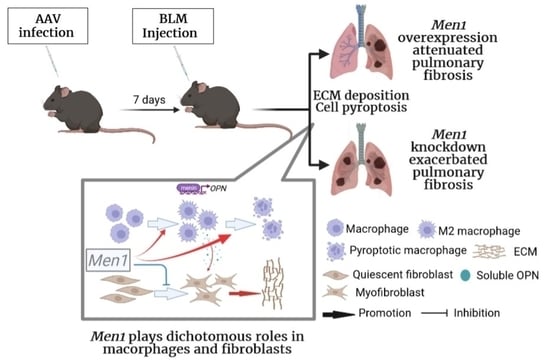
Dichotomous Roles of Men1 in Macrophages and Fibroblasts in Bleomycin—Induced Pulmonary Fibrosis
Abstract
:1. Introduction
2. Results
2.1. Menin Was Downregulated in BLM—Induced Pulmonary Fibrosis
2.2. Men1 Overexpression Improves BLM—Induced Pulmonary Fibrosis
2.3. Inhibition of Men1 Expression Exacerbates BLM—Induced Pulmonary Fibrosis
2.4. The Antifibrotic Role of Men1 in Fibroblasts
2.5. Men1 Has a Profibrotic Role in Macrophages
2.6. Men1 in Macrophages Promotes the Profibrotic Activity of Fibroblast
2.7. Men1 Promotes Inflammasome Activation and Cell Pyroptosis upon BLM-Stimulation
2.8. Men1 Transcriptionally Promotes OPN Expression upon BLM Administration
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animals
4.3. Induction of Pulmonary Fibrosis Model
4.4. Histological Staining
4.5. Immunofluorescence
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Cell Culture and Treatment
4.8. Isolation of Mouse Peritoneal Macrophages (PMC)
4.9. Lentivirus and Retrovirus Packaging
4.10. Transwell Invasion Assay
4.11. Scratch-Wound Healing Assay
4.12. Immunoblotting
4.13. Quantitative Real-Time PCR (qRT-PCR)
4.14. Dual-Luciferase Reporter Gene Assay
4.15. Chromatin Immunoprecipitation (ChIP)
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Arg-1 | Arginase 1 |
| AAV | Adeno associated virus |
| BLM | Bleomycin |
| ChIP | Chromatin immunoprecipitation |
| CM | Culture medium |
| ECM | Extracellular matrix |
| GSDMD | Gasdermin D |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| IL-10/1β/18 | Interleukin 101β/18 |
| IPF | Idiopathic pulmonary fibrosis |
| MEF | Mouse embryo fibroblasts |
| Men1 | Multiple endocrine neoplasia 1 |
| NLRP3 | NLR family pyrin domain containing 3 |
| OPN | Osteopontin |
| PMC | Peritoneal macrophages |
| TNF-α | Tumor necrosis factor |
| α-SMA | Smooth muscle actin α |
References
- Thannickal, V.J.; Toews, G.B.; White, E.S.; Lynch, J.P., III; Martinez, F.J. Mechanisms of pulmonary fibrosis. Annu. Rev. Med. 2004, 55, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.J.; Maher, T.M.; Lloyd, C.M. Pulmonary Macrophages: A New Therapeutic Pathway in Fibrosing Lung Disease? Trends Mol. Med. 2016, 22, 303–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M.; Clay, S.; Partolina, M.; Vuthoori, S.; Wu, S.; Lang, R.; Iredale, J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Investig. 2005, 115, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.Z.; Yao, B.; Yang, S.; Jiang, L.; Wang, S.; Fan, X.; Yin, H.; Wong, K.; Miyazawa, T.; Chen, J.; et al. CSF-1 signaling mediates recovery from acute kidney injury. J. Clin. Investig. 2012, 122, 4519–4532. [Google Scholar] [CrossRef] [Green Version]
- Rappolee, D.A.; Mark, D.; Banda, M.J.; Werb, Z. Wound macrophages express TGF-alpha and other growth factors in vivo: Analysis by mRNA phenotyping. Science 1988, 241, 708–712. [Google Scholar] [CrossRef]
- Shook, B.A.; Wasko, R.R.; Rivera-Gonzalez, G.C.; Salazar-Gatzimas, E.; López-Giráldez, F.; Dash, B.C.; Muñoz-Rojas, A.R.; Aultman, K.D.; Zwick, R.K.; Lei, V.; et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 2018, 362, eaar2971. [Google Scholar] [CrossRef] [Green Version]
- Willenborg, S.; Lucas, T.; Van Loo, G.; Knipper, J.A.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood J. Am. Soc. Hematol. 2012, 120, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Li, C.; Lu, Y.; Lei, X.; Zhang, Y.; Li, S.; Liu, F.; Chen, Y.; Weng, D.; Chen, J. Dioscin Alleviates Crystalline Silica−Induced Pulmonary Inflammation and Fibrosis through Promoting Alveolar Macrophage Autophagy. Theranostics 2019, 9, 1878–1892. [Google Scholar] [CrossRef]
- Larson-Casey, J.L.; Deshane, J.S.; Ryan, A.J.; Thannickal, V.J.; Carter, A.B. Macrophage Akt1 Kinase-Mediated Mitophagy Modulates Apoptosis Resistance and Pulmonary Fibrosis. Immunity 2016, 44, 582–596. [Google Scholar] [CrossRef] [Green Version]
- Belchamber, K.B.R.; Donnelly, L.E. Macrophage Dysfunction in Respiratory Disease. In Macrophages; Springer: Berlin/Heidelberg, Germany, 2017; Volume 62, pp. 299–313. [Google Scholar]
- Gasse, P.; Riteau, N.; Charron, S.; Girre, S.; Fick, L.; Pétrilli, V.; Tschopp, J.; Lagente, V.; Quesniaux, V.F.; Ryffel, B.; et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2009, 179, 903–913. [Google Scholar] [CrossRef]
- Gasse, P.; Mary, C.; Guenon, I.; Noulin, N.; Charron, S.; Schnyder-Candrian, S.; Schnyder, B.; Akira, S.; Quesniaux, V.F.; Lagente, V.; et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J. Clin. Investig. 2007, 117, 3786–3799. [Google Scholar] [CrossRef] [Green Version]
- Cassel, S.L.; Eisenbarth, S.C.; Iyer, S.S.; Sadler, J.J.; Colegio, O.R.; Tephly, L.A.; Carter, A.B.; Rothman, P.B.; Flavell, R.A.; Sutterwala, F.S. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA 2008, 105, 9035–9040. [Google Scholar] [CrossRef] [Green Version]
- Dostert, C.; Pétrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Enosi Tuipulotu, D.; Tan, W.H.; Kay, C.; Man, S.M. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef]
- Liang, Q.; Cai, W.; Zhao, Y.; Xu, H.; Tang, H.; Chen, D.; Qian, F.; Sun, L. Lycorine ameliorates bleomycin-induced pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and pyroptosis. Pharmacol. Res. 2020, 158, 104884. [Google Scholar] [CrossRef]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Luukkonen, J.; Hilli, M.; Nakamura, M.; Ritamo, I.; Valmu, L.; Kauppinen, K.; Tuukkanen, J.; Lehenkari, P. Osteoclasts secrete osteopontin into resorption lacunae during bone resorption. Histochem. Cell Biol. 2019, 151, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlin, D.; Berman, J.S. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 2001, 107, 1055–1061. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, F.; Takahashi, K.; Okazaki, T.; Maeda, K.; Ienaga, H.; Maeda, M.; Kon, S.; Uede, T.; Fukuchi, Y. Role of osteopontin in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2001, 24, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Berman, J.S.; Serlin, D.; Li, X.; Whitley, G.; Hayes, J.; Rishikof, D.C.; Ricupero, D.A.; Liaw, L.; Goetschkes, M.; O’Regan, A.W. Altered bleomycin-induced lung fibrosis in osteopontin-deficient mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L1311–L1318. [Google Scholar] [CrossRef] [Green Version]
- Morse, C.; Tabib, T.; Sembrat, J.; Buschur, K.L.; Bittar, H.T.; Valenzi, E.; Jiang, Y.; Kass, D.J.; Gibson, K.; Chen, W.; et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir. J. 2019, 54, 1802441. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirahara, K.; Kiuchi, M.; Wada, T.; Ichikawa, T.; Kanno, T.; Okano, M.; Kokubo, K.; Onodera, A.; Sakurai, D.; et al. Amphiregulin-Producing Pathogenic Memory T Helper 2 Cells Instruct Eosinophils to Secrete Osteopontin and Facilitate Airway Fibrosis. Immunity 2018, 49, 134–150.e6. [Google Scholar] [CrossRef] [Green Version]
- Matkar, S.; Thiel, A.; Hua, X. Menin: A scaffold protein that controls gene expression and cell signaling. Trends Biochem. Sci. 2013, 38, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Sowa, H.; Kaji, H.; Hendy, G.N.; Canaff, L.; Komori, T.; Sugimoto, T.; Chihara, K. Menin is required for bone morphogenetic protein 2- and transforming growth factor beta-regulated osteoblastic differentiation through interaction with Smads and Runx2. J. Biol. Chem. 2004, 279, 40267–40275. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Yuan, M.; Shen, S.; Ma, X.; Fang, J.; Zhu, L.; Sun, L.; Liu, Z.; He, X.; Huang, D.; et al. Menin enhances c-Myc-mediated transcription to promote cancer progression. Nat. Commun. 2017, 8, 15278. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Jothi, R. Genome-wide characterization of menin-dependent H3K4me3 reveals a specific role for menin in the regulation of genes implicated in MEN1-like tumors. PLoS ONE 2012, 7, e37952. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Juan, C.W.; Chen, K.Y.; Chang, Y.C.; Lee, J.C.; Chang, M.C. Upregulation of RPA2 promotes NF-κB activation in breast cancer by relieving the antagonistic function of menin on NF-κB-regulated transcription. Carcinogenesis 2017, 38, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Gurung, B.; Wan, B.; Matkar, S.; Veniaminova, N.A.; Wan, K.; Merchant, J.L.; Hua, X.; Lei, M. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature 2012, 482, 542–546. [Google Scholar] [CrossRef]
- Yokoyama, A.; Somervaille, T.C.; Smith, K.S.; Rozenblatt-Rosen, O.; Meyerson, M.; Cleary, M.L. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell 2005, 123, 207–218. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.H.; Zheng, R.; Gao, S.B.; Ding, L.H.; Yin, Z.Y.; Lin, X.; Feng, Z.J.; Zhang, S.; Wang, X.M.; et al. Menin promotes hepatocellular carcinogenesis and epigenetically up-regulates Yap1 transcription. Proc. Natl. Acad. Sci. USA 2013, 110, 17480–17485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, H.; Jin, B.M.; Wang, Z.F.; Xu, B.; Zheng, Q.F.; Zhang, L.; Zhu, L.Y.; Shi, S.; Yuan, J.B.; Lin, X.; et al. MEN1 deficiency leads to neuroendocrine differentiation of lung cancer and disrupts the DNA damage response. Nat. Commun. 2020, 11, 1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreijerink, K.M.A.; Groner, A.C.; Vos, E.S.M.; Font-Tello, A.; Gu, L.; Chi, D.; Reyes, J.; Cook, J.; Lim, E.; Lin, C.Y.; et al. Enhancer-Mediated Oncogenic Function of the Menin Tumor Suppressor in Breast Cancer. Cell Rep. 2017, 18, 2359–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, R.; Khan, A.P.; Asangani, I.A.; Cieślik, M.; Prensner, J.R.; Wang, X.; Iyer, M.K.; Jiang, X.; Borkin, D.; Escara-Wilke, J.; et al. Targeting the MLL complex in castration-resistant prostate cancer. Nat. Med. 2015, 21, 344–352. [Google Scholar] [CrossRef]
- Zindy, P.J.; L’helgoualc’h, A.; Bonnier, D.; Le Béchec, A.; Bourd-Boitin, K.; Zhang, C.X.; Musso, O.; Glaise, D.; Troadec, M.B.; Loréal, O.; et al. Upregulation of the tumor suppressor gene menin in hepatocellular carcinomas and its significance in fibrogenesis. Hepatology 2006, 44, 1296–1307. [Google Scholar] [CrossRef]
- Hall, C.; Ehrlich, L.; Meng, F.; Invernizzi, P.; Bernuzzi, F.; Lairmore, T.C.; Alpini, G.; Glaser, S. Inhibition of microRNA-24 increases liver fibrosis by enhanced menin expression in Mdr2−/− mice. J. Surg. Res. 2017, 217, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Zhang, H.Y.; Gong, X.K.; Dong, Z.; Chen, Z.Y.; Wang, R.; Yi, J.X.; Shen, Y.N.; Jin, S.Z. Mechanism of MEN1 gene in radiation-induced pulmonary fibrosis in mice. Gene 2018, 678, 252–260. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, Z.J.; Gao, S.B.; Matkar, S.; Xu, B.; Duan, H.B.; Lin, X.; Li, S.H.; Hua, X.; Jin, G.H. Interplay between menin and K-Ras in regulating lung adenocarcinoma. J. Biol. Chem. 2012, 287, 40003–40011. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.B.; Feng, Z.J.; Xu, B.; Wu, Y.; Yin, P.; Yang, Y.; Hua, X.; Jin, G.H. Suppression of lung adenocarcinoma through menin and polycomb gene-mediated repression of growth factor pleiotrophin. Oncogene 2009, 28, 4095–4104. [Google Scholar] [CrossRef] [Green Version]
- Ballester, B.; Milara, J.; Cortijo, J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2019, 20, 593. [Google Scholar] [CrossRef] [Green Version]
- Della Latta, V.; Cecchettini, A.; Del Ry, S.; Morales, M.A. Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacol. Res. 2015, 97, 122–130. [Google Scholar] [CrossRef]
- Hsu, H.S.; Liu, C.C.; Lin, J.H.; Hsu, T.W.; Hsu, J.W.; Su, K.; Hung, S.C. Involvement of ER stress, PI3K/AKT activation, and lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Sci. Rep. 2017, 7, 14272. [Google Scholar] [CrossRef]
- Ma, W.H.; Li, M.; Ma, H.F.; Li, W.; Liu, L.; Yin, Y.; Zhou, X.M.; Hou, G. Protective effects of GHK-Cu in bleomycin-induced pulmonary fibrosis via anti-oxidative stress and anti-inflammation pathways. Life Sci. 2020, 241, 117139. [Google Scholar] [CrossRef]
- Dong, R.; Liu, M.; Huang, X.X.; Liu, Z.; Jiang, D.Y.; Xiao, H.J.; Geng, J.; Ren, Y.H.; Dai, H.P. Water-Soluble C60 Protects Against Bleomycin-Induced Pulmonary Fibrosis in Mice. Int. J. Nanomed. 2020, 15, 2269–2276. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Li, J.; Tu, X.; Wu, C.; Liu, H.; Luo, Y.; Dong, X.; Li, X.; Pan, L.L.; Sun, J. Lysine-specific demethylase-1 regulates fibroblast activation in pulmonary fibrosis via TGF-β1/Smad3 pathway. Pharmacol. Res. 2020, 152, 104592. [Google Scholar] [CrossRef]
- Lis-Lopez, L.; Bauset, C.; Seco-Cervera, M.; Cosin-Roger, J. Is the Macrophage Phenotype Determinant for Fibrosis Development? Biomedicines 2021, 9, 1747. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Xiang, Z.; Ren, Y.; Zheng, X.; Zhao, Q.; Zhou, Q.; Zhou, Y.; Xu, L.; Wang, Y. Microcystin-LR ameliorates pulmonary fibrosis via modulating CD206+ M2-like macrophage polarization. Cell Death Dis. 2020, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Hatipoglu, O.F.; Uctepe, E.; Opoku, G.; Wake, H.; Ikemura, K.; Ohtsuki, T.; Inagaki, J.; Gunduz, M.; Gunduz, E.; Watanabe, S.; et al. Osteopontin silencing attenuates bleomycin-induced murine pulmonary fibrosis by regulating epithelial-mesenchymal transition. Biomed. Pharmacother. 2021, 139, 111633. [Google Scholar] [CrossRef]
- Hou, J.; Ji, J.; Chen, X.; Cao, H.; Tan, Y.; Cui, Y.; Xiang, Z.; Han, X. Alveolar epithelial cell-derived Sonic hedgehog promotes pulmonary fibrosis through OPN-dependent alternative macrophage activation. FEBS J. 2021, 288, 3530–3546. [Google Scholar] [CrossRef]
- La, P.; Desmond, A.; Hou, Z.; Silva, A.C.; Schnepp, R.W.; Hua, X. Tumor suppressor menin: The essential role of nuclear localization signal domains in coordinating gene expression. Oncogene 2006, 25, 3537–3546. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef] [Green Version]
- Naito, J.; Kaji, H.; Sowa, H.; Hendy, G.N.; Sugimoto, T.; Chihara, K. Menin suppresses osteoblast differentiation by antagonizing the AP-1 factor, JunD. J. Biol. Chem. 2005, 280, 4785–4791. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jia, Y.; Feng, Y.; Cui, R.; Miao, R.; Zhang, X.; Qu, K.; Liu, C.; Zhang, J. Methane alleviates sepsis-induced injury by inhibiting pyroptosis and apoptosis: In vivo and in vitro experiments. Aging 2019, 11, 1226–1239. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Y.; Zhang, Z.; He, L.; Zhu, J.; Zhang, M.; He, X.; Cheng, Z.; Ao, Q.; Cao, Y.; et al. Chop Deficiency Protects Mice Against Bleomycin-induced Pulmonary Fibrosis by Attenuating M2 Macrophage Production. Mol. Ther. 2016, 24, 915–925. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, J.; Zhao, J.; Song, Z.; Zhou, C.; Liu, X.; Teng, W.; Wang, W.; Zhang, Q.; Yan, W.; et al. The Role of MKP-5 in Adipocyte-Macrophage Interactions during Obesity. Obes. Facts 2020, 13, 86–101. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhao, J.; Tian, Y.; Shao, D.; Zhang, Z.; Li, S.; Li, J.; Zhang, H.; Wang, W.; Jiao, P.; et al. Dichotomous Roles of Men1 in Macrophages and Fibroblasts in Bleomycin—Induced Pulmonary Fibrosis. Int. J. Mol. Sci. 2022, 23, 5385. https://doi.org/10.3390/ijms23105385
Lu Y, Zhao J, Tian Y, Shao D, Zhang Z, Li S, Li J, Zhang H, Wang W, Jiao P, et al. Dichotomous Roles of Men1 in Macrophages and Fibroblasts in Bleomycin—Induced Pulmonary Fibrosis. International Journal of Molecular Sciences. 2022; 23(10):5385. https://doi.org/10.3390/ijms23105385
Chicago/Turabian StyleLu, Yuanhua, Jianan Zhao, Yafei Tian, Dan Shao, Zhiqi Zhang, Siqi Li, Jialin Li, Hugang Zhang, Wei Wang, Ping Jiao, and et al. 2022. "Dichotomous Roles of Men1 in Macrophages and Fibroblasts in Bleomycin—Induced Pulmonary Fibrosis" International Journal of Molecular Sciences 23, no. 10: 5385. https://doi.org/10.3390/ijms23105385
APA StyleLu, Y., Zhao, J., Tian, Y., Shao, D., Zhang, Z., Li, S., Li, J., Zhang, H., Wang, W., Jiao, P., & Ma, J. (2022). Dichotomous Roles of Men1 in Macrophages and Fibroblasts in Bleomycin—Induced Pulmonary Fibrosis. International Journal of Molecular Sciences, 23(10), 5385. https://doi.org/10.3390/ijms23105385