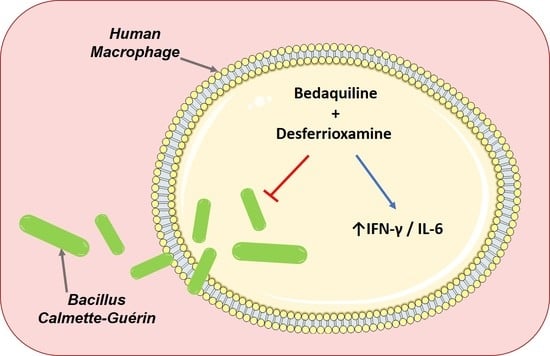
The Iron Chelator Desferrioxamine Increases the Efficacy of Bedaquiline in Primary Human Macrophages Infected with BCG
Abstract
:1. Introduction
2. Results
2.1. Determining Sub-Optimal Concentrations of the Antimicrobials Moxifloxacin, Bedaquiline, Amikacin, Clofazimine, Linezolid and Cycloserine in hMDMs Infected with BCG
2.2. Examining if the Iron Chelator, DFX, Can Increase the Efficacy of Moxifloxacin, Bedaquiline, Amikacin and Clofazimine in Primary hMDMs Infected with BCG
2.3. Investigating the Effect of Combined DFX-Bedaquiline Treatment on Secreted Chemokine and Cytokine Levels in Primary hMDMs Infected with BCG
3. Discussion
4. Materials and Methods
4.1. hMDM Cell Culture
4.2. Infection of hMDMs with M. Bovis BCG
4.3. Estimating Cell Viability and Cell Count Using Propidium Iodide (PI) Based Cell Exclusion Assays and Crystal Violet Assays
4.4. Mycobacterial Growth Inhibition Assay (MGIA)
4.5. Ex Vivo MSD Multiplex ELISA Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cahill, C.; Phelan, J.J.; Keane, J. Understanding and Exploiting the Effect of Tuberculosis Antimicrobials on Host Mitochondrial Function and Bioenergetics. Front. Cell. Infect. Microbiol. 2020, 10, 493. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report 2020; World Health Organisation: Geneva, Switzerland, 2020. [Google Scholar]
- De Voss, J.J.; Rutter, K.-L.; Schroeder, B.G.; Su, H.; Zhu, Y.; Barry, C.E. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. USA 2000, 97, 1252–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.; Dom, G.; Pedrosa, J.; Boelaert, J.; Appelberg, R. Effects of iron deprivation on Mycobacterium avium growth. Tuber. Lung Dis. 1999, 79, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.; Essler, L.; Giri, P.; Quinn, F. Interferon-gamma promotes iron export in human macrophages to limit intracellular bacterial replication. PLoS ONE 2020, 15, e0240949. [Google Scholar] [CrossRef]
- Siegrist, M.S.; Unnikrishnan, M.; McConnell, M.J.; Borowsky, M.; Cheng, T.-Y.; Siddiqi, N.; Fortune, S.M.; Moody, D.B.; Rubin, E.J. Mycobacterial Esx-3 is required for mycobactin-mediated iron acquisition. Proc. Natl. Acad. Sci. USA 2009, 106, 18792–18797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olakanmi, O.; Schlesinger, L.S.; Britigan, B.E. Hereditary hemochromatosis results in decreased iron acquisition and growth by Mycobacterium tuberculosis within human macrophages. J. Leukoc. Biol. 2007, 81, 195–204. [Google Scholar] [CrossRef]
- Cronin, S.J.F.; Woolf, C.J.; Weiss, G.; Penninger, J.M. The Role of Iron Regulation in Immunometabolism and Immune-Related Disease. Front. Mol. Biosci. 2019, 6, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggiali, E.; Cassinerio, E.; Zanaboni, L.; Cappellini, M.D. An update on iron chelation therapy. High Speed Blood Transfus. Equip. 2012, 10, 411–422. [Google Scholar]
- Cronjé, L.; Edmondson, N.; Eisenach, K.D.; Bornman, L. Iron and iron chelating agents modulate Mycobacterium tuberculosis growth and monocyte-macrophage viability and effector functions. FEMS Immunol. Med. Microbiol. 2005, 45, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelan, J.J.; Basdeo, S.A.; Tazoll, S.C.; McGivern, S.; Saborido, J.R.; Keane, J. Modulating Iron for Metabolic Support of TB Host Defense. Front. Immunol. 2018, 9, 2296. [Google Scholar] [CrossRef]
- Pereira, M.; Chen, T.-D.; Buang, N.; Olona, A.; Ko, J.-H.; Prendecki, M.; Costa, A.S.; Nikitopoulou, E.; Tronci, L.; Pusey, C.D.; et al. Acute Iron Deprivation Reprograms Human Macrophage Metabolism and Reduces Inflammation In Vivo. Cell Rep. 2019, 28, 498–511.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelan, J.J.; McQuaid, K.; Kenny, C.; Gogan, K.M.; Cox, D.J.; Basdeo, S.A.; O’Leary, S.; Tazoll, S.C.; Maoldomhnaigh, C.Ó.; O’Sullivan, M.P.; et al. Desferrioxamine Supports Metabolic Function in Primary Human Macrophages Infected With Mycobacterium tuberculosis. Front. Immunol. 2020, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.S.; Jou, R.; Wu, M.-H.; Dalton, T.; Kurbatova, E.; Ershova, J.; Cegielski, J.P. Global PETTS Investigators Susceptibilities of MDR Mycobacterium tuberculosis isolates to unconventional drugs compared with their reported pharmacokinetic/pharmacodynamic parameters. J. Antimicrob. Chemother. 2017, 72, 1678–1687. [Google Scholar] [CrossRef]
- Gillespie, S.H.; Billington, O. Activity of moxifloxacin against mycobacteria. J. Antimicrob. Chemother. 1999, 44, 393–395. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, N.; Goh, K.S.; Horgen, L.; Barrow, W.W. Synergistic activities of antituberculous drugs with cerulenin and trans-cinnamic acid against Mycobacterium tuberculosis. FEMS Immunol. Med. Microbiol. 1998, 21, 149–157. [Google Scholar] [CrossRef]
- Shetty, A.; Dick, T. Mycobacterial Cell Wall Synthesis Inhibitors Cause Lethal ATP Burst. Front. Microbiol. 2018, 9, 1898. [Google Scholar] [CrossRef] [Green Version]
- Singh, R. Determination of Minimum Inhibitory Concentration of Cycloserine in Multidrug Resistant Mycobacterium Tuberculosis Isolates. Jordan J. Biol. Sci. 2014, 7, 139–145. [Google Scholar] [CrossRef]
- Schaible, U.E.; Collins, H.L.; Priem, F.; Kaufmann, S.H. Correction of the iron overload defect in beta-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J. Exp. Med. 2002, 196, 1507–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobin, J.; Horwitz, M.A. Exochelins of Mycobacterium tuberculosis remove iron from human iron-binding proteins and donate iron to mycobactins in the M. tuberculosis cell wall. J. Exp. Med. 1996, 183, 1527–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakiba, Y. Application of Desferal to Treat COVID-19. Application of Desferal to Treat COVID-19: 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04333550 (accessed on 3 February 2021).
- Sandberg, A.; Jensen, K.S.; Baudoux, P.; Van Bambeke, F.; Tulkens, P.M.; Frimodt-Møller, N. Intra- and extracellular activity of linezolid against Staphylococcus aureus in vivo and in vitro. J. Antimicrob. Chemother. 2010, 65, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, D.; Alffenaar, J.-W.C.; Köser, C.U.; Dheda, K.; Chapagain, M.L.; Simbar, N.; Schön, T.; Sturkenboom, M.G.G.; McIlleron, H.; Lee, P.S.; et al. d-Cycloserine Pharmacokinetics/Pharmacodynamics, Susceptibility, and Dosing Implications in Multidrug-resistant Tuberculosis: A Faustian Deal. Clin. Infect. Dis. 2018, 67, S308–S316. [Google Scholar] [CrossRef]
- Song, T.; Lee, M.; Jeon, H.-S.; Park, Y.; Dodd, L.E.; Dartois, V.; Follman, D.; Wang, J.; Cai, Y.; Goldfeder, L.C.; et al. Linezolid Trough Concentrations Correlate with Mitochondrial Toxicity-Related Adverse Events in the Treatment of Chronic Extensively Drug-Resistant Tuberculosis. EBioMedicine 2015, 2, 1627–1633. [Google Scholar] [CrossRef] [Green Version]
- Apodaca, A.A.; Rakita, R.M. Linezolid-Induced Lactic Acidosis. N. Engl. J. Med. 2003, 348, 86–87. [Google Scholar] [CrossRef]
- Agyeman, A.A.; Ofori-Asenso, R. Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: A systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, R.S.; Lester, W. Clinical experience with cycloserine in the treatment of tuberculosis. Scand. J. Respir. Dis. Suppl. 1970, 71, 94–108. [Google Scholar] [PubMed]
- Degiacomi, G.; Sammartino, J.C.; Sinigiani, V.; Marra, P.; Urbani, A.; Pasca, M.R. In vitro Study of Bedaquiline Resistance in Mycobacterium tuberculosis Multi-Drug Resistant Clinical Isolates. Front. Microbiol. 2020, 11, 559469. [Google Scholar] [CrossRef] [PubMed]
- Beckert, P.; Sanchez-Padilla, E.; Merker, M.; Dreyer, V.; Kohl, T.A.; Utpatel, C.; Köser, C.U.; Barilar, I.; Ismail, N.; Omar, S.V.; et al. MDR M. tuberculosis outbreak clone in Eswatini missed by Xpert has elevated bedaquiline resistance dated to the pre-treatment era. Genome Med. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Domingo-Gonzalez, R.; Prince, O.; Cooper, A.; Khader, S.A. Cytokines and Chemokines in Mycobacterium tuberculosis Infection. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez-Méndez, R.; García-García, I.; Fernández-Olivera, N.; Valdés-Quintana, M.; Milanés-Virelles, M.T.; Carbonell, D.; Machado-Molina, D.; Valenzuela-Silva, C.M.; López-Saura, P.A. Adjuvant interferon gamma in patients with drug—Resistant pulmonary tuberculosis: A pilot study. BMC Infect. Dis. 2004, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Dawson, R.; Condos, R.; Tse, D.; Huie, M.L.; Ress, S.; Tseng, C.-H.; Brauns, C.; Weiden, M.; Hoshino, Y.; Bateman, E.; et al. Immunomodulation with recombinant interferon-gamma1b in pulmonary tuberculosis. PLoS ONE 2009, 4, e6984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupa, A.; Fol, M.; Dziadek, B.R.; Kepka, E.; Wojciechowska, D.; Brzostek, A.; Torzewska, A.; Dziadek, J.; Baughman, R.P.; Griffith, D.; et al. Binding of CXCL8/IL-8 toMycobacterium tuberculosisModulates the Innate Immune Response. Mediat. Inflamm. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.A.; Mazhar, H.; Saleha, S.; Tipu, H.N.; Muhammad, N.; Abbas, M.N. Interferon-Gamma Improves Macrophages Function against M. tuberculosis in Multidrug-Resistant Tuberculosis Patients. Chemother. Res. Pract. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cheallaigh, C.N.; Sheedy, F.J.; Harris, J.; Muñoz-Wolf, N.; Lee, J.; West, K.; McDermott, E.P.; Smyth, A.; Gleeson, L.E.; Coleman, M.; et al. A Common Variant in the Adaptor Mal Regulates Interferon Gamma Signaling. Immunity 2016, 44, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Okada, T.; Sawada, T.; Kubota, K. Deferoxamine enhances anti-proliferative effect of interferon-gamma against hepatocellular carcinoma cells. Cancer Lett. 2007, 248, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, J. Interleukin-6: An overview. Annu. Rev. Immunol. 1990, 8, 253–378. [Google Scholar] [CrossRef]
- Saunders, B.M.; Frank, A.A.; Orme, I.M.; Cooper, A.M. Interleukin-6 Induces Early Gamma Interferon Production in the Infected Lung but Is Not Required for Generation of Specific Immunity to Mycobacterium tuberculosisInfection. Infect. Immun. 2000, 68, 3322–3326. [Google Scholar] [CrossRef] [Green Version]
- Nagabhushanam, V.; Solache, A.; Ting, L.M.; Escaron, C.J.; Zhang, J.Y.; Ernst, J.D. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-gamma. J. Immunol. 2003, 171, 4750–4757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, R.K.; Kathania, M.; Raje, M.; Majumdar, S. IL-6 inhibits IFN-γ induced autophagy in Mycobacterium tuberculosis H37Rv infected macrophages. Int. J. Biochem. Cell Biol. 2012, 44, 942–954. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Dos Santos, M.S.; Huang, S.; Russell, M.R.G.; Collinson, L.M.; Macrae, J.I.; West, A.; Jiang, H.; Gutierrez, M.G. Subcellular antibiotic visualization reveals a dynamic drug reservoir in infected macrophages. Science 2019, 364, 1279–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fearns, A.; Greenwood, D.J.; Rodgers, A.; Jiang, H.; Gutierrez, M.G. Correlative light electron ion microscopy reveals in vivo localisation of bedaquiline in Mycobacterium tuberculosis–infected lungs. PLoS Biol. 2020, 18, e3000879. [Google Scholar] [CrossRef]
- Hartkoorn, R.C.; Uplekar, S.; Cole, S.T. Cross-Resistance between Clofazimine and Bedaquiline through Upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 2979–2981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hershko, C.; Link, G.; Konijn, A.M.; Cabantchik, Z.I. Objectives and Mechanism of Iron Chelation Therapy. Ann. N. Y. Acad. Sci. 2005, 1054, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Cumming, B.M.; Addicott, K.W.; Adamson, J.H.; Steyn, A.J. Mycobacterium tuberculosis induces decelerated bioenergetic metabolism in human macrophages. Elife 2018, 7, e39169. [Google Scholar] [CrossRef] [PubMed]
- Hackett, E.E.; Charles-Messance, H.; O’Leary, S.M.; Gleeson, L.E.; Muñoz-Wolf, N.; Case, S.; Wedderburn, A.; Johnston, D.G.; Williams, M.A.; Smyth, A.; et al. Mycobacterium tuberculosis Limits Host Glycolysis and IL-1β by Restriction of PFK-M via MicroRNA-21. Cell Rep. 2020, 30, 124–136.e4. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Gatineau, A.; Coya, J.M.; Maure, A.; Biton, A.; Thomson, M.; Bernard, E.M.; Marrec, J.; Gutierrez, M.G.; Larrouy-Maumus, G.; Brosch, R.; et al. The antibiotic bedaquiline activates host macrophage innate immune resistance to bacterial infection. Elife 2020, 9, e55692. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cahill, C.; O’Connell, F.; Gogan, K.M.; Cox, D.J.; Basdeo, S.A.; O’Sullivan, J.; Gordon, S.V.; Keane, J.; Phelan, J.J. The Iron Chelator Desferrioxamine Increases the Efficacy of Bedaquiline in Primary Human Macrophages Infected with BCG. Int. J. Mol. Sci. 2021, 22, 2938. https://doi.org/10.3390/ijms22062938
Cahill C, O’Connell F, Gogan KM, Cox DJ, Basdeo SA, O’Sullivan J, Gordon SV, Keane J, Phelan JJ. The Iron Chelator Desferrioxamine Increases the Efficacy of Bedaquiline in Primary Human Macrophages Infected with BCG. International Journal of Molecular Sciences. 2021; 22(6):2938. https://doi.org/10.3390/ijms22062938
Chicago/Turabian StyleCahill, Christina, Fiona O’Connell, Karl M. Gogan, Donal J. Cox, Sharee A. Basdeo, Jacintha O’Sullivan, Stephen V. Gordon, Joseph Keane, and James J. Phelan. 2021. "The Iron Chelator Desferrioxamine Increases the Efficacy of Bedaquiline in Primary Human Macrophages Infected with BCG" International Journal of Molecular Sciences 22, no. 6: 2938. https://doi.org/10.3390/ijms22062938
APA StyleCahill, C., O’Connell, F., Gogan, K. M., Cox, D. J., Basdeo, S. A., O’Sullivan, J., Gordon, S. V., Keane, J., & Phelan, J. J. (2021). The Iron Chelator Desferrioxamine Increases the Efficacy of Bedaquiline in Primary Human Macrophages Infected with BCG. International Journal of Molecular Sciences, 22(6), 2938. https://doi.org/10.3390/ijms22062938