coughlin, S.R. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Investig. 2009, 119, 1871–1879. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.J.; Holland, W.L.; Wilson, L.; Tanner, J.M.; Kearns, D.; Cahoon, J.M.; Pettey, D.; Losee, J.; Duncan, B.; Gale, D.; et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 2012, 61, 1848–1859. [Google Scholar] [CrossRef] [Green Version]
- Agatonovic-Kustrin, S.; Morton, D.W.; Smirnov, V.; Petukhov, A.; Gegechkori, V.; Kuzina, V.; Gorpinchenko, N.; Ramenskaya, G. Analytical Strategies in Lipidomics for Discovery of Functional Biomarkers from Human Saliva. Dis. Markers 2019, 2019, 6741518. [Google Scholar] [CrossRef] [Green Version]
- Area-Gomez, E.; Larrea, D.; Yun, T.; Xu, Y.; Hupf, J.; Zandkarimi, F.; Chan, R.B.; Mitsumoto, H. Lipidomics study of plasma from patients suggest that ALS and PLS are part of a continuum of motor neuron disorders. Sci. Rep. 2021, 11, 13562. [Google Scholar] [CrossRef]
- Wenk, M.R. Lipidomics: New tools and applications. Cell 2010, 143, 888–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, S.; Brietzke, E. Recent advances in lipidomics: Analytical and clinical perspectives. Prostaglandins Other Lipid Mediat. 2017, 128–129, 8–16. [Google Scholar] [CrossRef]
- Bersano, A.; on behalf of GEN-O-MA Study Group; Bedini, G.; Nava, S.; Acerbi, F.; Sebastiano, D.R.; Binelli, S.; Franceschetti, S.; Faragò, G.; Grisoli, M.; et al. GEN-O-MA project: An Italian network studying clinical course and pathogenic pathways of moyamoya disease—Study protocol and preliminary results. Neurol. Sci. 2019, 40, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahel, J.; Lehner, A.; Vogel, A.; Schleiffer, A.; Meinhart, A.; Haselbach, D.; Clausen, T. Moyamoya disease factor RNF213 is a giant E3 ligase with a dynein-like core and a distinct ubiquitin-transfer mechanism. eLife 2020, 9, e56185. [Google Scholar] [CrossRef]
- Habu, T.; Harada, K.H. UBC13 is an RNF213-associated E2 ubiquitin-conjugating enzyme, and Lysine 63-linked ubiquitination by the RNF213-UBC13 axis is responsible for angiogenic activity. FASEB Bioadv. 2021, 3, 243–258. [Google Scholar] [CrossRef]
- Scholz, B.; Korn, C.; Wojtarowicz, J.; Mogler, C.; Augustin, I.; Boutros, M.; Niehrs, C.; Augustin, H.G. Endothelial RSPO3 Controls Vascular Stability and Pruning through Non-canonical WNT/Ca(2+)/NFAT Signaling. Dev. Cell 2016, 36, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Mikami, T.; Suzuki, H.; Komatsu, K.; Mikuni, N. Influence of Inflammatory Disease on the Pathophysiology of Moyamoya Disease and Quasi-moyamoya Disease. Neurol. Med. Chir. 2019, 59, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Scott, R.M.; Smith, E.R. Moyamoya disease and moyamoya syndrome. N. Engl. J. Med. 2009, 360, 1226–1237. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, M.; Morito, D.; Ainuki, S.; Hirano, Y.; Ogino, K.; Kitamura, A.; Hirata, H.; Nagata, K. The AAA+ ATPase/ubiquitin ligase mysterin stabilizes cytoplasmic lipid droplets. J. Cell Biol. 2019, 218, 949–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.; Xie, Z.; Zhang, J.; Xu, H.; Su, H.; Tan, X.; Tian, D.; Su, M. Clinical and immunopathological features of Moyamoya disease. PLoS ONE 2012, 7, e36386. [Google Scholar] [CrossRef]
- Morgan, J.; Rouche, A.; Bausero, P.; Houssaïni, A.; Gross, J.; Fiszman, M.Y.; Alameddine, H.S. MMP-9 overexpression improves myogenic cell migration and engraftment. Muscle Nerve 2010, 42, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, J.; Lin, Z.; Shi, G.; Wang, R.; Zhao, Y.; Zhao, Y.; Zhao, J. MMP-9 as a Biomarker for Predicting Hemorrhagic Strokes in Moyamoya Disease. Front. Neurol. 2021, 12, 721118. [Google Scholar] [CrossRef]
- Blecharz-Lang, K.G.; Prinz, V.; Burek, M.; Frey, D.; Schenkel, T.; Krug, S.M.; Fromm, M.; Vajkoczy, P. Gelatinolytic activity of autocrine matrix metalloproteinase-9 leads to endothelial de-arrangement in Moyamoya disease. J. Cereb. Blood Flow Metab. 2018, 38, 1940–1953. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kim, J.H.; Phi, J.H.; Kim, Y.Y.; Kim, J.E.; Wang, K.C.; Cho, B.K.; Kim, S.K. Plasma matrix metalloproteinases, cytokines and angiogenic factors in moyamoya disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Watanabe, M.; Narisawa, A.; Shimizu, H.; Tominaga, T. Increased expression of serum Matrix Metalloproteinase-9 in patients with moyamoya disease. Surg. Neurol. 2009, 72, 476–480. [Google Scholar] [CrossRef]
- Ma, W.; Cui, C.; Feng, S.; Li, G.; Han, G.; Hu, Y.; Li, X.; Lv, J.; Liu, C.; Jin, F. Serum Uric Acid and Triglycerides in Chinese Patients with Newly Diagnosed Moyamoya Disease: A Cross-Sectional Study. Biomed. Res. Int. 2019, 2019, 9792412. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.M.; Zalos, G.; Halcox, J.P.J.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Inokuchi, J.I.; Kanoh, H.; Inamori, K.I.; Nagafuku, M.; Nitta, T.; Fukase, K. Homeostatic and pathogenic roles of the GM3 ganglioside. FEBS J. 2021. [Google Scholar] [CrossRef]
- Kim, H.S.; Han, M.; Park, I.H.; Park, C.H.; Kwak, M.S.; Shin, J.S. Sulfatide Inhibits HMGB1 Secretion by Hindering Toll-Like Receptor 4 Localization Within Lipid Rafts. Front. Immunol. 2020, 11, 1305. [Google Scholar] [CrossRef]
- Choi, J.W.; Son, S.M.; Mook-Jung, I.; Moon, Y.J.; Lee, J.Y.; Wang, K.C.; Kang, H.S.; Phi, J.H.; Choi, S.A.; Chong, S.; et al. Mitochondrial abnormalities related to the dysfunction of circulating endothelial colony-forming cells in moyamoya disease. J. Neurosurg. 2018, 129, 1151–1159. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Han, C.; Jia, Y.; Wang, J.; Ge, W.; Duan, L. Proteomic Profiling of Exosomes From Hemorrhagic Moyamoya Disease and Dysfunction of Mitochondria in Endothelial Cells. Stroke 2021, 52, 3351–3361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sakamoto, W.; Canals, D.; Ishibashi, M.; Matsuda, M.; Nishida, K.; Toyoshima, M.; Shigeta, S.; Taniguchi, M.; Senkal, C.E.; et al. Ceramide synthase 2-C24:1 -ceramide axis limits the metastatic potential of ovarian cancer cells. FASEB J. 2021, 35, e21287. [Google Scholar] [CrossRef]
- Öörni, K.; Jauhiainen, M.; Kovanen, P.T. Why and how increased plasma ceramides predict future cardiovascular events? Atherosclerosis 2020, 314, 71–73. [Google Scholar] [CrossRef]
- Newton, J.; Lima, S.; Maceyka, M.; Spiegel, S. Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Exp. Cell Res. 2015, 333, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, P.; Zhang, Q.; Ye, X.; Liu, X.; Deng, X.; Wang, J.; Wang, R.; Zhang, Y.; Zhang, D.; Zhao, J. Modifiable Risk Factors Associated With Moyamoya Disease: A Case-Control Study. Stroke 2020, 51, 2472–2479. [Google Scholar] [CrossRef]
- Church, E.W.; Bell-Stephens, T.E.; Bigder, M.G.; Gummidipundi, S.; Han, S.S.; Steinberg, G.K. Clinical Course of Unilateral Moyamoya Disease. Neurosurgery 2020, 87, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Miyawaki, S.; Imai, H.; Hongo, H.; Ohara, K.; Dofuku, S.; Teranishi, Y.; Nakatomi, H.; Saito, N. Association Between the Onset Pattern of Adult Moyamoya Disease and Risk Factors for Stroke. Stroke 2020, 51, 3124–3128. [Google Scholar] [CrossRef]
- Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis. Health labour sciences research grant for research on measures for intractable diseases guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of willis). Neurol. Med. Chir. 2012, 52, 245–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.W.; Huang, P.H.; Huang, S.S.; Leu, H.-B.; Huang, C.C.; Wu, T.C.; Chen, J.W.; Lin, S.J. Decreased circulating endothelial progenitor cell levels and function in essential hypertensive patients with electrocardiographic left ventricular hypertrophy. Hypertens. Res. 2011, 34, 999–1003. [Google Scholar] [CrossRef]
- Rossi, F.; Bertone, C.; Montanile, F.; Miglietta, F.; Lubrano, C.; Gandini, L.; Santiemma, V. HDL cholesterol is a strong determinant of endothelial progenitor cells in hypercholesterolemic subjects. Microvasc. Res. 2010, 80, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Miorin, M.; Facco, M.; Bonamico, S.; Baesso, I.; Grego, F.; Menegolo, M.; De Kreutzenberg, S.V.; Tiengo, A.; Agostini, C.; et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2005, 45, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Pucci, L.; Vanacore, R.; Baesso, I.; Penno, G.; Balbarini, A.; Di Stefano, R.; Miccoli, R.; De Kreutzenberg, S.; Coracina, A.; et al. Glucose tolerance is negatively associated with circulating progenitor cell levels. Diabetologia 2007, 50, 2156–2163. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, J.; Takaku, A. Cerebrovascular “moyamoya” disease. Arch. Neurol. 1969, 20, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Zulueta, A.; Mingione, A.; Caretti, A.; Ghidoni, R.; Signorelli, P.; Paroni, R. An Innovative Lipidomic Workflow to Investigate the Lipid Profile in a Cystic Fibrosis Cell Line. Cells 2020, 9, 1197. [Google Scholar] [CrossRef] [PubMed]





| CER 24:1 | Ceramide 24:1 | LPC | Lysophosphatidylcholine |
| CL 64:8 | Cardiolipin 64:8 | LPE | Lysophosphatidylethanolamine |
| DAG | Diacylglycerol | PC | Phosphatidylcholine |
| DHS1P | Dihydrosphingosine-1-phosphate | PE | Phosphatidylethanolamine |
| DHSph | Dihydrosphingosine | PI | Phosphatidylinositol |
| EtherPC | Phosphatidylcholine Ether | S1P | Sphingosine-1-phosphate |
| EtherPE | Phosphatidylethanolamine Ether | SM | Sphingomyelin |
| Gb3 | Globotriaosylceramide | Sph | Sphingosine |
| GM3 | Ganglioside GM3 18:0 | SULF 16:0 | Sulfatide 16:0 |
| HEXCER | Hexosylceramide | TAG | Triacylglycerol |
| LACCER | Lactosylceramide |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dei Cas, M.; Carrozzini, T.; Pollaci, G.; Potenza, A.; Nava, S.; Canavero, I.; Tinelli, F.; Gorla, G.; Vetrano, I.G.; Acerbi, F.; et al. Plasma Lipid Profiling Contributes to Untangle the Complexity of Moyamoya Arteriopathy. Int. J. Mol. Sci. 2021, 22, 13410. https://doi.org/10.3390/ijms222413410
Dei Cas M, Carrozzini T, Pollaci G, Potenza A, Nava S, Canavero I, Tinelli F, Gorla G, Vetrano IG, Acerbi F, et al. Plasma Lipid Profiling Contributes to Untangle the Complexity of Moyamoya Arteriopathy. International Journal of Molecular Sciences. 2021; 22(24):13410. https://doi.org/10.3390/ijms222413410
Chicago/Turabian StyleDei Cas, Michele, Tatiana Carrozzini, Giuliana Pollaci, Antonella Potenza, Sara Nava, Isabella Canavero, Francesca Tinelli, Gemma Gorla, Ignazio G. Vetrano, Francesco Acerbi, and et al. 2021. "Plasma Lipid Profiling Contributes to Untangle the Complexity of Moyamoya Arteriopathy" International Journal of Molecular Sciences 22, no. 24: 13410. https://doi.org/10.3390/ijms222413410
APA StyleDei Cas, M., Carrozzini, T., Pollaci, G., Potenza, A., Nava, S., Canavero, I., Tinelli, F., Gorla, G., Vetrano, I. G., Acerbi, F., Ferroli, P., Ciceri, E. F., Esposito, S., Saletti, V., Ciusani, E., Zulueta, A., Paroni, R., Parati, E. A., Ghidoni, R., ... Gatti, L. (2021). Plasma Lipid Profiling Contributes to Untangle the Complexity of Moyamoya Arteriopathy. International Journal of Molecular Sciences, 22(24), 13410. https://doi.org/10.3390/ijms222413410